Piper aduncum Essential Oil Rich in Dillapiole: Development of Hydrogel-Thickened Nanoemulsion and Nanostructured Lipid Carrier Intended for Skin Delivery
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Essential Oil from P. aduncum Leaves
2.3. Chromatographic and Analytical Conditions
2.4. Preparation of Nanoemulsion and Nanostructured Lipid Carrier
2.5. Preparation of Hydroxyethylcellulose Hydrogels Containing Lipid-Based Nanosystems
2.6. Characterization of Lipid Nanosystems and Derived Hydrogels
2.6.1. Evaluation of DIL Content in Lipid Nanosystems and Derived Hydrogels
2.6.2. Droplet Size, Polydispersity Index, and Zeta Potential
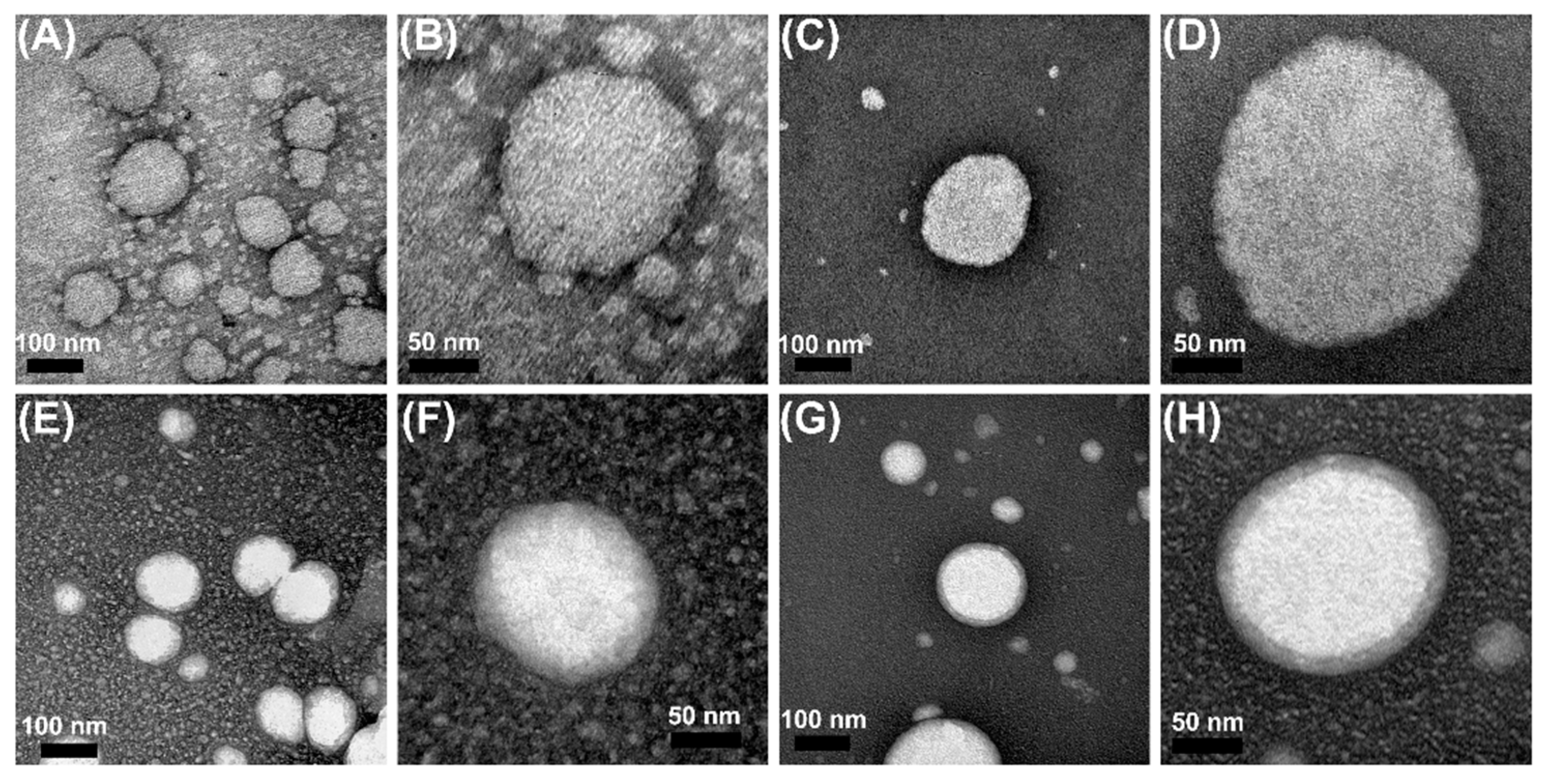
2.6.3. Morphology of Formulations
2.7. Rheological Behavior of Hydrogels
2.8. Bioadhesion Measurements
2.9. Release Studies
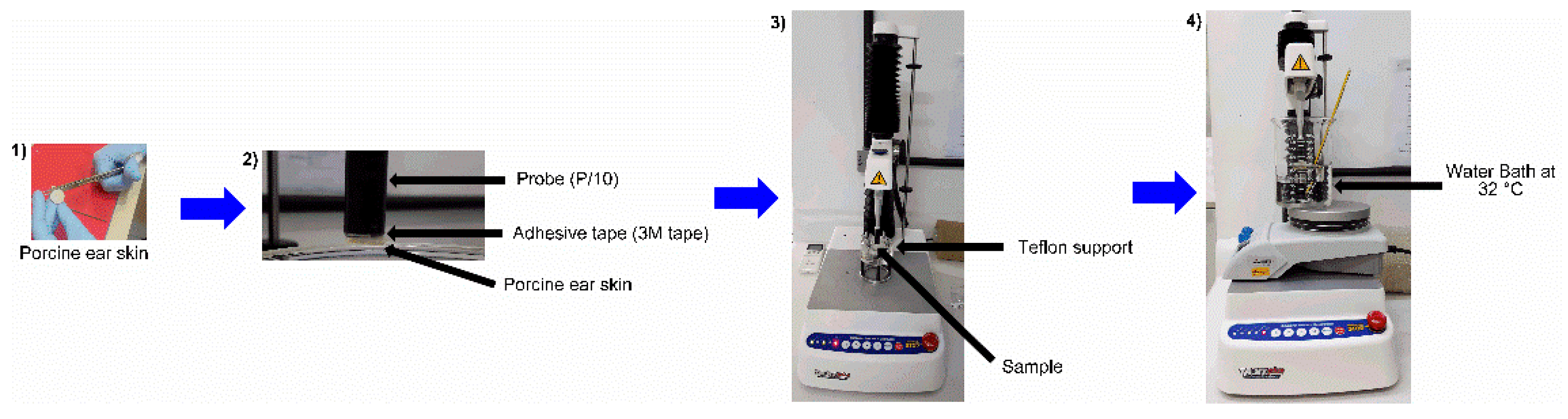
2.10. In Vitro Porcine Skin Permeation/Retention Study
2.11. HET-CAM Toxicity Test
+ [9 × (301 − coagulation time)/300]
2.12. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Nanoemulsions and Nanostructured Lipid Carriers Containing EOPA
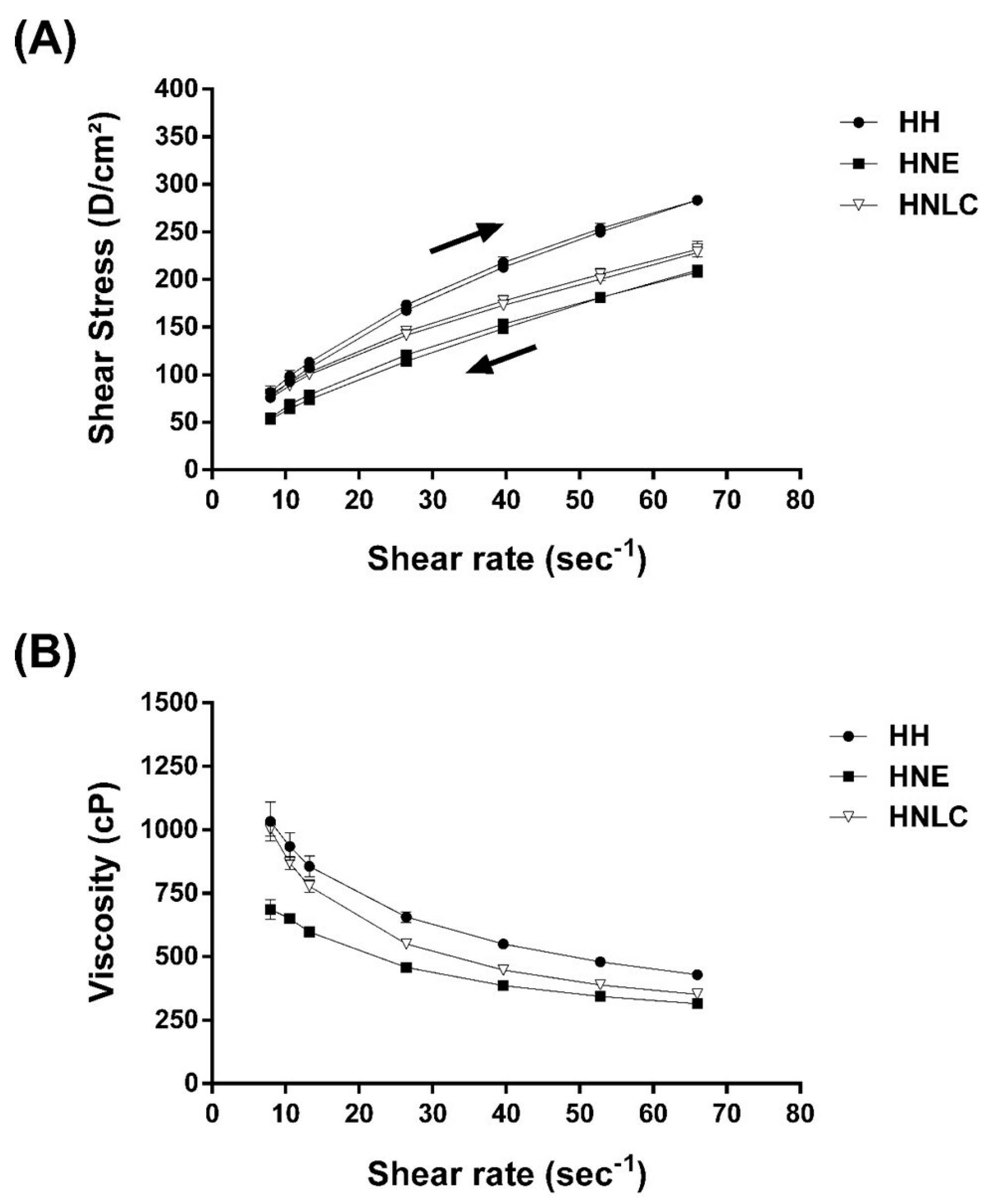
3.2. Rheological Behavior of Hydrogels Containing EOPA
3.3. Bioadhesion Measurements
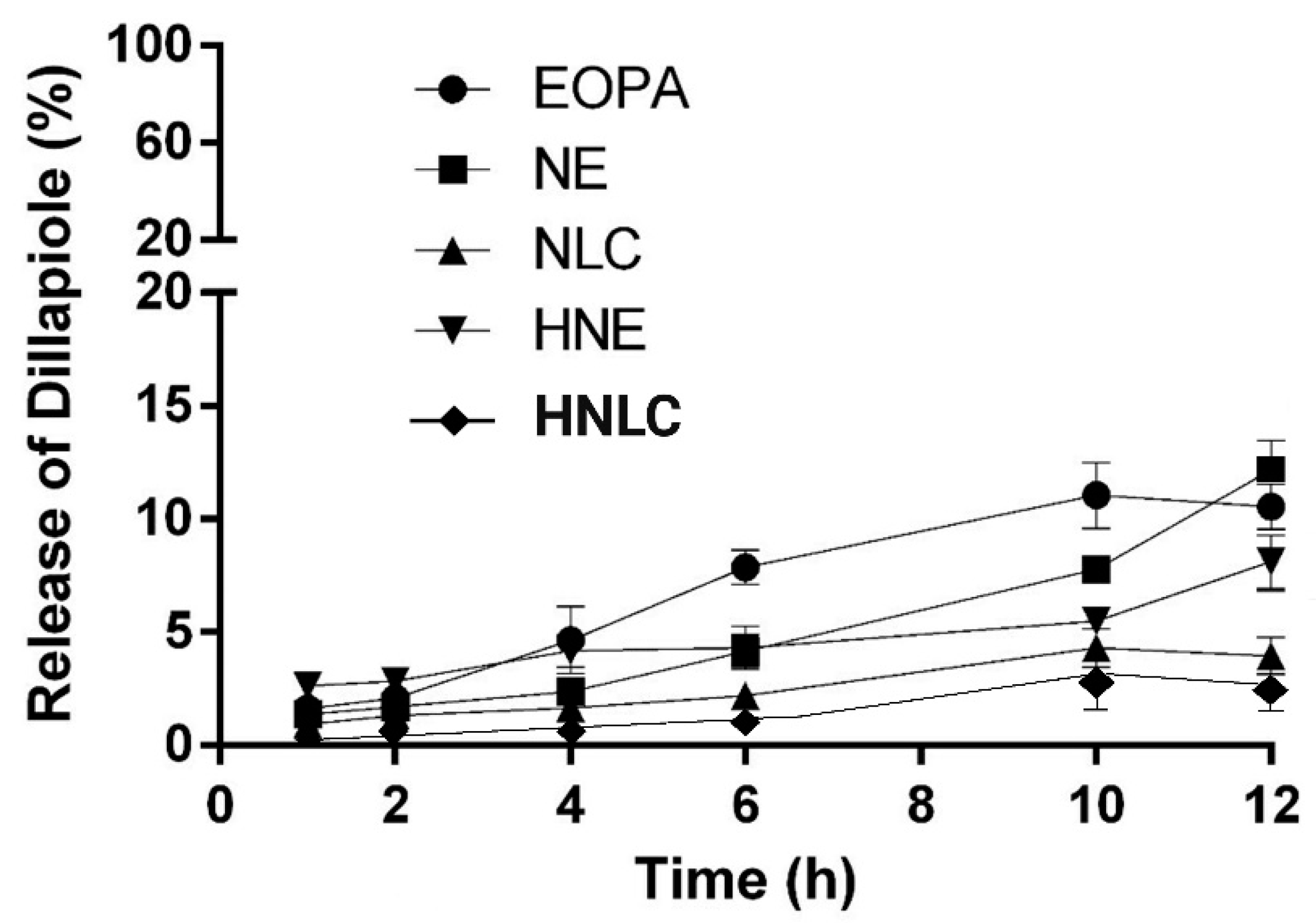
3.4. Dillapiole Release from the Formulations
3.5. In Vitro Permeation/Retention Studies
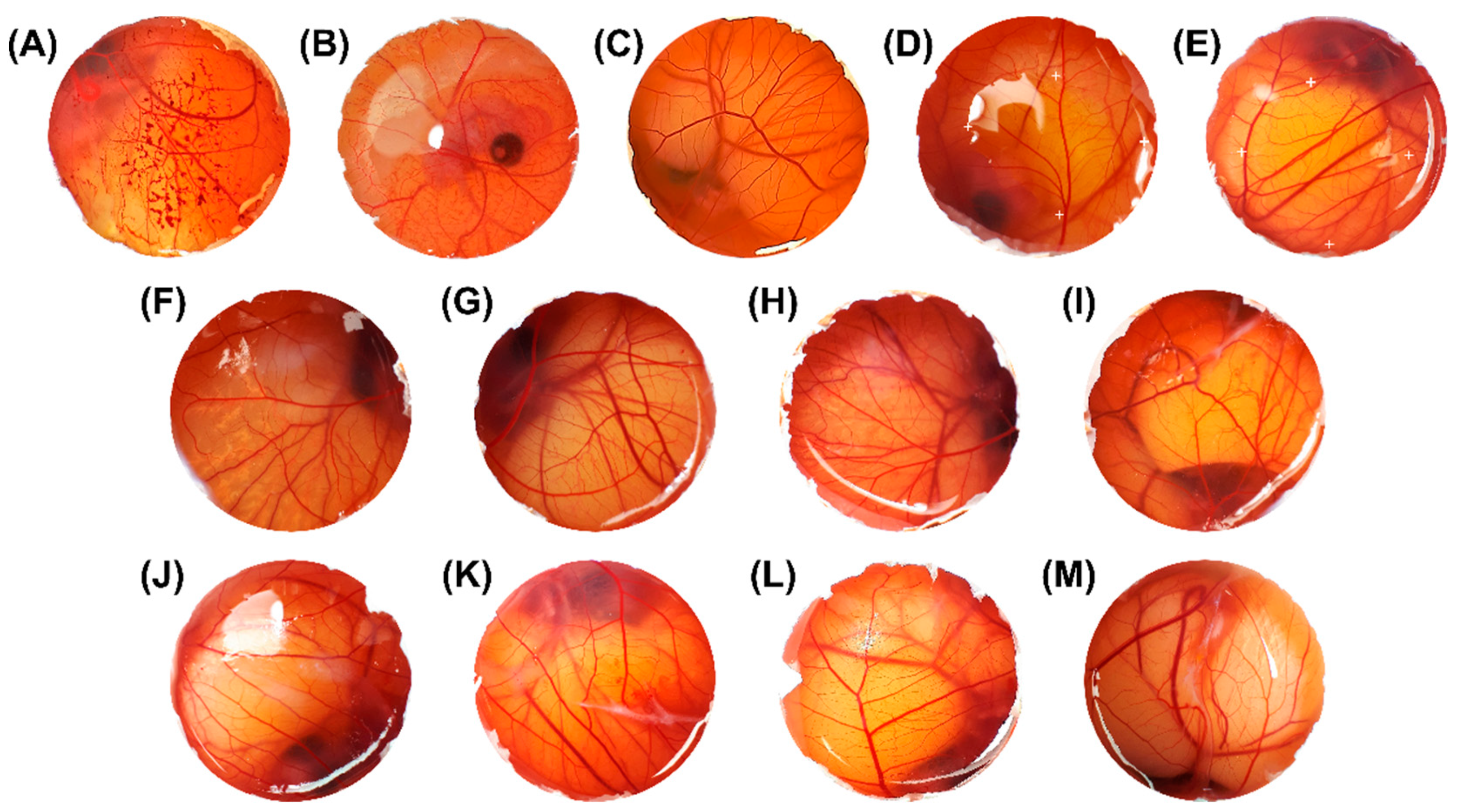
3.6. Safety Profile by HET-CAM
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Maia, J.G.S.; Zohhbi, M.D.G.B.; Andrade, E.H.A.; Santos, A.S.; Silva, M.H.L.; Luz, A.I.R.; Bastos, C.N. Constituents of the essential oil of Piper aduncum L. growing wild in the Amazon region. Flavour Fragr. J. 1998, 13, 269–272. [Google Scholar] [CrossRef]
- Maia, J.G.S.; Andrade, L.H.A. Database of the amazon aromatic plants and their essential oils. Quim. Nova 2009, 32, 595–622. [Google Scholar] [CrossRef]
- Salehi, B.Z.A.; Zakaria, R.; Gyawali, S.A.; Ibrahim, J.; Rajkovic, Z.K.; Shinwari, T.; Khan, J.; Sharifi-Rad, A.; Ozleyen, E.; Turkdonmez, M.; et al. Piper species: A comprehensive review on their phytochemistry, biological activities and applications. Molecules 2019, 24, 1364. [Google Scholar] [CrossRef] [PubMed]
- Taher, M.; Amri, M.S.; Susanti, D.; Kudos, M.B.A.; Nor, N.F.A.; Syukri, Y. Medicinal Uses, Phytochemistry, and Pharmacological Properties of Piper aduncum L. Sains Malays. 2020, 49, 1829–1851. [Google Scholar] [CrossRef]
- Durofil, A.; Radice, M.; Blanco-Salas, J.; Ruiz-Téllez, T. Piper aduncum essential oil: A promising insecticide, acaricide and antiparasitic. A review. Parasite 2021, 28, 42. [Google Scholar] [CrossRef] [PubMed]
- Navickiene, H.M.D.; Morandim, A.A.; Alécio, A.C.; Regasini, L.O.; Bergamo, D.C.B.; Telascrea, M.; Cavalheiro, A.J.; Lopes, M.N.; Bolzani, V.S.; Furlan, M.; et al. Composition and antifungal activity of essential oils from Piper aduncum, Piper arboreum and Piper tuberculatum. Quím. Nova 2006, 29, 467–470. [Google Scholar] [CrossRef]
- Guerrini, A.; Sacchetti, G.; Rossi, D.; Paganetto, G.; Muzzoli, M.; Andreotti, E. Bioactivities of Piper aduncum L. and Piper obliquum Ruiz & Pavon (Piperaceae) essential oils from Eastern Ecuador. Environ. Toxicol. Pharmacol. 2009, 27, 39–48. [Google Scholar] [CrossRef]
- Ferreira, R.; Monteiro, M.; Silva, J.; Maia, J. Antifungal Action of the Dillapiole-rich Oil of Piper aduncum against Dermatomycoses Caused by Filamentous Fungi. Br. J. Med. Med. Res. 2016, 15, 1–10. [Google Scholar] [CrossRef]
- Volpe, H.X.; Fazolin, M.; Garcia, R.B.; Magnani, R.F.; Barbosa, J.C.; Miranda, M.P. Efficacy of essential oil of Piper aduncum against nymphs and adults of Diaphorina citri. Pest Manag. Sci. 2016, 72, 1242–1249. [Google Scholar] [CrossRef]
- Brazão, M.A.B.; Maia, J.G.S.; Monteiro, M.C.; Brazão, F.V. Antibacterial activity of the Piper aduncum oil and dillapiole, Its main constituent, Against multidrug-resistant strains. Bol. Latinoam. Caribe Plantas Med. Aromat. 2014, 13, 517–526. [Google Scholar]
- Monzote, L.; Scull, R.; Cos, P.; Setzer, W. Essential Oil from Piper aduncum: Chemical Analysis, Antimicrobial Assessment, and Literature Review. Medicines 2017, 4, 49. [Google Scholar] [CrossRef]
- Parise-Filho, R.; Pastrello, M.; Camerlingo, P.C.E.; Silva, G.J.; Agostinho, L.A.; Souza, T. The anti-inflammatory activity of dillapiole and some semisynthetic analogues. Pharm. Biol. 2011, 49, 1173–1179. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.M.N. Nano and microparticles as controlled drug delivery devices. J. Pharm. Pharm. Sci. 2000, 3, 234–258. [Google Scholar]
- Bilia, A.R.; Guccione, C.; Isacchi, B.; Righeschi, C.; Firenzuoli, F.; Bergonzi, M.C. Essential oils loaded in nanosystems: A developing strategy for a successful therapeutic approach. Evid. Based Complement. Altern. Med. 2014, 2014, 1593. [Google Scholar] [CrossRef]
- Matos, S.P.; Teixeira, H.F.; Lima, Á.A.N.; Veiga-Junior, V.F.; Koester, L.S. Essential Oils and Isolated Terpenes in Nanosystems Designed for Topical Administration: A Review. Biomolecules 2019, 9, 138. [Google Scholar] [CrossRef] [PubMed]
- Gunasekaran, T.; Haile, T.; Nigusse, T.; Dhanaraju, M.D. Nanotechnology: An effective tool for enhancing bioavailability and bioactivity of phytomedicine. Asian Pac. J. Trop. Biomed. 2014, 4, S1–S7. [Google Scholar] [CrossRef]
- Tyagi, V.; Singh, V.K.; Sharma, P.K.; Singh, V.D. Essential oil-based nanostructures for inflammation and rheumatoid arthritis. J. Drug Deliv. Sci. Technol. 2020, 60, 101983. [Google Scholar] [CrossRef]
- Baser, K.H.C.; Buchbauer, G. Handbook of Essential Oils Science, Technology, and Applications; CRC Press: Boca Raton, FL, USA, 2010; p. 1120. [Google Scholar]
- Turek, C.; Stintzing, C. Stability of essential oils: A review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 40–53. [Google Scholar] [CrossRef]
- El Asbahani, A.; Miladi, K.; Badri, W.; Sala, M.; Addi, E.H.A.; Casabianca, H.; El Mousadik, A.; Hartmann, D.; Jilale, A.; Renaud, F.N.R.; et al. Essential oils: From extraction to encapsulation. Int. J. Pharm. 2015, 483, 220–243. [Google Scholar] [CrossRef]
- Jaiswal, M.; Dudhe, R.; Sharma, P.K. Nanoemulsion: An advanced mode of drug delivery system. 3 Biotech 2015, 5, 123. [Google Scholar] [CrossRef]
- Singh, Y.; Meher, J.G.; Raval, K.; Khan, F.A.; Chaurasia, M.; Jain, N.K.; Chourasia, M.K. Nanoemulsion: Concepts, development and applications in drug delivery. J. Control. Release 2017, 252, 28–49. [Google Scholar] [CrossRef]
- Kreutz, T.; Carneiro, S.B.; Soares, K.D.; Limberger, R.P.; Apel, M.A.; Veiga-Junior, V.F.; Koester, L.S. Aniba canelilla (Kunth) Mez essential oil-loaded nanoemulsion: Improved stability of the main constituents and in vitro antichemotactic activity. Ind. Crops Prod. 2021, 171, 113949. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, M.; Kai, C.; Mingqi, W. Nano-emulsion prepared by high pressure homogenization method as a good carrier for Sichuan pepper essential oil: Preparation, stability, and bioactivity. LWT 2022, 154, 112779. [Google Scholar] [CrossRef]
- Jenning, V.; Thünemann, A.F.; Gohla, S.H. Characterisation of a novel solid lipid nanoparticle carrier system based on binary mixtures of liquid and solid lipids. Int. J. Pharm. 2000, 199, 167–177. [Google Scholar] [CrossRef]
- Obeidat, W.M.; Schwabe, K.; Müller, R.H.; Keck, C.M. Preservation of nanostructured lipid carriers (NLC). Eur. J. Pharm. Biopharm. 2010, 76, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Cai, T.; Huang, Y.; Xia, X.; Cole, S.P.C.; Cai, Y. A review of the structure, preparation, and application of NLCs, PNPs, and PLNs. Nanomaterials 2017, 7, 122. [Google Scholar] [CrossRef]
- Montenegro, L.; Lai, F.; Offerta, A.; Sarpietro, M.G.; Micicchè, L.; Maccioni, A.M.; Valenti, D.; Fadda, A.M. From nanoemulsions to nanostructured lipid carriers: A relevant development in dermal delivery of drugs and cosmetics. J. Drug Deliv. Sci. Technol. 2016, 32, 100–112. [Google Scholar] [CrossRef]
- Katopodi, A.; Detsi, A. Solid Lipid Nanoparticles and Nanostructured Lipid Carriers of natural products as promising systems for their bioactivity enhancement: The case of essential oils and flavonoids. Col. Surf. A Physicochem. Eng. Asp. 2021, 630, 127529. [Google Scholar] [CrossRef]
- Gomes, G.V.L.; Sola, M.R.; Marostegan, L.F.P.; Jange, C.G.; Cazado, C.P.S.; Pinheiro, A.C.; Vicente, A.A.; Pinho, S.C. Physico-chemical stability and in vitro digestibility of beta-carotene-loaded lipid nanoparticles of cupuacu butter (Theobroma grandiflorum) produced by the phase inversion temperature (PIT) method. J. Food Eng. 2017, 192, 93–102. [Google Scholar] [CrossRef]
- Soldati, P.P.; Polonini, H.C.; Paes, C.Q.; Restrepob, J.A.S.; Creczynksi-Pasa, T.B.; Chaves, M.G.A.M.; Brandão, M.A.F.; Pittella, F.; Raposo, N.R.B. Controlled release of resveratrol from lipid nanoparticles improves antioxidant effect. IFAC PapersOnLine 2018, 51, 16–21. [Google Scholar] [CrossRef]
- Alam, M.S.; Ali, M.S.; Alam, N.; Alam, M.I.; Anwer, T.; Imam, F.; Ali, M.D.; Siddiqui, M.R.; Shamim, M. Design and characterization of nanostructure topical gel of betamethasone dipropionate for psoriasis. J. Appl. Pharm. Sci. 2012, 2, 148–158. [Google Scholar] [CrossRef]
- Barradas, T.N.; Senna, J.P.; Cardoso, S.A.; Holanda, K.G.; Silva, C.R. Elias Mansur, Formulation characterization and in vitro drug release of hydrogel-thickened nanoemulsions for topical delivery of 8-methoxypsoralen. Mater. Sci. Eng. C 2018, 92, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Buwalda, S.J.; Boere, K.W.M.; Dijkstra, P.J.; Feijen, J.; Vermonden, T.; Hennink, W.E. Hydrogels in a historical perspective: From simple networks to smart materials. J. Control. Release 2014, 190, 254–273. [Google Scholar] [CrossRef]
- Lucca, L.G.; Matos, S.P.; Kreutz, T.; Teixeira, H.F.; Veiga-Junior, V.F.; Araújo, B.V.; Limberger, R.P.; Koester, L.S. Anti-inflammatory Effect from a Hydrogel Containing Nanoemulsified Copaiba oil (Copaifera multijuga Hayne). AAPS PharmSciTech 2017, 19, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectroscopy, 4th ed.; Allured: Northbrook, IL, USA, 2017. [Google Scholar]
- Nep, E.I.; Conway, B.R. Grewia gum 2: Mucoadhesive properties of compacts and gels. Trop. J. Pharm. Res. 2011, 10, 393–401. [Google Scholar] [CrossRef][Green Version]
- Klang, V.; Schwarz, J.C.; Lenobel, B.; Nadj, M.; Auböck, J.; Wolzt, M.; Valenta, C. In vitro vs. in vivo tape stripping: Validation of the porcine ear model and penetration assessment of novel sucrose stearate emulsions. Eur. J. Pharm. Biopharm. 2012, 80, 604–614. [Google Scholar] [CrossRef] [PubMed]
- Luepke, N.P. Hen’s egg chorioallantoic membrane test for irritation potential. Food Chem. Toxicol. 1985, 23, 287–291. [Google Scholar] [CrossRef]
- Pereira, R.L.; Leites, F.I.; Paese, K.; Sponchiado, R.M.; Michalowski, C.B.; Guterres, S.S.; Schapoval, E.E.S. Hydrogel containing adapalene- and dapsone-loaded lipid-core nanocapsules for cutaneous application: Development, characterization, in vitro irritation and permeation studies. Drug Dev. Ind. Pharm. 2016, 42, 2001–2008. [Google Scholar] [CrossRef]
- Koch, C.; Reichling, J.; Kehm, R.; Sharaf, M.M.; Zentgraf, H.; Schneele, J.; Schnitzler, P. Efficacy of anise oil, dwarf-pine oil and chamomile oil against thymidine-kinase-positive and thymidine-kinase-negative herpesviruses. J. Pharm. Pharmacol. 2008, 60, 1545–1550. [Google Scholar] [CrossRef]
- Muller, R.H.; Shegokar, R.; Keck, C.M. 20 Years of Lipid Nanoparticles (SLN & NLC): Present State of Development & Industrial Applications. Curr. Drug Discov. Technol. 2011, 8, 207–227. [Google Scholar] [CrossRef]
- Vitorino, C.; Almeida, J.; Gonçalves, L.M.; Almeida, A.J.; Sousa, J.J.; Pais, A.A.C.C. Co-encapsulating nanostructured lipid carriers for transdermal application: From experimental design to the molecular detail. J. Control. Release 2013, 167, 301–314. [Google Scholar] [CrossRef]
- McClements, D.J.; Jafari, S.M. General Aspects of Nanoemulsions and Their Formulation; Elsevier Inc.: Amsterdam, The Netherlands, 2018. [Google Scholar] [CrossRef]
- Gündel, S.S.; Souza, M.E.; Quatrin, P.M.; Klein, B.; Wagner, R.; Gündel, A.; Vaucher, R.A.; Santos, R.C.V.; Ourique, A.F. Nanoemulsions containing Cymbopogon flexuosus essential oil: Development, characterization, stability study and evaluation of antimicrobial and antibiofilm activities. Microb. Pathog. 2018, 118, 268–276. [Google Scholar] [CrossRef]
- Chetoni, P.; Burgalassi, S.; Monti, D.; Tampucci, S.; Tullio, V.; Cuffini, A.M.; Muntoni, E.; Spagnolo, R.; Zara, G.P.; Cavalli, R. Solid lipid nanoparticles as promising tool for intraocular tobramycin delivery: Pharmacokinetic studies on rabbits. Eur. J. Pharm. Biopharm. 2016, 109, 214–223. [Google Scholar] [CrossRef]
- Gündel, S.S.; Velho, M.C.; Diefenthaler, M.K.; Favarin, F.R.; Copetti, P.M.; Oliveira, A.; Fogaça, B.; Klein, R.; Wagner, A.; Gündel, M.R.; et al. Basil oil-nanoemulsions: Development, cytotoxicity and evaluation of antioxidant and antimicrobial potential. J. Drug Deliv. Sci. Technol. 2018, 46, 378–383. [Google Scholar] [CrossRef]
- Nasseri, M.; Golmohammadzadeh, S.; Arouiee, H.; Jaafari, M.R.; Neamati, H. Antifungal activity of Zataria multiflora essential oil-loaded solid lipid nanoparticles in-vitro condition. Iran. J. Basic Med. Sci. 2016, 19, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.K.; Kreutz, T.; Danielli, L.J.; Marchi, J.G.B.; Pippi, B.; Koester, L.S.; Fuentefria, A.M.; Limberger, R.P. A chitosan hydrogel-thickened nanoemulsion containing Pelargonium graveolens essential oil for treatment of vaginal candidiasis. J. Drug Deliv. Sci. Technol. 2020, 56, 101527. [Google Scholar] [CrossRef]
- Gull, A.; Ahmed, S.; Ahmad, J.F.; Nagaich, U.; Chandra, A. Hydrogel thickened microemulsion; a local cargo for the co- delivery of cinnamaldehyde and berberine to treat acne vulgaris. J. Drug Deliv. Sci. Technol. 2020, 58, 101835. [Google Scholar] [CrossRef]
- Aulton, M.E.; Taylor, K.M.G. Pharmaceutics: The Science of Dosagem Form Design, 2nd ed.; Churchill Livingstone: London, UK, 2001. [Google Scholar] [CrossRef]
- Carvalho, F.C.; Calixto, G.; Hatakeyama, I.N.; Luz, G.M.; Gremião, M.P.D.; Chorilli, M. Rheological, mechanical, and bioadhesive behavior of hydrogels to optimize skin delivery systems. Drug Dev. Ind. Pharm. 2013, 39, 1750–1757. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Zhang, X.; Ma, X.; Wang, W.; Yan, F.; Zhao, X.; Chu, X.; Xu, W.; Sun, C. A review of the properties and applications of bioadhesive hydrogels. Polym. Chem. 2021, 12, 3721–3739. [Google Scholar] [CrossRef]
- Rai, V.K.; Mishra, N.; Yadav, K.S.; Yadav, N.P. Nanoemulsion as pharmaceutical carrier for dermal and transdermal drug delivery: Formulation development, stability issues, basic considerations and applications. J. Control. Release 2018, 270, 203–225. [Google Scholar] [CrossRef] [PubMed]
- Üner, M.; Yener, G.; Erguven, M.; Karaman, E.; Utku, E. Solid Lipid Nanoparticles and Nanostructured Lipid Carriers of Celecoxib for Topical Application—Preparation, Characterization and Drug Penetration Through Rat Skin. Curr. Nanosci. 2014, 10, 532–542. [Google Scholar] [CrossRef]
- Contri, R.V.; Katzer, T.; Pohlmann, A.R.; Guterres, S.S. Chitosan hydrogel containing capsaicinoids-loaded nanocapsules: An innovative formulation for topical delivery. Soft Mater. 2010, 8, 370–385. [Google Scholar] [CrossRef]
- Asbill, C.S.; Michniak, B.B. Percutaneous penetration enhancers: Local versus transdermal activity. Pharm. Sci. Technol. Today 2000, 3, 36–41. [Google Scholar] [CrossRef]
- Roberts, M.S.; Mohammed, Y.; Pastore, M.N.; Namjoshi, S.; Yousef, S.; Alinaghi, A.; Haridass, I.N.; Abd, E.; Leite-Silva, V.R.; Benson, H.A.E.; et al. Topical and cutaneous delivery using nanosystems. J. Control. Release 2017, 247, 86–105. [Google Scholar] [CrossRef] [PubMed]
- Benson, H.A.E.; Watkinson, A.C. Topical and Transdermal Drug Delivery; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011. [Google Scholar] [CrossRef]
- Vogt, A.; Wischke, A.T.; Neffe, N.; Ma, U.; Alexiev, A. Nanocarriers for drug delivery into and through the skin—Do existing technologies match clinical challenges? J. Control. Release 2016, 242, 3–15. [Google Scholar] [CrossRef]
- Duharte, B.A.; Murillo, J.G.; Pérez, U.M.; Tur, E.N.; Portuondo, D.F.; Martínez, B.T.; Téllez-Martínez, D.; Betancourt, J.E.; Pérez, O. The Hen’s Egg Test on Chorioallantoic Membrane: An Alternative Assay for the Assessment of the Irritating Effect of Vaccine Adjuvants. Int. J. Toxicol. 2016, 35, 627–633. [Google Scholar] [CrossRef]
- Vargas, A.; Zeisser-Labouèbe, M.; Lange, N.; Gurny, R.; Delie, F. The chick embryo and its chorioallantoic membrane (CAM) for the in vivo evaluation of drug delivery systems. Adv. Drug Deliv. Rev. 2007, 59, 1162–1176. [Google Scholar] [CrossRef]
- Villone, D.; Fritsch, A.; Koch, M.; Bruckner-Tuderman, L.; Hansen, U.; Bruckner, P. Supramolecular interactions in the dermo-epidermal junction zone: Anchoring fibril-collagen VII tightly binds to banded collagen fibrils. J. Biol. Chem. 2008, 283, 24506–24513. [Google Scholar] [CrossRef]
- Silva, W.C.; Martins, S.J.R.; Souza, H.E.M.; Heinzen, H.; Cesio, M.V.; Mato, M.; Albrecht, F.; Azevedo, J.L.; Barros, N.M. Toxicity of Piper aduncum L. (Piperales: Piperaceae) from the Amazon forest for the cattle tick Rhipicephalus (Boophilus) microplus (Acari: Ixodidae). Vet. Parasitol. 2009, 164, 267–274. [Google Scholar] [CrossRef]
- Barros, F.J.; Costa, R.J.O.; Cesário, F.R.A.S.; Rodrigues, L.B.; Costa, J.G.M.; Coutinho, H.D.M.; Galvao, H.B.F.; Menezes, I.R.A. Activity of essential oils of Piper aduncum anf and Cinnamomum zeylanicum by evaluating osmotic and morphologic fragility of erythrocytes. Eur. J. Integr. Med. 2016, 8, 505–512. [Google Scholar] [CrossRef]
- Oliveira, A.G.L.; Silva, R.S.; Alves, E.N.; Presgrave, R.F.; Presgrave, O.A.F.; Delgado, I.F. Chorioallantoic membrane assays (HET-CAM and CAM-TBS): Alternative tests for performing toxicological evaluation of products with low potential for ocular irritation. Rev. Inst. Adolfo Lutz. 2012, 71, 153–159. Available online: https://www.arca.fiocruz.br/bitstream/handle/icict/8985/RIAL_71_153-159.pdf?sequence=2 (accessed on 7 January 2020).





| Constituents | B-NE | B-NLC | NE | NLC | HB | HB-NE | HB-NLC | HNE | HNLC |
|---|---|---|---|---|---|---|---|---|---|
| EOPA | - | - | 5 | 5 | - | - | - | 5 | 5 |
| MCT | 10 | 5 | 5 | - | - | 10 | 5 | 5 | - |
| Span 80® | 2.5 | 2.5 | 2.5 | 2.5 | - | 2.5 | 2.5 | 2.5 | 2.5 |
| Tween 20® | 2.5 | 2.5 | 2.5 | 2.5 | - | 2.5 | 2.5 | 2.5 | 2.5 |
| Cupuaçu butter | - | 5 | - | 5 | - | - | 5 | - | 5 |
| Hydroxyethylcellulose | - | - | - | - | 1 | 1 | 1 | 1 | 1 |
| Water q.s. | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Formulation | Bingham Model | Ostwald Model | Casson Model | Herschel-Bulkley Model | ||||
|---|---|---|---|---|---|---|---|---|
| Equation | r2 | Equation | r2 | Equation | r2 | Equation | r2 | |
| HH | y = 3.4917x + 66.59 | 0.98 | y = 0.5886x + 1.3917 | 0.9988 | y = 1.4749x + 5.2063 | 0.9919 | y = 1.3241x − 0.0281 | 0.9667 |
| HNE | y = 2.6076x + 42.919 | 0.9868 | y = 0.62x + 1.1939 | 0.9983 | y = 1.3043x + 4.0222 | 0.9941 | y = 1.258x − 0.0475 | 0.9791 |
| HNLC | y = 2.6088x + 67.061 | 0.9868 | y = 0.503x + 1.4476 | 0.9999 | y = 1.1831x + 5.7667 | 0.9965 | y = 1.3193x − 0.1526 | 0.9718 |
| IS (RSD%) | Results | |
|---|---|---|
| NaCl 0.9% m/v | 0 (0) | Non-irritant |
| Lauryl sodium sulfate 1% m/v | 11.14 (0.62) | Extremely irritant |
| NaOH 0.1 M | 13.51 (1.15) | Extremely irritant |
| Olive oil | 0 (0) | Non-irritant |
| EOPA: Olive oil (1:20) | 4.38 (3.61) | Slightly irritant |
| B-NE | 0 (0) | Non-irritant |
| NE | 4.08 (9.88) | Slightly irritant |
| HB-NE | 0 (0) | Non-irritant |
| HNE | 4.81 (0.38) | Slightly irritant |
| B-NLC | 0 (0) | Non-irritant |
| NLC | 4.68 (6.77) | Slightly irritant |
| HB-NLC | 0 (0) | Non-irritant |
| HNLC | 4.82 (0.29) | Slightly irritant |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carneiro, S.B.; Kreutz, T.; Limberger, R.P.; Teixeira, H.F.; da Veiga Júnior, V.F.; Koester, L.S. Piper aduncum Essential Oil Rich in Dillapiole: Development of Hydrogel-Thickened Nanoemulsion and Nanostructured Lipid Carrier Intended for Skin Delivery. Pharmaceutics 2022, 14, 2525. https://doi.org/10.3390/pharmaceutics14112525
Carneiro SB, Kreutz T, Limberger RP, Teixeira HF, da Veiga Júnior VF, Koester LS. Piper aduncum Essential Oil Rich in Dillapiole: Development of Hydrogel-Thickened Nanoemulsion and Nanostructured Lipid Carrier Intended for Skin Delivery. Pharmaceutics. 2022; 14(11):2525. https://doi.org/10.3390/pharmaceutics14112525
Chicago/Turabian StyleCarneiro, Simone Braga, Tainá Kreutz, Renata Pereira Limberger, Helder Ferreira Teixeira, Valdir Florêncio da Veiga Júnior, and Letícia Scherer Koester. 2022. "Piper aduncum Essential Oil Rich in Dillapiole: Development of Hydrogel-Thickened Nanoemulsion and Nanostructured Lipid Carrier Intended for Skin Delivery" Pharmaceutics 14, no. 11: 2525. https://doi.org/10.3390/pharmaceutics14112525
APA StyleCarneiro, S. B., Kreutz, T., Limberger, R. P., Teixeira, H. F., da Veiga Júnior, V. F., & Koester, L. S. (2022). Piper aduncum Essential Oil Rich in Dillapiole: Development of Hydrogel-Thickened Nanoemulsion and Nanostructured Lipid Carrier Intended for Skin Delivery. Pharmaceutics, 14(11), 2525. https://doi.org/10.3390/pharmaceutics14112525







