Nipah and Hendra Viruses: Deadly Zoonotic Paramyxoviruses with the Potential to Cause the Next Pandemic
Abstract
:1. Introduction
2. Henipaviruses: A Cause of Grave Concern
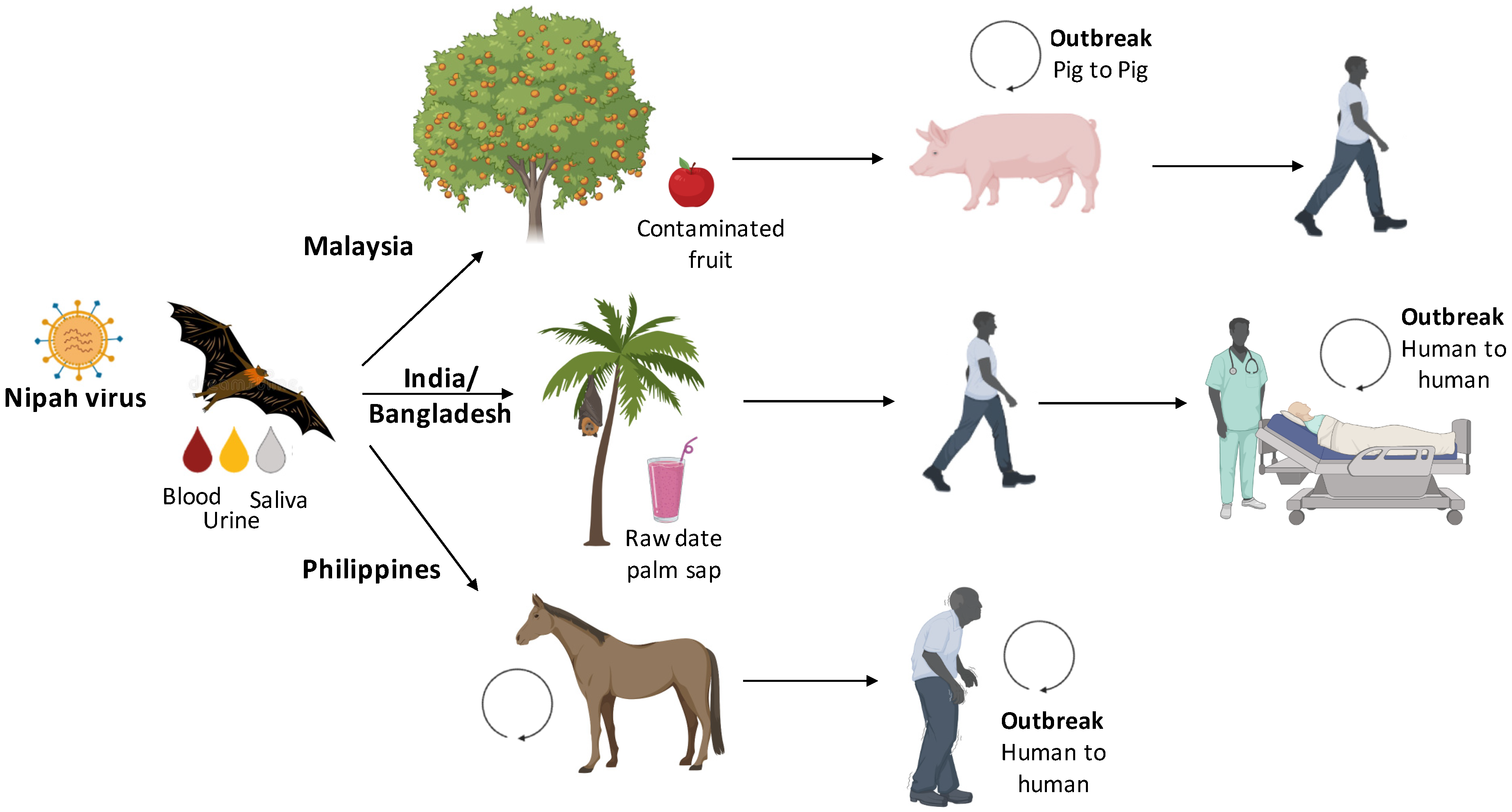
3. Transmission Cycle from Bats to Humans
4. The Virus: Unique Features of Henipa Virions
4.1. Structure and Genome Organization
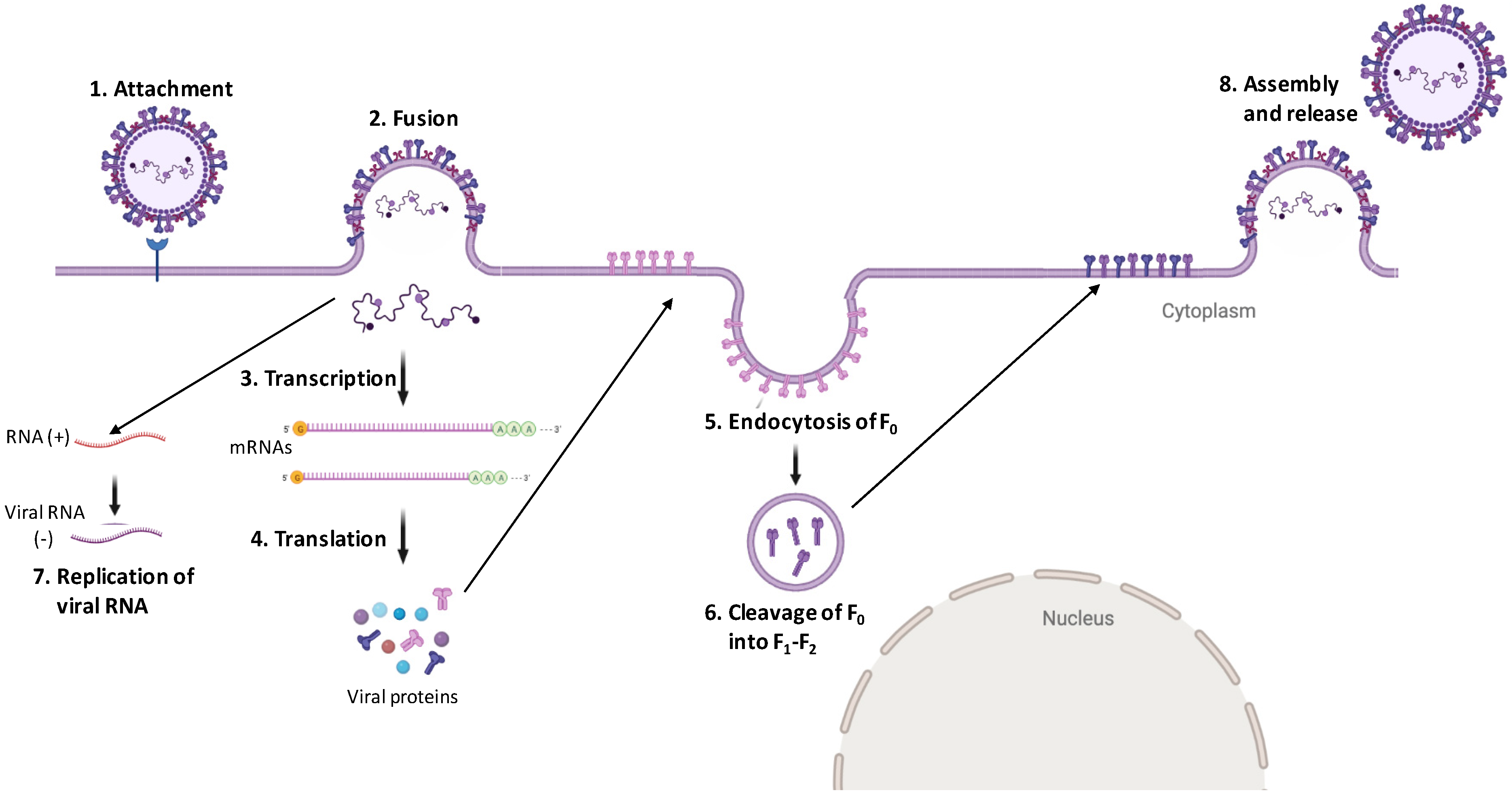
4.2. Digging Deep into Henipavirus Replication Cycle
5. Traditional and Novel Diagnostic Tests
6. Recent Strategies for the Control of the Nipah and Hendra Viruses
6.1. Passive Immunization Using Monoclonal Antibodies
6.2. Recent Developments in Vaccine Production
6.2.1. Subunit Vaccines
6.2.2. Vectored Vaccines
6.3. Virus-like Particles
6.4. Single-Dose Lipid Nanoparticle mRNA Vaccine
7. Other Recently Discovered Henipaviruses
8. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rogers, R.J.; Douglas, I.C.; Baldock, F.C.; Glanville, R.J.; Seppanen, K.T.; Gleeson, L.J.; Selleck, P.N.; Dunn, K.J. Investigation of a second focus of equine morbillivirus infection in coastal Queensland. Aust. Veter. J. 1996, 74, 243–244. [Google Scholar] [CrossRef] [PubMed]
- Chua, K.B.; Goh, K.J.; Wong, K.T.; Kamarulzaman, A.; Tan, P.S.K.; Ksiazek, T.G.; Zaki, S.R.; Paul, G.; Lam, S.K.; Tan, C.T. Fatal encephalitis due to Nipah virus among pig-farmers in Malaysia. Lancet 1999, 354, P1257–P12593. [Google Scholar] [CrossRef] [PubMed]
- WHO. Nipah Virus. 2018. Available online: https://www.who.int/news-room/fact-sheets/detail/nipah-virus (accessed on 6 August 2022).
- Epstein, J.H.; Anthony, S.J.; Islam, A.; Kilpatrick, A.M.; Khan, S.A.; Balkey, M.D.; Ross, N.; Smith, I.; Zambrana-Torrelio, C.; Tao, Y.; et al. Nipah virus dynamics in bats and implications for spillover to humans. Proc. Natl. Acad. Sci. USA 2020, 117, 29190–29201. [Google Scholar] [CrossRef] [PubMed]
- Luby, S.P.; Hossain, M.J.; Gurley, E.S.; Ahmed, B.N.; Banu, S.; Khan, S.U.; Homaira, N.; Rota, P.A.; Rollin, P.E.; Comer, J.A.; et al. Recurrent zoonotic transmission of Nipah virus into humans, Bangladesh, 2001. Emerg. Infect. Dis. 2009, 15, 1229–1235. [Google Scholar] [CrossRef] [PubMed]
- Thakur, V.; Thakur, P.; Ratho, R.K. Nipah Outbreak: Is it the beginning of another pandemic in the era of COVID-19 and Zika. Brain. Behav. Immun. 2022, 99, 25–26. [Google Scholar] [CrossRef]
- Chua, K.B. Nipah virus outbreak in Malaysia. J. Clin. Virol. 2003, 26, 265–275. [Google Scholar] [CrossRef]
- Satterfield, B.A.; Cross, R.W.; Fenton, K.A.; Borisevich, V.; Agans, K.N.; Deer, D.J.; Graber, J.; Basler, C.F.; Geisbert, T.W.; Mire, C.E. Nipah virus C and W proteins contribute to respiratory disease in ferrets. J. Virol. 2016, 90, 6326–6343. [Google Scholar] [CrossRef] [Green Version]
- Arankalle, V.A.; Bandyopadhyay, B.T.; Ramdasi, A.Y.; Jadi, R.; Patil, D.R.; Rahman, M.; Majumdar, M.; Banerjee, P.S.; Hati, A.K.; Goswami, R.P.; et al. Genomic Characterization of Nipah Virus, West Bengal, India. Emerg. Infec. Dis. 2011, 17, 907–909. [Google Scholar] [CrossRef]
- Aditi; Shariff, M. Nipah virus infection: A review. Epidemiol. Infect. 2019, 147, 95. [Google Scholar] [CrossRef]
- Murray, K.; Rogers, R.; Selvey, L.; Selleck, P.; Hyatt, A.; Gould, A.; Gleeson, L.; Hooper, P.; Westbury, H. A novel morbillivirus pneumonia of horses and its transmission to humans. Emerg. Infect. Dis. 1995, 1, 31–33. [Google Scholar] [CrossRef]
- Murray, K.; Selleck, P.; Hooper, P.; Hyatt, A.; Gould, A.; Gleeson, L.; Westbury, H.; Hiley, L.R.; Selvey, L.; Rodwell, B.; et al. A morbilli virus that caused fatal disease in horses and humans. Science 1995, 268, 94–97. [Google Scholar] [CrossRef] [PubMed]
- Selvey, L.A.; Wells, R.M.; McCormack, J.G.; Ansford, A.J.; Murray, K.; Rogers, R.J.; Lavercombe, P.S.; Selleck, P.; Sherdian, J.W. Infection of humans and horses by a newly described morbillivirus. Med. J. Aust. 1995, 162, 642–645. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, J.D.; Allworth, A.M.; Paterson, D.L.; Snow, T.M.; Boots, R.; Geelson, L.J.; Gould, A.R.; Hyatt, A.D.; Bradfield, J. Fatal encephalitis due to novel paramyxovirus transmitted from horses. Lancet 1997, 349, 93–95. [Google Scholar] [CrossRef] [PubMed]
- Queensland Government. Summary of Hendra Virus Incidents in Horses. 2019. Available online: https://www.business.qld.gov.au/industries/service-industries-professionals/service-industries/veterinary-surgeons/guidelines-hendra/incident-summary (accessed on 10 April 2022).
- Playford, E.G.; McCall, B.; Smith, G.; Slinko, V.; Allen, G.; Smith, I.; Moore, F.; Taylor, C.; Kung, H.H.; Field, H. Human Hendra virus encephalitis associated with equine outbreak, Australia. Emerg. Infect. Dis. 2010, 16, 219–223. [Google Scholar] [CrossRef]
- Dimmock, N.J.; Easton, A.J.; Leppard, K.N. (Eds.) Introduction to Modern Virology; School of Life Sciences University of Warwick: Coventry, UK; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Hosono, H.; Kono, H.; Ito, S.; Shirai, J. Economic impact of Nipah virus infection outbreak in Malaysia, Obihiro University of Agriculture and Veterinary Medicine; National Institute of Animal Health. In Proceedings of the 11th International Symposium on Veterinary Epidemiology and Economics, Cairns, Australia, 6–11 August 2006. [Google Scholar]
- Gurley, E.S.; Hegde, S.T.; Hossain, K.; Sazzad, H.M.S.; Hossain, M.J.; Rahman, M.; Sharker, M.A.Y.; Salje, H.; Islam, M.S.; Epstein, J.H.; et al. Convergence of humans, bats, trees, and culture in Nipah virus transmission, Bangladesh. Emerg. Infect. Dis. 2017, 23, 1446–1453. [Google Scholar] [CrossRef] [Green Version]
- Chanchal, D.K.; Alok, S.; Sabharwal, M.; Bijauliya, R.K.; Rashi, S. Nipah: Silently rising infection. Int. J. Pharm. Sci. Res. 2018, 9, 3128–3135. [Google Scholar]
- Luby, S.P.; Gurley, E.S.; Hossain, M.J. Transmission of human infection with Nipah virus. Clin. Infect. Dis. 2009, 49, 1743–1748. [Google Scholar] [CrossRef] [Green Version]
- Hassan, M.Z.; Sazzad, H.M.S.; Luby, S.P.; Sturm-Ramirez, K.; Bhuiyan, M.U.; Rahman, M.Z.; Islam, M.M.; Stroher, U.; Sultana, S.; Kafi, M.A.H.; et al. Nipah virus contamination of hospital surfaces during outbreaks, Bangladesh, 2013–2014. Emerg. Infect. Dis. 2018, 24, 15–21. [Google Scholar] [CrossRef] [Green Version]
- Hedge, S.T.; Sazzas, H.M.S.; Hossain, M.J.; Alam, M.U.; Kenah, E.; Daszak, P.; Rollin, P.; Rahman, M.; Luby, S.P.; Gurley, E.S. Investigating Rare Risk Factors for Nipah Virus in Bangladesh: 2001. Ecohealth 2016, 13, 720–728. [Google Scholar]
- Sun, B.; Jia, L.; Liang, B.; Chen, Q.; Liu, D. Phylogeography, Transmission, and Viral Proteins of Nipah Virus. VirologicaSinica 2018, 33, 385–393. [Google Scholar] [CrossRef] [Green Version]
- Lamb, R.A.; Parks, G.D. Paramyxoviridae: The viruses and their replication. In Fields Virology, 6th ed.; Knipe, D.M., Howley, P.M., Cohen, J.I., Griffin, D.E., Lamb, R.A., Martin, M.A., Racaniello, V.R., Roizman, B., Eds.; Lippincott, Williams and Wilkins: Philadelphia, PA, USA, 2013; p. 957. [Google Scholar]
- Harcourt, B.H.; Tamin, A.; Ksiazek, T.G.; Rollin, P.E.; Anderson, L.J.; Bellini, W.J.; Rota, P.A. Molecular characterization of Nipah virus, a newly emergent paramyxovirus. Virology 2002, 271, 334–349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.F.; Yu, M.; Hansson, E.; Pritchard, L.I.; Shiell, B.; Michalski, W.P.; Eaton, B.T. The exceptionally large genome of Hendra virus: Support for creation of a new genus within the family Paramyxoviridae. J. Virol. 2002, 74, 9972–9979. [Google Scholar] [CrossRef] [PubMed]
- Harcourt, B.H.; Tamin, A.; Halpin, K.; Ksiazek, T.G.; Rollin, P.E.; Bellini, W.J.; Rota, P.A. Molecular characterization of the polymerase gene and genomic termini of Nipah virus. Virology 2001, 287, 192–201. [Google Scholar] [CrossRef] [Green Version]
- Peret, T.C.; Boivin, G.; Li, Y.; Couillard, M.; Humphery, C.; Osterhaus, A.D.; Erdman, D.D.; Anderson, L.J. Electron cryomicroscopy reveals different F1+F2 protein states in intact parainfluenza virions. J. Virol. 2008, 82, 3775–3781. [Google Scholar]
- El Najjar, F.; Schmitt, A.; Dutch, R. Paramyxovirus glycoprotein incorporation, assembly and budding: A three-way dance for infectious particle production. Viruses 2014, 6, 3019–3054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamb, R.A.; Parks, G.D. Paramyxoviridae: The viruses and their replication. In Fields Virology, 5th ed.; Knipe, D.M., Howley, P.M., Eds.; Lippincott, Williams and Wilkins: Philadelphia, PA, USA, 2006; pp. 1449–1496. [Google Scholar]
- Rodriguez, J.J.; Parisien, J.P.; Horvath, C.M. Nipah virus Vproteins evades Alpha and Gamma interferons by preventing STAT1 and STAT2 activation and nuclear accumulation. J. Virol. 2002, 76, 11476–11483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horvath, C.M. Weapons of STAT destruction Interferon evasion by paramyxovirus V proteins Curt M. Horvath. Eur. J. Biochem. 2004, 271, 4621–4628. [Google Scholar] [CrossRef]
- Negrete, O.A.; Levroney, E.L.; Aguilar, H.C.; Bertolotti-Ciarlet, A.; Nazarian, R.; Tajyar, S.; Lee, B. EphrinB2 is the entry receptor for Nipah virus, an emergent deadly paramyxovirus. Nature 2005, 436, 401–405. [Google Scholar] [CrossRef]
- Lamb, R.A.; Paterson, R.G.; Jardetzky, T.S. Paramyxovirus membrane fusion: Lessons from the F and HN atomic structures. Virology 2006, 344, 30–37. [Google Scholar] [CrossRef] [Green Version]
- Klenk, H.D.; Garten, W. Host cell proteases controlling virus pathogenicity. Trends Microbiol. 1994, 2, 39–43. [Google Scholar] [CrossRef]
- Diederich, S.; Moll, M.; Klenk, H.D.; Maisner, A. The nipah virus fusion protein is cleaved within the endosomal compartment. J. Biol. Chem. 2005, 280, 29899–29903. [Google Scholar] [CrossRef] [Green Version]
- Pager, C.T.; Dutch, R.E. Cathepsin L is involved in proteolytic processing of the Hendra virus fusion protein. J. Virol. 2005, 79, 12714–12720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lyles, D.S. Assembly and budding of negative-strand RNA viruses. In Advances in Virus Research; Academic Press: Cambridge, MA, USA, 2013; pp. 57–90. [Google Scholar]
- Guillaume, V.; Contamin, H.; Loth, P.; Georges-Courbot, M.C.; Lefeuvre, A.; Marianneau, P.; Wild, T.F. Nipah virus: Vaccination and passive protection studies in a hamster model. J. Virol. 2004, 78, 834–840. [Google Scholar] [CrossRef] [PubMed]
- Mazzola, L.T.; Kelly-Cirino, C. Diagnostics for Nipah virus: A zoonotic pathogen endemic to Southeast Asia. BMJ Global Health 2019, 4, 001118. [Google Scholar] [CrossRef]
- Liu, J.; Ochieng, C.; Wiersma, S.; Stroher, U.; Towner, J.S.; Whitmer, S.; Nichol, S.T.; Moore, C.C.; Kersh, G.J.; Kato, C.; et al. Development of a TaqMan Array Card for acute-febrile-illness outbreak investigation and surveillance of emerging pathogens, including Ebola virus. J. Clin. Microbiol. 2016, 54, 49–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ong, K.C.; Wong, K.T. Henipavirusencephalitis: Recent developments and advances. Brain Pathol. 2015, 25, 605–613. [Google Scholar] [CrossRef]
- Wang, L.F.; Daniels, P. Diagnosis of Henipavirus infection: Current capabilities and future directions. Curr. Top. Microbiol. Immunol. 2012, 359, 179–196. [Google Scholar] [PubMed]
- Chiang, C.F.; Lo, M.K.; Rota, P.A.; Spiropoulou, C.F.; Rollin, P.E. Use of monoclonal antibodies against Hendra and Nipah viruses in an antigen capture ELISA. J. Virol. 2010, 7, 115. [Google Scholar] [CrossRef] [Green Version]
- Tiong, V.; Lam, C.W.; Phoon, W.H.; AbuBakar, S.; Chang, L.Y. Serum from Nipah virus patients recognises recombinant viral proteins produced in Escherichia coli. Jpn. J. Infect. Dis. 2017, 70, 26–31. [Google Scholar] [CrossRef] [Green Version]
- Defang, G.N.; Khetawat, D.; Broder, C.C.; Quinnan, G.V., Jr. Induction of neutralizing antibodies to Hendra and Nipah glycoproteins using a Venezuelan equine encephalitis virus in vivo expression system. Vaccines 2010, 29, 212–220. [Google Scholar] [CrossRef] [Green Version]
- Kulkarni, D.D.; Tosh, C.; Venkatesh, G.; Senthil, K.D. Nipah virus infection: Current scenario. Indian J. Virol. 2013, 24, 398–408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daniels, P.; Ksiazek, T.; Eaton, B.T. Laboratory diagnosis of Nipah and Hendra virus infections. Microb. Infect. 2001, 3, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Bossart, K.N.; Zhu, Z.; Middleton, D.; Klippel, J.; Crameri, G.; Bingham, J.; McEachern, J.A.; Green, D.; Hancock, T.J.; Chan, Y.P.; et al. A neutralizing human monoclonal antibody protects against lethal disease in a new ferret model of acute Nipah virus infection. PLoS Pathog. 2008, 5, 1000642. [Google Scholar] [CrossRef]
- Xu, K.; Rockx, B.; Xie, Y.; Debuysscher, B.L.; Fusco, D.L.; Zhu, Z.; Chan, Y.P.; Xu, Y.; Luu, T.; Cer, R.Z.; et al. Crystal structure of the Hendra virusattachment G glycoprotein bound to a potent cross-reactive neutralizing human monoclonal antibody. PLoS Pathog. 2013, 9, 1003684. [Google Scholar] [CrossRef] [Green Version]
- Playford, E.G.; Munro, T.; Mahler, S.M.; Elliott, S.; Gerometta, M.; Hoger, K.L.; Jones, M.L.; Griffin, P.; Lynch, K.D.; Carroll, H.; et al. Safety, tolerability, pharmacokinetics, and immunogenicity of a human monoclonal antibody targeting the G glycoprotein of henipaviruses in healthy adults: A first-in-human, randomised, controlled, phase 1 study. Lancet Infect. Dis. 2020, 20, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Dang, H.V.; Chan, Y.P.; Park, Y.J.; Snijder, J.; DaSilva, S.C.; Vu, B.; Yan, L.; Feng, Y.R.; Rockx, B.; Geisbert, T.W.; et al. An antibody against the F glycoprotein inhibits Nipah and Hendra virus infections. Nat. Struct. Mol. Biol. 2019, 26, 980–987. [Google Scholar] [CrossRef] [PubMed]
- Amaya, M.; Broder, C.C. Vaccines to Emerging Viruses: Nipah and Hendra. Ann. Rev. Virol. 2020, 7, 447–473. [Google Scholar] [CrossRef]
- Broder, C.C.; Xu, K.; Nikolov, D.B.; Zhu, Z.; Dimitrov, D.S.; Middleton, D. A treatment for and vaccine against the deadly Hendra and Nipah viruses. Antivir. Res. 2013, 100, 8–13. [Google Scholar] [CrossRef] [Green Version]
- Pickering, B.S.; Hardham, J.M.; Smith, G.; Weingartl, E.T.; Dominowski, P.J.; Foss, D.L.; Mwangi, D.; Broder, C.C.; Roth, J.A.; Weingartl, H.M. Protection against henipaviruses in swine requires both, cell-mediated and humoral immune response. Vaccine 2016, 34, 4777–4786. [Google Scholar] [CrossRef]
- Weingartl, H.M.; Berhane, Y.; Caswell, J.L. Recombinantnipah virus vaccines protect pigs against challenge. J. Virol. 2006, 80, 7929–7938. [Google Scholar] [CrossRef] [Green Version]
- Ploquin, A.; Szecsi, J.; Mathieu, C.; Guillaume, V.; Barateau, V.; Ong, K.C.; Wong, K.T.; Cosset, F.L.; Horvat, B.; Salvetti, A. Protection against henipavirus infection by use of recombinant adeno-associated virus–vector vaccines. J. Infect. Dis. 2013, 207, 469–478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ewer, K.; Sebastian, S.; Spencer, A.J.; Gilbert, S.; Hill, A.V.S.; Lambe, T. Chimpanzee adenoviral vectors as vaccines for outbreak pathogens. Hum. Vaccines Immunother. 2017, 13, 3020–3032. [Google Scholar] [CrossRef] [PubMed]
- Van Doremalen, N.; Lambe, T.; Sebastian, S.; Bushmaker, T.; Fischer, R.; Feldmann, F.; Haddock, E.; Letko, M.; Avanzato, V.A.; Rissanen, I.; et al. single-dose ChAdOx1- vectored vaccine provides complete protection against Nipah Bangladesh and Malaysia in Syrian golden hamsters. PLoS Negl. Trop. Dis. 2019, 13, 0007462. [Google Scholar] [CrossRef] [PubMed]
- Mire, C.E.; Versteeg, K.M.; Cross, R.W. Single injection recombinant vesicular stomatitis virus vaccines protect ferrets against lethal Nipah virus disease. J. Virol. 2013, 10, 353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lo, M.K.; Bird, B.H.; Chattopadhyay, A.; Drew, C.P.; Martin, B.E.; Coleman, J.D.; Rose, J.K.; Nichol, S.T.; Spiropoulou, C.F. Single-dose replication-defective VSV-based Nipah virus vaccines provide protection from lethal challenge in Syrian hamsters. Antiviral Res. 2014, 101, 26–29. [Google Scholar] [CrossRef] [Green Version]
- Prescott, J.; DeBuysscher, B.L.; Feldmann, F.; Gardner, D.J.; Haddock, E.; Martellaro, C.; Scott, D.; Feldmann, H. Single-dose live-attenuated vesicular stomatitis virus-based vaccine protects African green monkeys from Nipah virus disease. Vaccine 2015, 4, 2823–2829. [Google Scholar] [CrossRef] [Green Version]
- DeBuysscher, B.L.; Scott, D.; Marzi, A.; Prescott, J.; Feldmann, H. Single-dose live-attenuated Nipah virus vaccines confer complete protection by eliciting antibodies directed against surface glycoproteins. Vaccine 2014, 32, 2637–2644. [Google Scholar] [CrossRef] [Green Version]
- Kurup, D.; Wirblich, C.; Feldmann, H.; Marzi, A.; Schnell, M.J. Rhabdovirus-based vaccine platforms against henipaviruses. J. Virol. 2015, 89, 144–154. [Google Scholar] [CrossRef] [Green Version]
- Shuai, L.; Ge, J.; Wen, Z.; Wang, J.; Wang, X.; Bu, Z. Immune responses in mice and pigs after oral vaccination with rabies virus vectored Nipah disease vaccines. Vet. Microbiol. 2020, 241, 108549. [Google Scholar] [CrossRef]
- Yoneda, M.; Georges-Courbot, M.C.; Ikeda, F.; Ishii, M.; Nagata, N.; Jacquot, F.; Raoul, H.; Sato, H.; Kai, C. Recombinant measles virus vaccine expressing the Nipah virus glycoprotein protects against lethal Nipah virus challenge. PLoS ONE 2013, 8, 58414. [Google Scholar] [CrossRef]
- Kong, D.; Wen, Z.; Su, H.; Ge, J.; Chen, W.; Wang, X.; Wu, C.; Yang, C.; Chen, H.; Bu, Z. Newcastle disease virus-vectored Nipah encephalitis vaccines induce B and T cell responses in mice and long-lasting neutralizing antibodies in pigs. Virology 2012, 25, 327–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walpita, P.; Cong, Y.; Jahrling, P.B.; Rojas, O.; Postnikova, E.; Yu, S.; Johns, L.; Holbrook, M.R. A VLP-based vaccine provides complete protection against Nipah virus challenge following multiple-dose or single-dose vaccination schedules in a hamster model. NPJ Vaccines 2017, 2, 21. [Google Scholar] [CrossRef] [PubMed]
- Walpita, P.; Barr, J.; Sherman, M.; Basler, C.F.; Wang, L. Vaccine potential of Nipah Virus Like Particles. PLoS ONE 2011, 6, 18437. [Google Scholar] [CrossRef]
- National Institute of Allergy and Infectious Diseases. Available online: https://www.niaid.nih.gov/news-events/nih-launches-clinical-trial-mrna-nipah-virus-vaccine (accessed on 11 November 2022).
- Marsh, G.A.; De Jong, C.; Barr, J.A.; Tachedjian, M.; Smith, C.; Middleton, D.; Yu, M.; Todd, S.; Foord, A.J.; Haring, V. Cedar virus: A novel Henipavirus isolated from Australian bats. PLoS Pathog. 2012, 8, 1002836. [Google Scholar] [CrossRef]
- Drexler, J.F.; Corman, V.M.; Muller, M.A.; Maganga, G.D.; Vallo, P.; Binger, T.; Gloza-Rausch, F.; Cottontail, V.M.; Rasche, A.; Yordanov, S.; et al. Bats host major mammalian paramyxoviruses. Nat. Commun. 2012, 3, 796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rissanen, I.; Ahmed, A.; Azarm, K.; Beaty, S.; Hong, P.; Nambulli, S.; Duprex, W.P.; Lee, B.; Bowden, T.A. Idiosyncratic Mojiang virus attachment glycoprotein directs a host-cell entry pathway distinct from genetically related henipaviruses. Nat. Commun. 2017, 8, 16060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geisbert, T.W.; Bobb, K.; Borisevich, V.; Geisbert, J.B.; Agans, K.N.; Cross, R.W. A single dose investigational subunit vaccine for human use against Nipah virus and Hendra virus. Vaccines 2021, 6, 23. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gazal, S.; Sharma, N.; Gazal, S.; Tikoo, M.; Shikha, D.; Badroo, G.A.; Rashid, M.; Lee, S.-J. Nipah and Hendra Viruses: Deadly Zoonotic Paramyxoviruses with the Potential to Cause the Next Pandemic. Pathogens 2022, 11, 1419. https://doi.org/10.3390/pathogens11121419
Gazal S, Sharma N, Gazal S, Tikoo M, Shikha D, Badroo GA, Rashid M, Lee S-J. Nipah and Hendra Viruses: Deadly Zoonotic Paramyxoviruses with the Potential to Cause the Next Pandemic. Pathogens. 2022; 11(12):1419. https://doi.org/10.3390/pathogens11121419
Chicago/Turabian StyleGazal, Sabahat, Neelesh Sharma, Sundus Gazal, Mehak Tikoo, Deep Shikha, Gulzar Ahmed Badroo, Mohd Rashid, and Sung-Jin Lee. 2022. "Nipah and Hendra Viruses: Deadly Zoonotic Paramyxoviruses with the Potential to Cause the Next Pandemic" Pathogens 11, no. 12: 1419. https://doi.org/10.3390/pathogens11121419
APA StyleGazal, S., Sharma, N., Gazal, S., Tikoo, M., Shikha, D., Badroo, G. A., Rashid, M., & Lee, S.-J. (2022). Nipah and Hendra Viruses: Deadly Zoonotic Paramyxoviruses with the Potential to Cause the Next Pandemic. Pathogens, 11(12), 1419. https://doi.org/10.3390/pathogens11121419






