Bioabsorbable Magnesium-Based Materials Potential and Safety in Bone Surgery: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
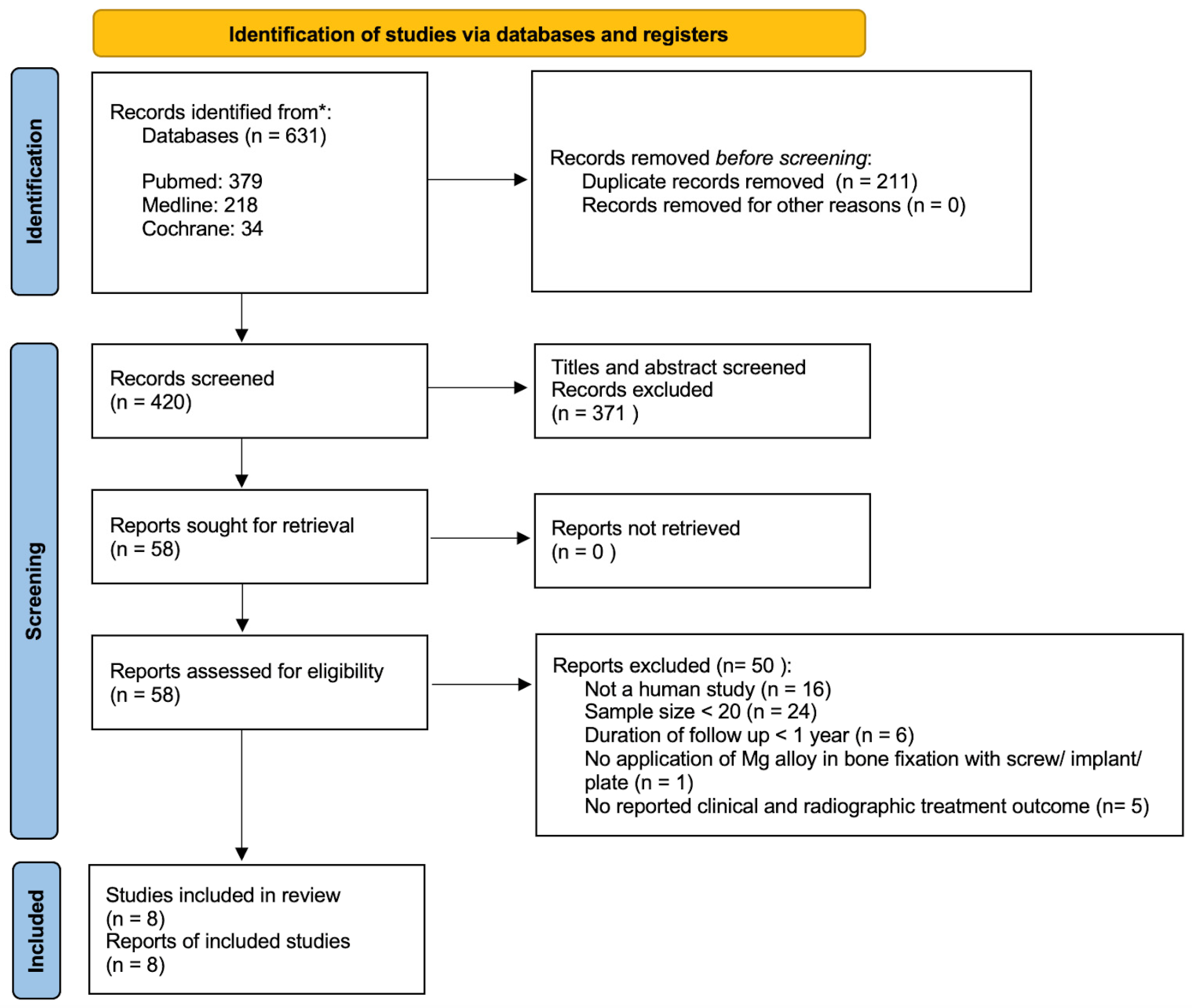
2.2. Study Selection
2.3. Search Strategy
2.3.1. First Round Search
2.3.2. Second Round Search
2.3.3. Third Round Search
2.4. Data Extraction
3. Results
| Studies | D1 | D2 | D3 | D4 | D5 | D6 | D7 | Overall |
|---|---|---|---|---|---|---|---|---|
| Stürznickel J et al. [12] | No | No | No | No | No | No | No | Low |
| May H et al. [13] | No | No | No | No | Yes | Yes | No | Moderate |
| Zhao D et al. [8] | No | No | No | No | No | No | No | Low |
| Lee JW et al. [14] | No | No | No | No | Yes | Yes | No | Moderate |
| Polat O et al. [15] | No | No | No | No | No | No | No | Low |
| Herber V et al. [16] | No | No | No | No | No | No | No | Low |
| Choo JT et al. [17] | No | No | No | No | Yes | Yes | No | Moderate |
| Lee CH et al. [18] | No | No | No | No | Yes | Yes | No | Moderate |
| Authors | Year | Title | Study Design | Number of Participants | Patient Mean Age | Anatomical Site | Type of Mg Used | Titanium Screws Use | Outcome Measures | Follow-Up Time Point | Country |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Stürznickel et al. [12] | 2021 | Safety and performance of biodegradable magnesium-based implants in children and adolescents | Case series | Mg: 89 | Mg: 12.9 ± 3.2 | Proximal tibia Elbow Upper Ankle joint Patella Distal fermur | MgYREZr | / | Combination of clinical (range of motion, functions, pain) and radiographic (X-rays and/or MRI) findings. | 4th–8th weeks, 3rd–6th months | Germany |
| May et al. [13] | 2020 | Bioabsorbable magnesium screw versus conventional titanium screw fixation for medial malleolar fractures | Case series | Mg: 23 Ti: 25 | Mg: 37.9 ± 17.7 Ti: 45 ± 15.7 | Medial malleolar | MgYREZr | Yes | Combination of clinical (infection, wound problem, ankle instability, tendon subluxation, AOFAS, LK grading), and radiographic (X-rays and CT) findings. | Mean 24.7 ± 12.0 months (no scheduled follow-up due to retrospective in nature) | Turkey |
| Zhao et al. [8] | 2015 | Vascularized bone grafting fixed by biodegradable magnesium screw for treating osteonecrosis of the femoral head | RCT | Mg: 23 | Mg: 30 ± 7 | Femoral head | Pure Mg (99.99 wt.%) | / | Combination of clinical (Harris hip score) and radiographic (X-rays and CT) findings and blood test (trace element detector for serum level). | 1st, 3rd, 6th, and 12th month | China |
| Lee JW et al. [14] | 2016 | Long-term clinical study and multiscale analysis of in vivo biodegradation mechanism of Mg alloy | Case series | Mg: 53 | N/A | Scaphoid, distal radius | 5 wt% Ca, 1 wt% Zn screw | / | Combination of clinical (VAS, DASH, range of motion, grip power) and radiographic (X-rays) findings. | 1st and 2nd week, 1st, 2nd, 3rd, and 6th month, 1st year | Republic of Korea |
| Polat et al. [15] | 2021 | Surgical outcomes of scaphoid fracture osteosynthesis with magnesium screws | Case series | Mg: 21 | Mg: 28.5 ± 5.8 | Scaphoid | MgYREZr | / | Combination of clinical (grip strength, pinch strength, and range of motion) and radiographic (X-rays) findings. | Mean: 43.3 ± 5.3 months (no scheduled follow-up due to retrospective nature of study) | Turkey |
| Herber et al. [16] | 2021 | Can Hardware Removal be Avoided Using Bioresorbable Mg-Zn-Ca Screws After Medial Malleolar Fracture Fixation? Mid-Term Results of a First-In-Human Study | Non-randomized cohort study | Mg: 20 Ti: 17 | Mg: 40.1 ±14.5 Ti: N/A | Medial malleolar | ZX00 rods (99.1 wt% Mg, 0.45 wt% Zn, and 0.45 wt% Ca) | Yes | Combination of clinical (VAS, AOFAS, blood analysis, and range of motion) and radiographic (X-rays) findings. | 6th and 12th month | Austria |
| Choo et al. [17] | 2018 | Magnesium-based bioabsorbable screw fixation for hallux valgus surgery—A suitable alternative to metallic implants | Case series | Mg: 24 Ti: 69 | Mg: 54.5 ± 12.0 Ti: N/A | Hallux Vagus | MgYREZr | Yes | Combination of short form 36 (SF-36), AOFAS-HMI, VAS, and radiological evaluation (pre-op, 3 and 12 months); limited field forefoot CT scan was performed at 12 months. | 3rd and 12th month | Singapore |
| Lee CH et al. [18] | 2023 | Results of the Use of Bioabsorbable Magnesium Screws for Surgical Treatment of Mason Type II Radial Head Fractures | Case series | Mg: 22 | Mg: 52.3 | Radial head | Mg Screw (Resomet: U&I Corp Seoul, Republic of Korea) | / | Combination of disabilities of the arm, shoulder, and hand (DASH) score, mayo elbow performance score (MEPS), range of joint motion and hand grip power, measured at 6 months post-op; radiographic tests performed at 2 weeks, 4 weeks, 8 weeks, 12 weeks, 6 months, and 1 year. | 2nd, 4th, 8th, 12th, and 6th month, 1st year | Republic of Korea |
3.1. Types of Magnesium Used
3.2. Anatomical Sites Treated
3.3. Outcome Measures of Included Studies
3.4. Clinical Outcomes
3.4.1. Infection Rate and Serum Level
3.4.2. Need for Revision
3.5. Radiographic Outcome
3.5.1. Bone Union
3.5.2. Radiolucent Zone
3.6. Comparison with Titanium Implants
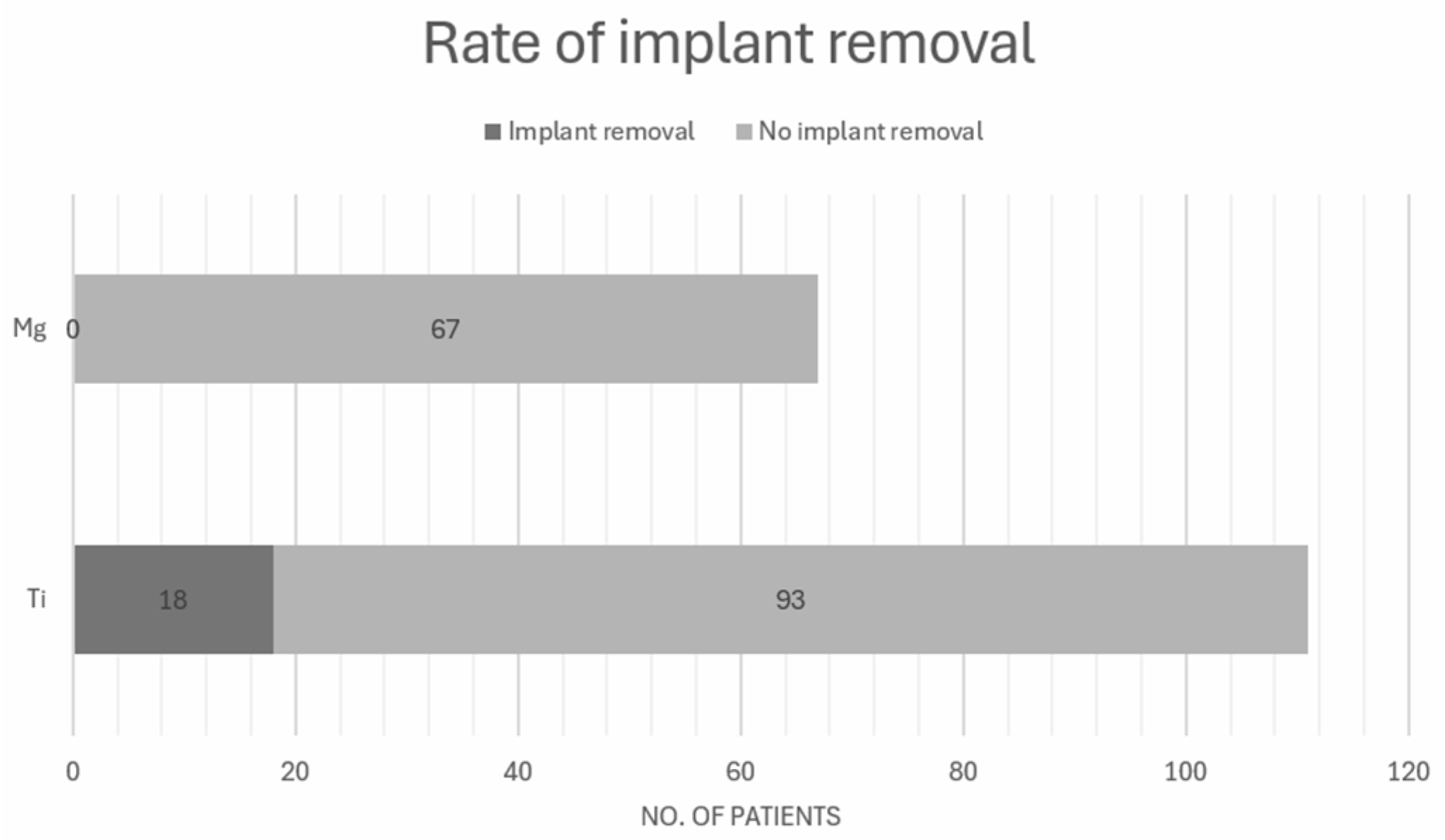
3.6.1. Rate of Implant Removal
3.6.2. AOFAS Score
3.7. Rate of Mg Implant Degradation (Table 5)
| Author | Title | Types of Mg Used | Dimension | Anatomical Stie | Rate of Degradation |
|---|---|---|---|---|---|
| Stürznickel et al. [12] | Safety and performance of biodegradable magnesium-based implants in children and adolescents | MgYREZr | Diameter: 4.8 mm Length: Not mentioned | Patella | One patient had visible degradation of screw head at 1 year; another patient had degradation of the magnesium-based implants that was observed after 15 months. |
| Zhao et al. [8] | Vascularized bone grafting fixed by biodegradable magnesium screw for treating osteonecrosis of the femoral head | Pure Mg (99.99 wt.%) | Diameter: 4 mm Length: 40 mm | Femoral head | A decrease in screw diameter of around 25% over a period of 12 months (3.7%, 9.3%, 13.7% and 25.2% reduction at 1, 3, 6, and 12 months postoperatively). |
| Herber et al. [16] | Can Hardware Removal be Avoided Using Bioresorbable Mg-Zn-Ca Screws After Medial Malleolar Fracture Fixation? Mid-Term Results of a First-In-Human Study | ZX00 rods (99.1 wt% Mg, 0.45 wt% Zn and 0.45 wt% Ca) | Diameter: 3.5 mm Length: 40 mm | Medial malleolar | Of the included patients, 17 (90%) had a radiographic disappearance of the screw head at one year; shafts of screw were still visible after 6 and 12 months. |
| Choo et al. [17] | Magnesium-based bioabsorbable screw fixation for hallux valgus surgery—A suitable alternative to metallic implants | MgYREZr | Diameter: 3.2 mm Length: Not mentioned | Hallux valgus | A few patients had almost full absorption of the screw which occurred at one year. * |
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Park, Y.W. Bioabsorbable osteofixation for orthognathic surgery. Maxillofac. Plast. Reconstr. Surg. 2015, 37, 6. [Google Scholar] [CrossRef] [PubMed]
- Luthringer, B.J.; Feyerabend, F.; Willumeit-Romer, R. Magnesium-based implants: A mini-review. Magnes. Res. 2014, 27, 142–154. [Google Scholar] [CrossRef] [PubMed]
- Geetha, M.; Singh, A.K.; Asokamani, R.; Gogia, A.K. Ti based biomaterials, the ultimate choice for orthopaedic implants—A review. Prog. Mater. Sci. 2009, 54, 397–425. [Google Scholar] [CrossRef]
- Niinomi, M.; Nakai, M. Titanium-Based Biomaterials for Preventing Stress Shielding between Implant Devices and Bone. Int. J. Biomater. 2011, 2011, 836587. [Google Scholar] [CrossRef] [PubMed]
- Vos, D.I.; Verhofstad, M.H. Indications for implant removal after fracture healing: A review of the literature. Eur. J. Trauma Emerg. Surg. 2013, 39, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Tang, N.; Ngai, T.; Wu, C.; Ruan, Y.; Huang, L.; Qin, L. Hybrid fracture fixation systems developed for orthopaedic applications: A general review. J. Orthop. Translat. 2019, 16, 1–13. [Google Scholar] [CrossRef]
- You, S.; Huang, Y.; Kainer, K.U.; Hort, N. Recent research and developments on wrought magnesium alloys. J. Magnes. Alloys 2017, 5, 239–253. [Google Scholar] [CrossRef]
- Zhao, D.; Huang, S.; Lu, F.; Wang, B.; Yang, L.; Qin, L.; Yang, K.; Li, Y.; Li, W.; Wang, W.; et al. Vascularized bone grafting fixed by biodegradable magnesium screw for treating osteonecrosis of the femoral head. Biomaterials 2016, 81, 84–92. [Google Scholar] [CrossRef]
- Shan, Z.; Xie, X.; Wu, X.; Zhuang, S.; Zhang, C. Development of degradable magnesium-based metal implants and their function in promoting bone metabolism (A review). J. Orthop. Translat. 2022, 36, 184–193. [Google Scholar] [CrossRef]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef]
- Sterne, J.A.; Hernan, M.A.; Reeves, B.C.; Savovic, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar]
- Sturznickel, J.; Delsmann, M.M.; Jungesblut, O.D.; Stucker, R.; Knorr, C.; Rolvien, T.; Kertai, M.; Rupprecht, M. Safety and performance of biodegradable magnesium-based implants in children and adolescents. Injury 2021, 52, 2265–2271. [Google Scholar] [PubMed]
- May, H.; Alper Kati, Y.; Gumussuyu, G.; Yunus Emre, T.; Unal, M.; Kose, O. Bioabsorbable magnesium screw versus conventional titanium screw fixation for medial malleolar fractures. J. Orthop. Traumatol. 2020, 21, 9. [Google Scholar] [PubMed]
- Lee, J.W.; Han, H.S.; Han, K.J.; Park, J.; Jeon, H.; Ok, M.R.; Seok, H.-K.; Ahn, J.-P.; Lee, K.E.; Lee, D.-H.; et al. Long-term clinical study and multiscale analysis of in vivo biodegradation mechanism of Mg alloy. Proc. Natl. Acad. Sci. USA 2016, 113, 716–721. [Google Scholar] [PubMed]
- Polat, O.; Toy, S.; Kibar, B. Surgical outcomes of scaphoid fracture osteosynthesis with magnesium screws. Jt. Dis. Relat. Surg. 2021, 32, 721–728. [Google Scholar] [PubMed]
- Herber, V.; Labmayr, V.; Sommer, N.G.; Marek, R.; Wittig, U.; Leithner, A.; Seibert, F.; Holweg, P. Can Hardware Removal be Avoided Using Bioresorbable Mg-Zn-Ca Screws After Medial Malleolar Fracture Fixation? Mid-Term Results of a First-In-Human Study. Injury 2022, 53, 1283–1288. [Google Scholar]
- Choo, J.T.; Lai, S.H.S.; Tang, C.Q.Y.; Thevendran, G. Magnesium-based bioabsorbable screw fixation for hallux valgus surgery—A suitable alternative to metallic implants. Foot Ankle Surg. 2019, 25, 727–732. [Google Scholar]
- Lee, C.H.; Woo, S.; Choi, H.D. Results of the Use of Bioabsorbable Magnesium Screws for Surgical Treatment of Mason Type II Radial Head Fractures. Clin. Orthop. Surg. 2023, 15, 1013–1021. [Google Scholar] [PubMed]
- Van Lieshout, E.M.; De Boer, A.S.; Meuffels, D.E.; Den Hoed, P.T.; Van der Vlies, C.H.; Tuinebreijer, W.E.; Verhofstad, M.H.J. American Orthopaedic Foot and Ankle Society (AOFAS) Ankle-Hindfoot Score: A study protocol for the translation and validation of the Dutch language version. BMJ Open 2017, 7, e012884. [Google Scholar]
- Gareb, B.; Van Bakelen, N.B.; Vissink, A.; Bos, R.R.M.; Van Minnen, B. Titanium or Biodegradable Osteosynthesis in Maxillofacial Surgery? In Vitro and In Vivo Performances. Polymers 2022, 14, 2782. [Google Scholar] [CrossRef]
- Lee, B.J.; Lee, C.H.; Lee, Y.H.; Woo, S. Intramedullary fixation of metacarpal and phalangeal bone fractures with bioabsorbable Mg K-wire in 20 cases. Eur. J. Orthop. Surg. Traumatol. 2023, 33, 2911–2920. [Google Scholar] [CrossRef] [PubMed]
- Kose, O. Magnesium (MgYREZr) Bioabsorbable Screws in Orthopedic Surgery; Military Medicine Worldwide: Belgium, Brussels, 2019. [Google Scholar]
- Baldini, M.; Coppa, V.; Falcioni, D.; Senigagliesi, E.; Marinelli, M.; Gigante, A.P. Use of resorbable magnesium screws in children: Systematic review of the literature and short-term follow-up from our series. J. Child. Orthop. 2021, 15, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Wang, G.X.; Wang, C.G.; Li, Z.J. Comparison between bioabsorbable magnesium and titanium compression screws for hallux valgus treated with distal metatarsal osteotomies: A meta-analysis. Jt. Dis. Relat. Surg. 2023, 34, 289–297. [Google Scholar] [PubMed]
- Plaass, C.; von Falck, C.; Ettinger, S.; Sonnow, L.; Calderone, F.; Weizbauer, A.; Reifenrath, J.; Claassen, L.; Waizy, H.; Daniilidis, K.; et al. Bioabsorbable magnesium versus standard titanium compression screws for fixation of distal metatarsal osteotomies—3 year results of a randomized clinical trial. J. Orthop. Sci. 2018, 23, 321–327. [Google Scholar] [CrossRef]
- Konneker, S.; Schachinger, U.; Vogt, P.M. Magnesium-Based Compression Screws in Acute Scaphoid Fractures and Nonunions. World J. Surg. 2023, 47, 1129–1135. [Google Scholar]
- Unal, M.; Demirayak, E.; Ertan, M.B.; Kilicaslan, O.F.; Kose, O. Bioabsorbable magnesium screw fixation for tibial tubercle osteotomy; a preliminary study. Acta Biomed. 2022, 92, e2021263. [Google Scholar]
- Acar, B.; Unal, M.; Turan, A.; Kose, O. Isolated Lateral Malleolar Fracture Treated with a Bioabsorbable Magnesium Compression Screw. Cureus 2018, 10, e2539. [Google Scholar]
- Anderson, T.; Lee, J.; Johnston, P.; Torreggiani, W.; Ryan, M. Magnesium implants in orthopaedic surgery create a diagnostic conundrum: A radiology case series and literature review. Ir. J. Med. Sci. 2023, 192, 1381–1385. [Google Scholar] [CrossRef]
- Gutiérrez Púa, L.D.C.; Rincón Montenegro, J.C.; Fonseca Reyes, A.M.; Zambrano Rodríguez, H.; Paredes Méndez, V.N. Biomaterials for orthopedic applications and techniques to improve corrosion resistance and mechanical properties for magnesium alloy: A review. J. Mater. Sci. 2023, 58, 3879–3908. [Google Scholar]
- Acar, B.; Kose, O.; Turan, A.; Unal, M.; Kati, Y.A.; Guler, F. Comparison of Bioabsorbable Magnesium versus Titanium Screw Fixation for Modified Distal Chevron Osteotomy in Hallux Valgus. Biomed. Res. Int. 2018, 2018, 5242806. [Google Scholar]
- Plaass, C.; Ettinger, S.; Sonnow, L.; Koenneker, S.; Noll, Y.; Weizbauer, A.; Reifenrath, J.; Claassen, L.; Daniilidis, K.; Stukenborg-Colsman, C.; et al. Early results using a biodegradable magnesium screw for modified chevron osteotomies. J. Orthop. Res. 2016, 34, 2207–2214. [Google Scholar] [CrossRef] [PubMed]
- Meier, R.; Panzica, M. First results with a resorbable MgYREZr compression screw in unstable scaphoid fractures show extensive bone cysts. Handchir Mikrochir Plast Chir. 2017, 49, 37–41. [Google Scholar]
- Delsmann, M.M.; Sturznickel, J.; Kertai, M.; Stucker, R.; Rolvien, T.; Rupprecht, M. Radiolucent zones of biodegradable magnesium-based screws in children and adolescents-a radiographic analysis. Arch. Orthop. Trauma Surg. 2023, 143, 2297–2305. [Google Scholar] [CrossRef]
- Ng, W.F.; Chiu, K.Y.; Cheng, F.T. Effect of pH on the in vitro corrosion rate of magnesium degradable implant material. Mater. Sci. Eng. C 2010, 30, 898–903. [Google Scholar] [CrossRef]
- Antoniac, I.; Miculescu, M.; Manescu, P.V.; Stere, A.; Quan, P.H.; Paltanea, G.; Robu, A.; Earar, K. Magnesium-Based Alloys Used in Orthopedic Surgery. Materials 2022, 15, 1148. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Witte, F.; Lu, F.; Wang, J.; Li, J.; Qin, L. Current status on clinical applications of magnesium-based orthopaedic implants: A review from clinical translational perspective. Biomaterials 2017, 112, 287–302. [Google Scholar] [CrossRef]
- On, S.W.; Cho, S.W.; Byun, S.H.; Yang, B.E. Bioabsorbable Osteofixation Materials for Maxillofacial Bone Surgery: A Review on Polymers and Magnesium-Based Materials. Biomedicines 2020, 8, 300. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.; Shadanbaz, S.; Woodfield, T.B.; Staiger, M.P.; Dias, G.J. Magnesium biomaterials for orthopedic application: A review from a biological perspective. J. Biomed. Mater. Res. B Appl. Biomater. 2014, 102, 1316–1331. [Google Scholar] [CrossRef] [PubMed]
- Windhagen, H.; Radtke, K.; Weizbauer, A.; Diekmann, J.; Noll, Y.; Kreimeyer, U.; Schavan, R.; Stukenborg-Colsman, C.; Waizy, H. Biodegradable magnesium-based screw clinically equivalent to titanium screw in hallux valgus surgery: Short term results of the first prospective, randomized, controlled clinical pilot study. Biomed. Eng. Online 2013, 12, 62. [Google Scholar] [CrossRef]
- Sun, J.; Li, Z.; Liu, S.; Xia, T.; Shen, J. Biodegradable magnesium screw, titanium screw and direct embedding fixation in pedicled vascularized iliac bone graft transfer for osteonecrosis of the femoral head: A randomized controlled study. J. Orthop. Surg. Res. 2023, 18, 523. [Google Scholar] [CrossRef] [PubMed]
- Seitz, J.-M.; Lucas, A.; Kirschner, M. Magnesium-Based Compression Screws: A Novelty in the Clinical Use of Implants. Jom 2016, 68, 1177–1182. [Google Scholar] [CrossRef]
- Leonhardt, H.; Ziegler, A.; Lauer, G.; Franke, A. Osteosynthesis of the Mandibular Condyle with Magnesium-Based Biodegradable Headless Compression Screws Show Good Clinical Results During a 1-Year Follow-Up Period. J. Oral Maxillofac. Surg. 2021, 79, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Leonhardt, H.; Franke, A.; McLeod, N.M.H.; Lauer, G.; Nowak, A. Fixation of fractures of the condylar head of the mandible with a new magnesium-alloy biodegradable cannulated headless bone screw. Br. J. Oral Maxillofac. Surg. 2017, 55, 623–625. [Google Scholar] [CrossRef] [PubMed]
- Al-Moraissi, E.A.; Ellis, E., 3rd. Biodegradable and Titanium Osteosynthesis Provide Similar Stability for Orthognathic Surgery. J. Oral Maxillofac. Surg. 2015, 73, 1795–1808. [Google Scholar] [PubMed]
- Cheung, L.K.; Chow, L.K.; Chiu, W.K. A randomized controlled trial of resorbable versus titanium fixation for orthognathic surgery. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2004, 98, 386–397. [Google Scholar] [PubMed]
- Ueki, K.; Yoshizawa, K.; Moroi, A. Bone healing after Le Fort I osteotomy with SSRO, using uHA/PLLA plates and screws, in class II and III patients. J. Craniomaxillofac. Surg. 2019, 47, 1338–1342. [Google Scholar] [PubMed]
- Choi, J.Y.; Kim, J.W.; Yoo, C.K.; Yun, P.Y.; Baek, S.H.; Kim, Y.K. Evaluation of post-surgical relapse in maxillary surgery using resorbable plate. J. Craniomaxillofac. Surg. 2011, 39, 578–582. [Google Scholar] [CrossRef]
- Iizuka, T.; Lindqvist, C. Rigid internal fixation of mandibular fractures. An analysis of 270 fractures treated using the AO/ASIF method. Int. J. Oral Maxillofac. Surg. 1992, 21, 65–69. [Google Scholar]
- Chow, L.K.; Singh, B.; Chiu, W.K.; Samman, N. Prevalence of postoperative complications after orthognathic surgery: A 15-year review. J. Oral Maxillofac. Surg. 2007, 65, 984–992. [Google Scholar] [CrossRef]
| Author | Title | Type of Mg Used | Anatomical Site | Infection | Serum Level | Revision Surgery Performed (Per Patient) | Bone Union | Time for Bone Union (Weeks) | Presence of Radiolucent Zones |
|---|---|---|---|---|---|---|---|---|---|
| Stürznickel et al. [12] | Safety and performance of biodegradable magnesium-based implants in children and adolescents | MgYREZr | Proximal tibia; elbow; upper ankle joint; patella; distal femur | 0/89 (0%) | Not mentioned | 1/89 | 88/89 (98.9%) patients achieved bone union | N/A | All patients 89/89 and decrease in size during follow-up examinations (mean follow-up duration was 8.2 months). |
| May et al. [13] | Bioabsorbable magnesium screw versus conventional titanium screw fixation for medial malleolar fractures | MgYREZr | Medial malleolar | 0/23 (0%) | Not mentioned | 0/23 | All patients (23/23) (100%) achieved bone union | N/A | All patients (23/23), the amount of gas increased in the first 4 months and began to decrease after the 6th month and disappeared within 1 year. |
| Zhao et al. [8] | Vascularized bone grafting fixed by biodegradable magnesium screw for treating osteonecrosis of the femoral head | Pure Mg (99.99 wt.%) | Femoral head | 0/23 (0%) | No significant changes of serum Ca, Mg, and P at 1, 7, and 14 days postoperation | 0/23 | Mg screw promoted bone mineral density | N/A | Not observed. |
| Lee JW et al. [14] | Long-term clinical study and multiscale analysis of in vivo biodegradation mechanism of Mg alloy | 5 wt% Ca, 1 wt% Zn screw | Scaphoid, distal radius | 0/53 (0%) | Not mentioned | 0/53 | All patients (53/53) (100%) achieved bone union | Range: 4–6 | After 6 months of surgical fixation, the distal radius fracture was completely healed with a small radiolucent area; completely healed in 1 year. |
| Polat et al. [15] | Surgical outcomes of scaphoid fracture osteosynthesis with magnesium screws | MgYREZr | Scaphoid | 0/21 (0%) | Not mentioned | 0/21 | All patients (21/21) (100%) achieved bone union | Mean: 11.2 (range: 9–14) | All patients (21/21), radiolucent zone decreased in size at 9 months and completely disappeared within 18 months. |
| Herber et al. [16] | Can Hardware Removal be Avoided Using Bioresorbable Mg-Zn-Ca Screws After Medial Malleolar Fracture Fixation? Mid-Term Results of a First-In-Human Study | ZX00 rods (99.1 wt% Mg, 0.45 wt% Zn, and 0.45 wt% Ca) | Medial malleolar | 0/20 (0%) | Normal levels of Mg and Ca; renal function remained stable | 0/20 | All patients (20/20) (100%) achieved bone union | N/A | 19/19 had a radiolucent zone noted, slightly decreased from 6 months to 1 year. |
| Choo et al. [17] | Magnesium-based bioabsorbable screw fixation for hallux valgus surgery—A suitable alternative to metallic implants | MgYREZr | Hallux Vagus | 3/24 (12.5%) | Not mentioned | 0/24 | All patients (24/24) (100%) achieved bone union | N/A | Soft tissue gas shadows were observed on 3-month X-rays, all of which were resolved on the 12-month postoperative X-ray. |
| Lee CH et al. [18] | Results of the Use of Bioabsorbable Magnesium Screws for Surgical Treatment of Mason Type II Radial Head Fractures | Mg Screw (Resomet:U&I Corp Seoul, Republic of Korea) | Radial head | 0/22 (0%) | Not mentioned | 0/22 | All patients (22/22) (100%) achieved bone union | Mean: 10.2 (range 8–16) | First appeared at an average of 2 weeks after the surgery, peaked at an average of 8.6 weeks (range, 8–12 weeks), and gradually absorbed and disappeared. |
| Author | Title | Type of Ti Used | Types of Mg Used | Anatomical Site | Mg rate of Implant Removal (Per Patient) | Ti Rate of Implant Removal (Per Patient) | AOFAS Ti (1 Year Post-op) | AOFAS Mg (1 Year Post-op) | p Value |
|---|---|---|---|---|---|---|---|---|---|
| May et al. [13] | Bioabsorable magnesium screw versus conventional titanium screw fixation for medial malleolar fractures | Conventional titanium screw | MgYREZr | Medial malleolar | 0/23 (0%) | 5/25 (20%) | 90.0 ± 10.7 | 93.7 ± 8.8 | 0.161 |
| Herber et al. [16] | Can Hardware Removal be Avoided Using Bioresorbable Mg-Zn-Ca Screws After Medial Malleolar Fracture Fixation? Mid-Term Results of a First-In-Human Study | Conventional titanium screw | ZX00 rods (99.1 wt% Mg, 0.45 wt% Zn and 0.45 wt% Ca) | Medial malleolar | 0/20 (0%) | 12/17 (71%) | / | 89.8 ± 7.1 | / |
| Choo et al. [17] | Magnesium-based bioabsorbable screw fixation for hallux valgus surgery—A suitable alternative to metallic implants | Conventional titanium screw | MgYREZr | Hallux Valgus | 0/24 (0%) | 1/69 (1.4%) | 83.6 ± 14.1 | 89.5 ± 11.6 | 0.065 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the AO Foundation. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hung, C.H.; Kwok, Y.C.; Yip, J.; Wong, H.H.; Leung, Y.Y. Bioabsorbable Magnesium-Based Materials Potential and Safety in Bone Surgery: A Systematic Review. Craniomaxillofac. Trauma Reconstr. 2025, 18, 24. https://doi.org/10.3390/cmtr18020024
Hung CH, Kwok YC, Yip J, Wong HH, Leung YY. Bioabsorbable Magnesium-Based Materials Potential and Safety in Bone Surgery: A Systematic Review. Craniomaxillofacial Trauma & Reconstruction. 2025; 18(2):24. https://doi.org/10.3390/cmtr18020024
Chicago/Turabian StyleHung, Chun Ho, Yui Chit Kwok, Jason Yip, Ho Hin Wong, and Yiu Yan Leung. 2025. "Bioabsorbable Magnesium-Based Materials Potential and Safety in Bone Surgery: A Systematic Review" Craniomaxillofacial Trauma & Reconstruction 18, no. 2: 24. https://doi.org/10.3390/cmtr18020024
APA StyleHung, C. H., Kwok, Y. C., Yip, J., Wong, H. H., & Leung, Y. Y. (2025). Bioabsorbable Magnesium-Based Materials Potential and Safety in Bone Surgery: A Systematic Review. Craniomaxillofacial Trauma & Reconstruction, 18(2), 24. https://doi.org/10.3390/cmtr18020024







