Performance Comparison and Light Reflectance of Al, Cu, and Fe Metals in Direct Contact Flat Solar Heating Systems
Abstract
1. Introduction
2. Heat Conduction
- The vibration of molecules or atoms of solid materials, as a result of heating, causes micro-mechanical waves (phonons) because of their lattice structure vibration. Thus, they transmit thermal energy toward the temperature gradient from one point to another in the heated substance. This process depends on the lattice structure and temperatures of heated materials [22].
- Free electrons in solid materials play a vital role in heat transfer. When the substance is heated up, the kinetic energy of its free electrons increases. The free electrons then move away from the region of higher temperature to the region of lower temperature to lose their thermal energy, and then return to the higher-temperature region, and so on. Consequently, the formation of a structure resembling a continuous electronic vortex transfers thermal energy if a temperature difference exists in the used material. The thermal conductivities and specific heat capacities of Al, Cu, and Fe are reported in Table 1. Essential details about heat transfer in materials can be found in the literature [22].
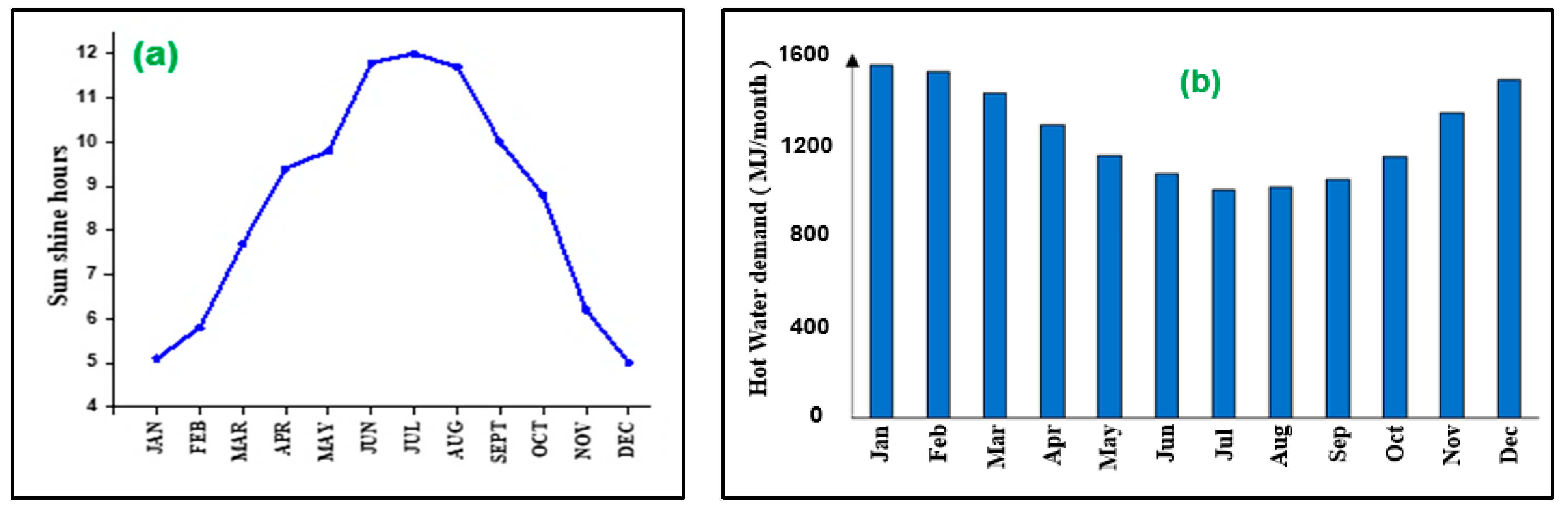
3. Brief Solar Energy Information about Jordan
4. Materials and Methods
4.1. Experimental Apparatus and Materials
4.2. Experimental Procedure
4.2.1. Al and B. Al Containers Solar Heating
4.2.2. Effect of Metal Sheet Thickness of DCFSHS on Water Temperature
4.2.3. Three Metal Types of DCFSHS with a Unified Thickness of Approximately 1 mm
4.2.4. Effect of Inside Wooden Box Color on Water Temperature of Solar Heater
4.2.5. Percent Reflectance (Reflectance %) (R%) Measurement
5. Results and Discussion
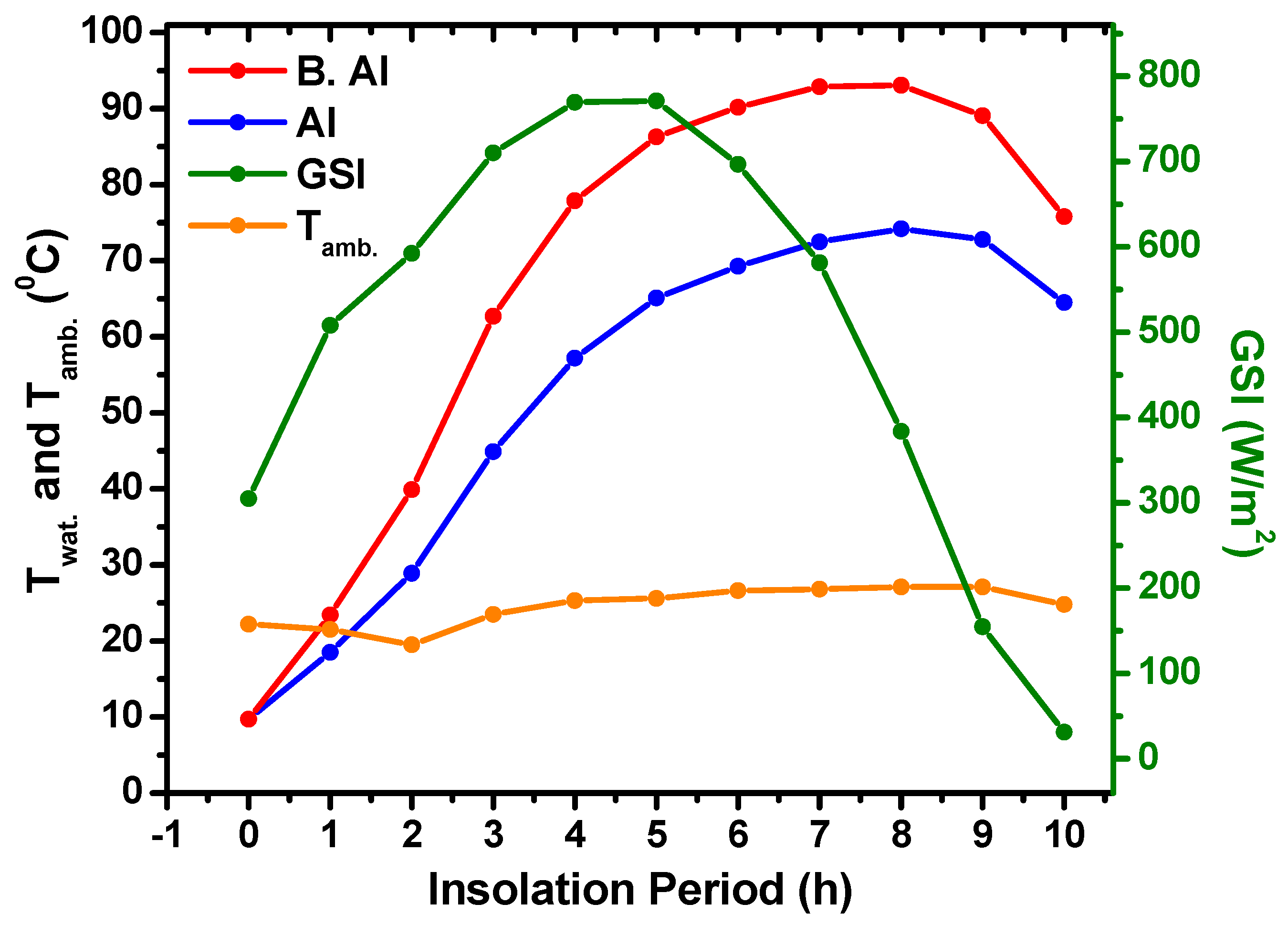
5.1. Al and B. Al Containers Solar Heating
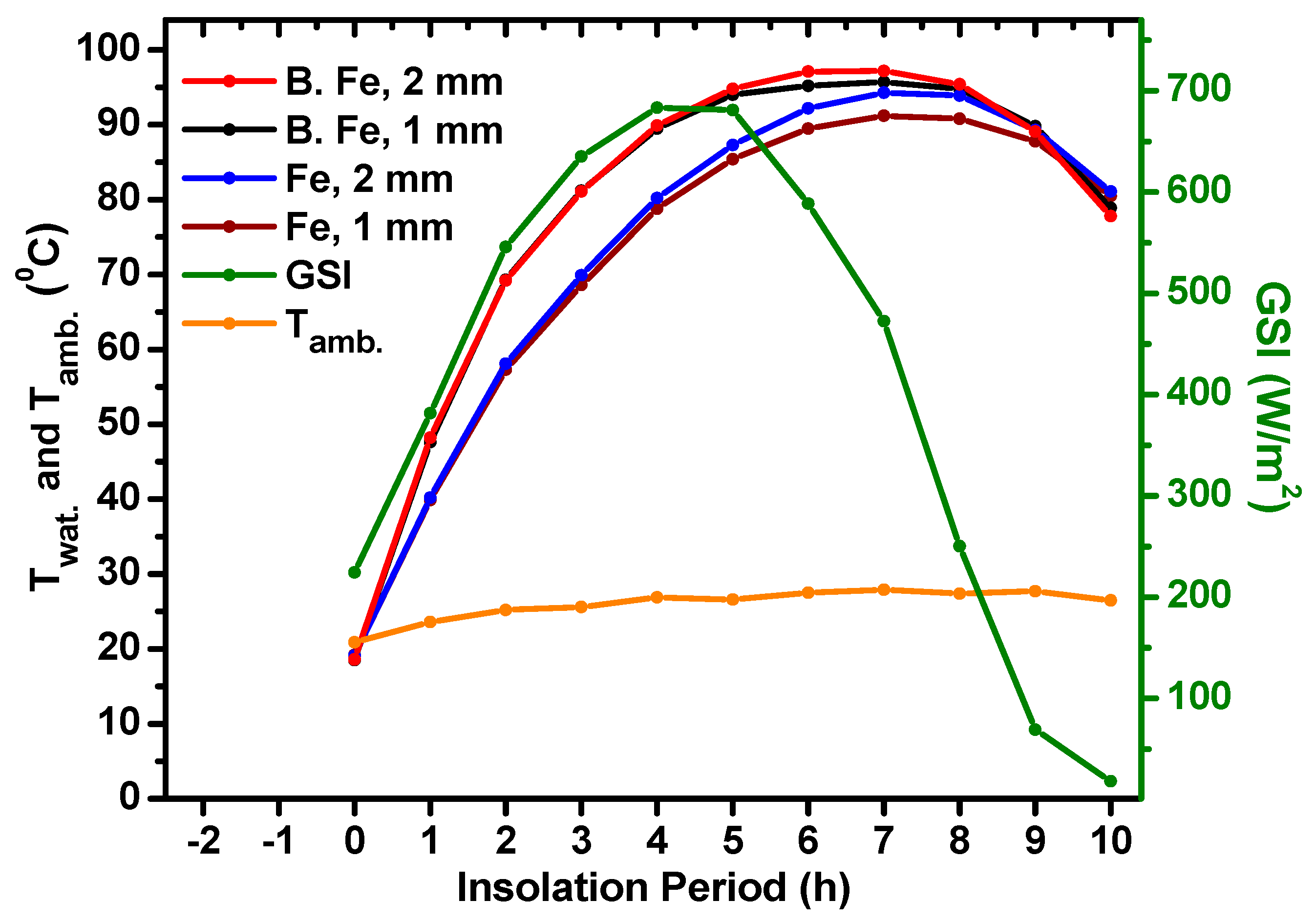
5.2. Effect of Metal Sheet Thickness of DCFSHS on Water Temperatures
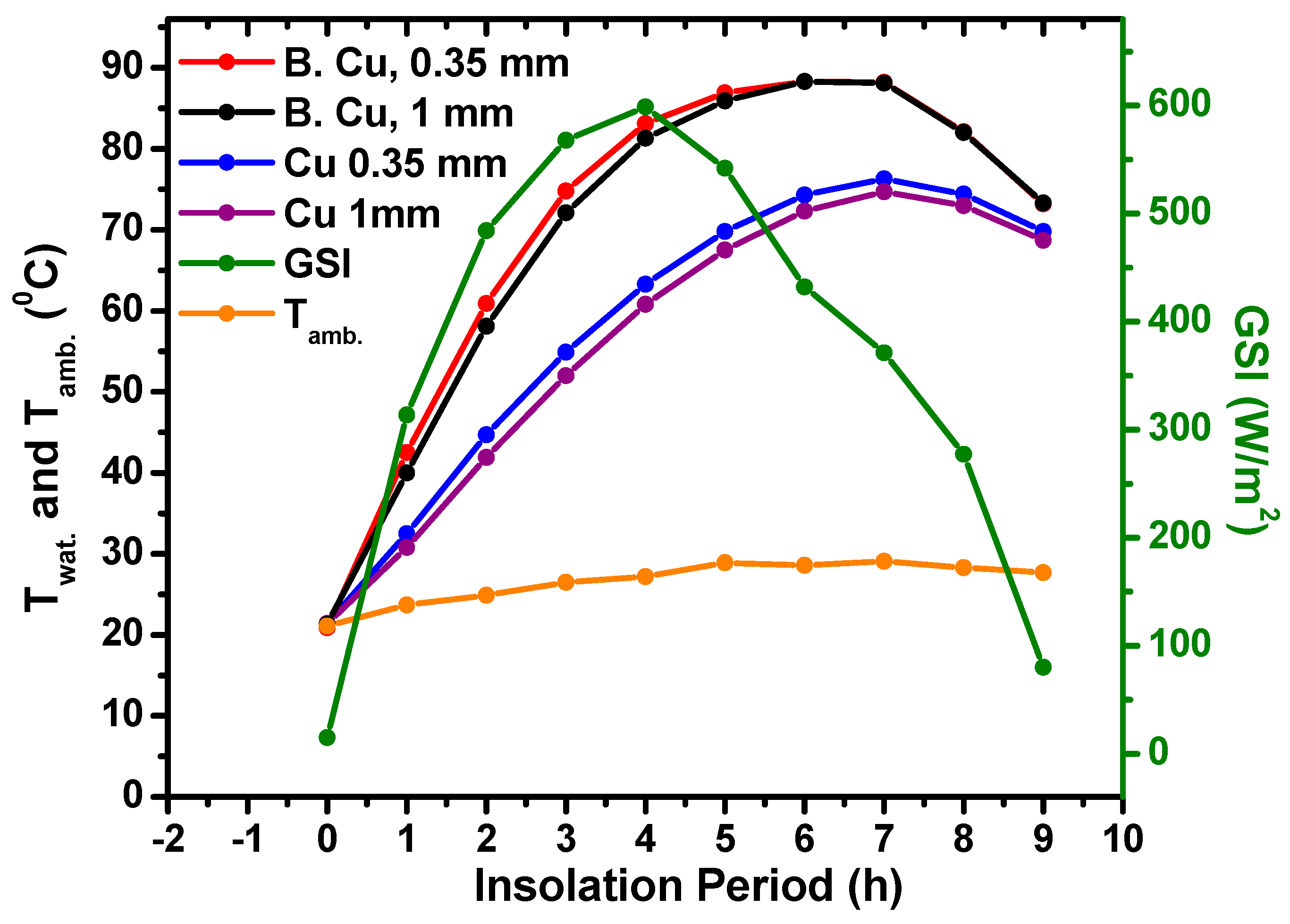
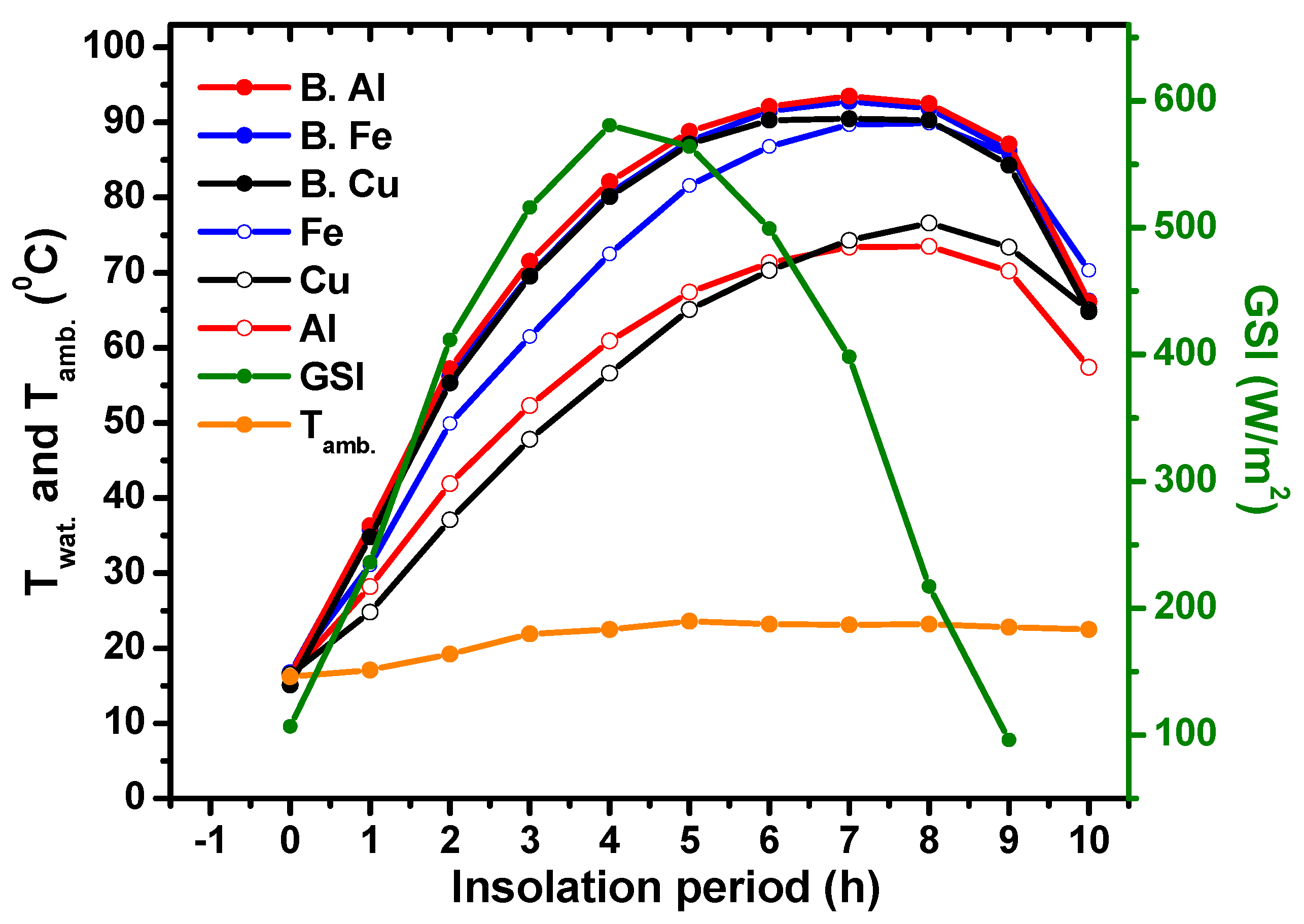
5.3. Three Metal Types of DCFSHS with a Unified Thickness of Approximately 1 mm
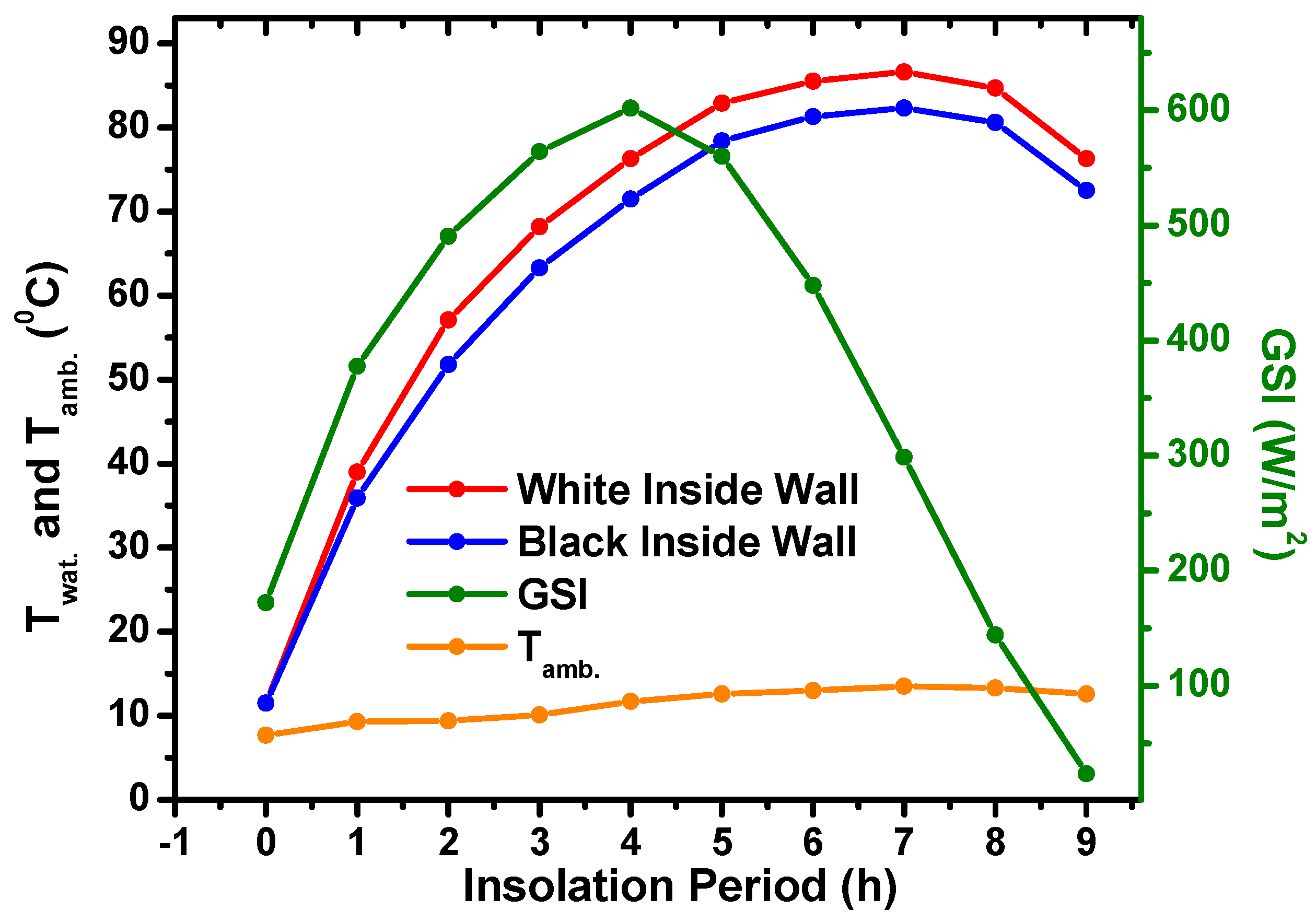
5.4. Effect of Inside Wooden Box Color on Water Temperature of Solar Heater
5.5. Percent Reflectance (Reflectance %) (R%) Measurement
6. Conclusions
- The obtained results confirmed the feasibility of using the DCFSHS technique as an efficient water heating mechanism, regardless of the thermal conductivities of the metals used in solar heating system production.
- Water temperatures higher than 90 °C were possible with Al, Cu, and Fe metals colored with matte black paint, whereas water temperatures in the range of 65–70 °C were possible using stock metals without any paint. These temperatures are well suited for domestic home usage.
- The thickness of the selected metals used in the construction of DCFSHS is irrelevant and high water temperatures can be easily reached in all cases. This was confirmed by using 0.35 mm and 1 mm thicknesses in the Cu containers. The almost identical heating profiles of both of these thicknesses allow for a reduction in the cost of solar heating system construction.
- Matte-black-painted anodized Al is an excellent choice for the construction of water heaters due to its good corrosion resistance, availability as a lightweight metal, and high thermal conductivity.
- It was also found that white inner walls in the solar heater can lead to an increase in the water temperature by approximately 4 °C compared to the black color. This improved the heat trapping process in the container.
- The R% test in the wavelength range of 240–840 nm for Al, Cu, and Fe metals showed tangible differences. The matte-black-painted metals gave almost zero R%, which makes them a better choice for solar-radiation-absorbing surfaces.
- The above-mentioned points highlight the innovative design of our solar water heating system due to the improvements in many aspects, such as design, production costs, environment, and weight.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vengadesan, E.; Ramalingam, S. A review on recent development of thermal performance enhancement methods of flat plate solar water heater. Sol. Energy 2020, 206, 935–961. [Google Scholar] [CrossRef]
- Dehghan, M.; Pfeiffer, C.; Rakhshani, E.; Bakhshi-Jafarabadi, R. Review on Techno-Economic Assessment of Solar Water Heating Systems in the Middle East. Energies 2021, 14, 4944. [Google Scholar] [CrossRef]
- Sethupathi, N.; Vikramprasad, K.; Maadeswaran, P. A review of efficiency factors and equations of various solar water heaters and their relative field components. Rasayan J. Chem. 2017, 10, 778–783. [Google Scholar]
- Hohne, P.; Kusakana, K.; Numbi, B. A review of water heating technologies: An application to the South African context. Energy Rep. 2019, 5, 1–19. [Google Scholar] [CrossRef]
- Oliva, A.I.; Maldonado, R.D.; Díaz, E.A.; Montalvo, A.I. A high absorbance material for solar collectors’ applications. IOP Conf. Ser.: Mater. Sci. Eng. 2013, 45, 012019. [Google Scholar] [CrossRef]
- Yassen, T.; Mokhlif, N.; Eleiwi, M. Performance investigation of an integrated solar water heater with corrugated absorber surface for domestic use. Renew. Energy 2019, 138, 852–860. [Google Scholar] [CrossRef]
- Li Voti, R. Optimization of a perfect absorber multilayer structure by genetic algorithms. J. Eur. Opt. Soc. 2018, 14, 1–12. [Google Scholar] [CrossRef]
- Pinho, D.; Freire, F.; Araújo, F.; Dutra, K.; Teixeira, E.; da Silva, M.; Costa Rocha, P. Characterization and application of a selective coating for solar collectors from of the cashew nutshell liquid. Proc. Inst. Mech. Eng. Part L 2020, 234, 167–174. [Google Scholar]
- De Maio, D.; D’Alessandro, C.; Caldarelli, A.; De Luca, D.; Di Gennaro, E.; Russo, R.; Musto, M. A selective solar absorber for unconcentrated solar thermal panels. Energies 2021, 14, 900. [Google Scholar] [CrossRef]
- AlShamaileh, E. Testing of a new solar coating for solar water heating applications. Sol. Energy 2010, 84, 1637–1643. [Google Scholar] [CrossRef]
- Moosa, I. Effect of Galena powder of 63 μm micrometer particle size and less on the absorptivity of black paint mixture. Int. J. Adv. Res. Eng. Technol. 2015, 6, 60–68. [Google Scholar]
- Wang, X.; Ouyang, T.; Duan, X.; Ke, C.; Zhang, X.; Min, J.; Li, A.; Guo, W.; Cheng, X. Improved Solar Absorptance of WC/Co Solar Selective Absorbing Coating with Multimodal WC Particles. Metals 2017, 137, 137. [Google Scholar] [CrossRef]
- David, H.; Barrera-Calva, E.; González, F.; Rentería Tapia, V. Ultrasonic spray pyrolysis technique to generate a solar absorber coating of Mn-doped α-Fe2O3. Renew. Energy Environ. Sustain. 2021, 6, 3. [Google Scholar]
- Jin, X.; Lin, G.; Jin, H.; Fu, Z.; Sun, H. Experimental Research on the Selective Absorption of Solar Energy by Hybrid Nanofluids. Energies 2021, 14, 8186. [Google Scholar] [CrossRef]
- Faizal, M.; Saidur, R.; Mekhilef, S. Potential of Size Reduction of Flat-plate Solar Collectors When Applying Al2O3 Nanofluid. Adv. Mater. Res. 2014, 832, 149–153. [Google Scholar] [CrossRef]
- Bhalla, V.; Tyagi, H. Solar energy harvesting by cobalt oxide nanoparticles, a nanofluid absorption-based system. Sustain. Energy Technol. Assess. 2017, 24, 45–54. [Google Scholar] [CrossRef]
- Mohaghegh, M.R. Nanofluids Applications in Solar Energy Systems: A Review. J. Sol. Energy Res. 2018, 3, 57–65. [Google Scholar]
- Abu Shadate, F.; Kamarulzaman, M.; Wan Harun, W.S.; Kadirgama, K.; Ramasamy, D.; Farhana, K.; Abu Bakar, R.; Yusaf, T.; Subramanion, S.; Yousif, B. A Comprehensive Review on Efficiency Enhancement of Solar Collectors Using Hybrid Nanofluids. Energies 2022, 15, 1391. [Google Scholar]
- Ofoegbu, S.; Fernandes, F.; Pereira, A. The sealing step in aluminum anodizing: A focus on sustainable strategies for enhancing both energy efficiency and corrosion resistance. Coatings 2020, 10, 226. [Google Scholar] [CrossRef]
- Ramesh, C.; Vijayakumar, M.; Alshahrani, S.; Navaneethakrishnan, G.; Palanisamy, R.; Natrayan, L.; Saleel, C.; Afzal, A.; Shaik, S.; Panchal, H. Performance enhancement of selective layer coated on solar absorber panel with reflector for water heater by response surface method: A case study. Therm. Eng. 2022, 36, 102093. [Google Scholar] [CrossRef]
- Sørensen, B.; Breeze, P.; Storvick, T.; Yang, S.; da Rosa, A.; Gupta, H.; Sukanta, R.; Doble, M.; Maegaard, P.; Pistoia, G.; et al. Renewable Energy Focus Handbook, 1st ed.; Academic Press: New York, NY, USA, 2009; p. 343. [Google Scholar]
- Serway, R.; Jewett, J. Physics for Scientists and Engineers, 9th ed.; Cengage Learning: Boston, MA, USA, 2014; pp. 594, 609 and 811. [Google Scholar]
- Fraihat, H.; Almbaideen, A.; Al-Odienat, A.; Al-Naami, B.; De Fazio, R.; Visconti, P. Solar Radiation Forecasting by Pearson Correlation Using LSTM Neural Network and ANFIS Method: Application in the West-Central Jordan. Future Internet 2022, 14, 79. [Google Scholar] [CrossRef]
- Alrwashdeh, S.; Alsaraireh, F.; Saraireh, M. Solar radiation map of Jordan governorates. Int. J. Eng. Technol. 2018, 7, 1664–1667. [Google Scholar] [CrossRef]
- Etier, I.; Al Tarabsheh, A.; Ababneh, M. Analysis of Solar Radiation in Jordan. Jordan J. Mech. Ind. Eng. 2010, 4, 190–197. [Google Scholar]
- Qasaimeh, A.; Qasaimeh, M.; Abu-Salem, Z.; Momani, M. Solar Energy Sustainability in Jordan. Comput. Water Energy Environ. Eng. 2014, 3, 41–47. [Google Scholar] [CrossRef]
- International Renewable Energy Agency (IRENA). Renewable Readiness Assessment: The Hashemite Kingdom of Jordan; International Renewable Energy Agency: Abu Dhabi, United Arab Emirates, 2021; p. 32. [Google Scholar]
- Kalogirou, S. The potential of solar industrial process heat applications. Appl. Energy 2003, 76, 337–361. [Google Scholar] [CrossRef]
- Ahmad, Z. Aluminium Alloys in Solar Power—Benefits and Limitations; InTech: Rijeka, Croatia, 2013; Available online: https://doi.org/10.5772/3354 (accessed on 24 October 2022).
- AlShamaileh, E.; Altwaiq, A.; Esaifan, M.; Al-Fayyad, H.; Shraideh, Z.; Moosa, I.; Hamadneh, I. Study of the microstructure, corrosion and optical properties of anodized aluminum for solar heating applications. Metals 2022, 12, 1635. [Google Scholar] [CrossRef]
- Ashtiani, A.; Faraji, S.; Iranagh, S.; Faraji, A. The study of electroless Ni–P alloys with different complexing agents on Ck45 steel substrate. Arabian J. Chem. 2017, 10, 1541–1545. [Google Scholar] [CrossRef]






| Metals | Thermal Conductivity W/m. °C, Page 609 | Specific Heat Capacity J/kg. °C, Page 594 |
|---|---|---|
| Aluminum | 238 | 900 |
| Copper | 397 | 387 |
| Iron | 79.5 | 448 |
| Used Metals | Container Type | Metal Thickness (mm) | Symbolize |
|---|---|---|---|
| Commercial anodized Al | As it is Al Matte-Black-Painted Al | 1 | Al B. Al |
| Commercial Cu | As it is Cu Matte-Black-Painted Cu | 0.35 0.35 | Cu, 0.35 mm B. Cu, 0.35 mm |
| As it is Cu Matte-Black-Painted Cu | 1 1 | Cu, 1 mm B. Cu, 1 mm | |
| Commercial Fe | As it is Fe Matte-Black-Painted Fe | 1 1 | Fe, 1 mm B. Fe, 1 mm |
| As it is Fe Matte-Black-Painted Fe | 2 2 | Fe, 2 mm B. Fe, 2 mm |
| Container Type | Container Sheet Thickness (mm) | ~ Max. Water Temp. (°C) | Mean Values of Tamb. and GSI |
|---|---|---|---|
| Fe B. Fe Fe B. Fe | 1 1 2 2 | 91 96 94 97 | 26 °C, 414 W/m2 Sunny day |
| Cu B. Cu | 0.35 0.35 | 76 88 | 27 °C, 368 W/m2 Partially cloudy day |
| Cu B. Cu | 1 1 | 75 88 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
AlShamaileh, E.; Moosa, I.S.; Al-Fayyad, H.; Lahlouh, B.; Kazem, H.A.; Abu-Afifeh, Q.; Al-Saqarat, B.S.; Esaifan, M.; Hamadneh, I. Performance Comparison and Light Reflectance of Al, Cu, and Fe Metals in Direct Contact Flat Solar Heating Systems. Energies 2022, 15, 8888. https://doi.org/10.3390/en15238888
AlShamaileh E, Moosa IS, Al-Fayyad H, Lahlouh B, Kazem HA, Abu-Afifeh Q, Al-Saqarat BS, Esaifan M, Hamadneh I. Performance Comparison and Light Reflectance of Al, Cu, and Fe Metals in Direct Contact Flat Solar Heating Systems. Energies. 2022; 15(23):8888. https://doi.org/10.3390/en15238888
Chicago/Turabian StyleAlShamaileh, Ehab, Iessa Sabbe Moosa, Heba Al-Fayyad, Bashar Lahlouh, Hussein A. Kazem, Qusay Abu-Afifeh, Bety S. Al-Saqarat, Muayad Esaifan, and Imad Hamadneh. 2022. "Performance Comparison and Light Reflectance of Al, Cu, and Fe Metals in Direct Contact Flat Solar Heating Systems" Energies 15, no. 23: 8888. https://doi.org/10.3390/en15238888
APA StyleAlShamaileh, E., Moosa, I. S., Al-Fayyad, H., Lahlouh, B., Kazem, H. A., Abu-Afifeh, Q., Al-Saqarat, B. S., Esaifan, M., & Hamadneh, I. (2022). Performance Comparison and Light Reflectance of Al, Cu, and Fe Metals in Direct Contact Flat Solar Heating Systems. Energies, 15(23), 8888. https://doi.org/10.3390/en15238888









