Canine Distemper Virus Infection in the Free-Living Wild Canines, the Red Fox (Vulpes vulpes) and Jackal (Canis aureus moreoticus), in Croatia
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Preparation
2.2. Detection of CDV RNA
2.3. Sequence and Phylogenetic Analysis
3. Results
3.1. Detection of CDV Specific RNA Fragments in Brain Samples
3.2. The Partial Sequencing of H Gene Region
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Howard, C.R.; Fletcher, N.F. Emerging virus diseases: Can we ever expect the unexpected? Emerg. Microbes Infect. 2012, 1, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Marston, H.D.; Folkers, G.K.; Morens, D.M.; Fauci, A.S. Emerging viral diseases: Confronting threats with new technologies. Sci. Trans. Med. 2014, 6, 253. [Google Scholar] [CrossRef] [PubMed]
- ICTV (2021): ICTV Virus Taxonomy-2021 Release. Available online: https://ictv.global/taxonomy (accessed on 11 April 2023).
- Martinez-Gutierrez, M.; Ruiz-Saenz, J. Diversity of susceptible hosts in canine distemper virus infection: A systemic review and data synthesis. BMC Vet. Res. 2016, 12, 78. [Google Scholar] [CrossRef] [PubMed]
- Parardo, I.D.R.; Johnson, G.C.; Kleiboeker, S.B. Phylogenetic characterization of canine distemper viruses detected in naturally infected dogs in North America. J. Clin. Microbiol. 2005, 43, 5009–5017. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, M.; Une, Y.; Mochizuki, M. Hemagglutinin genotype profiles of canine distemper virus from domestic dogs in Japan. Arch. Virol. 2001, 146, 149–155. [Google Scholar] [CrossRef]
- Von Messling, V.; Zimmer, G.; Herrler, G.; Haas, L.; Cattaneo, R. The hemagglutinin of canine distemper virus determines tropism and cytopathogenicity. J. Virol. 2001, 75, 6418–6427. [Google Scholar] [CrossRef]
- Bhatt, M.; Rajak, K.K.; Chakravarti, S.; Yadav, A.K.; Kumar, A.; Gupta, V.; Chander, V.; Mathesh, K.; Chandramohan, S.; Sharma, A.K.; et al. Phylogenetic analysis of haemagglutinin gene deciphering a new genetically distinct lineage of canine distemper virus circulating among domestic dogs in India. Transbound. Emerg. Dis. 2019, 66, 1252–1267. [Google Scholar] [CrossRef]
- Jo, W.K.; Peters, M.; Kydyrmanov, A.; van de Bildt, M.W.G.; Kuiken, T.; Osterhaus, A.; Ludlow, M. The canine morbillivirus strain associated with an epizootic in caspian seals provides new insights into the evolutionary history of this virus. Viruses 2019, 11, 894. [Google Scholar] [CrossRef]
- Piewbang, C.; Radtanakatikanon, A.; Puenpa, J.; Poovorawan, Y.; Techangamsuwan, S. Genetic and evolutionary analysis of a new Asia-4 lineage and naturally recombinant canine distemper virus strains from Thailand. Sci. Rep. 2019, 9, 3198. [Google Scholar] [CrossRef]
- Rendon-Marin, S.; Da-Fontoura-Budaszewski, R.; Canal, C.W.; Ruiz-Saenz, J. Tropism and molecular pathogenesis of canine distemper virus. Virol. J. 2019, 16, 1. [Google Scholar] [CrossRef]
- Wang, R.; Wang, X.; Zhai, J.; Zhang, P.; Irwin, D.M.; Shen, X.; Chen, W.; Shen, Y. A new canine distemper virus lineage identifed from red pandas in China. Transbound. Emerg. Dis. 2022, 69, e944–e952. [Google Scholar] [CrossRef]
- Sekulin, K.; Hafner-Marx, A.; Kolodziejek, J.; Janik, D.; Schmidt, P.; Nowotny, N. Emergence of Canine Distemper in Bavarian wildlife associated with a specific amino acid exchange in the haemagglutinin protein. Vet. J. 2011, 187, 399–401. [Google Scholar] [CrossRef]
- Denzin, N.; Herwig, V.; Van Der Grinten, E. Occurrence and geographical distribution of Canine Distemper Virus infection in red foxes (Vulpes vulpes) of Saxony-Anhalt, Germany. Vet. Microbiol. 2013, 162, 214–218. [Google Scholar] [CrossRef]
- Nouvellet, P.; Donnelly, C.A.; De Nardi, M.; Rhodes, C.J.; De Benedictis, P.; Citterio, C.; Obber, F.; Lorenzetto, M.; Pozza, M.D.; Cauchemez, S.; et al. Rabies and canine distemper virus epidemics in the red fox population of northern Italy (2006–2010). PLoS ONE 2013, 8, e61588. [Google Scholar] [CrossRef]
- Akdesir, E.; Origgi, F.C.; Wimmershoff, J.; Frey, J.; Frey, C.F.; Ryser-Degiorgis, M.P. Causes of mortality and morbidity in free-ranging mustelids in Switzerland: Necropsy data from over 50 years of general health surveillance. BMC Vet. Res. 2018, 14, 195. [Google Scholar] [CrossRef]
- Balboni, A.; Savini, F.; Scagliarini, A.; Berti, E.; Naldi, M.; Urbani, L.; Fontana, M.C.; Carra, E.; Gibelli, L.R.M.; Gobbo, F.; et al. Natural distemper infection in stone martens (Martes foina): From infection to neutralizing antibodies. Res. Vet. Sci. 2021, 138, 196–200. [Google Scholar] [CrossRef]
- Di Sabatino, D.; Di Francesco, G.; Zaccaria, G.; Malatesta, D.; Brugnola, L.; Marcacci, M.; Portanti, O.; De Massis, F.; Savini, G.; Teodori, L.; et al. Lethal distemper in badgers (Meles meles) following epidemic in dogs and wolves. Infect. Genet. Evol. 2016, 46, 130–137. [Google Scholar] [CrossRef]
- Garigliany, M.; Sarlet, M.; Franssen, M.; Desmecht, D.; Volpe, R.; Lesenfants, C.; Paternostre, J.; Linden, A. Re-emergence of canine distemper in wildlife in Belgium. Vet. Rec. 2018, 182, 439. [Google Scholar] [CrossRef]
- Hammer, A.S.; Dietz, H.H.; Andersen, T.H.; Nielsen, L.; Blixenkrone-Møller, M. Distemper virus as a cause of central nervous disease and death in badgers (Meles meles) in Denmark. Vet. Rec. 2004, 154, 527–530. [Google Scholar] [CrossRef]
- Origgi, F.C.; Plattet, P.; Sattler, U.; Robert, N.; Casaubon, J.; Mavrot, F.; Pewsner, M.; Wu, N.; Giovannini, S.; Oevermann, A.; et al. Emergence of canine distemper virus strains with modified molecular signature and enhanced neuronal tropism leading to high mortality in wild carnivores. Vet. Path. 2012, 49, 913–929. [Google Scholar] [CrossRef]
- Pavlacik, L.; Celer, V.; Koubek, P.; Literak, I. Prevalence of canine distemper virus in wild mustelids in the Czech Republic and a case of canine distemper in young stone martens. Vet. Med. 2007, 52, 69–73. [Google Scholar] [CrossRef]
- López-Peña, M.; Vázquez, S.; Alemán, N.; López-Beceiro, A.; Muñoz, F.; Pereira, J.L.; Nieto, J.M. Canine distemper in a genet (Gennetta gennetta), associated with endogenous lipid pneumonia. J. Comp. Pathol. 2001, 124, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Meli, M.L.; Simmler, P.; Cattori, V.; Martínez, F.; Vargas, A.; Palomares, F.; López-Bao, J.V.; Simón, M.A.; López, G.; León-Vizcaino, L.; et al. Importance of canine distemper virus (CDV) infection in free-ranging Iberian lynxes (Lynx pardinus). Vet. Microbiol. 2010, 146, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Oleaga, A.; Vicente, J.; Ferroglio, E.; De Macedo, M.R.P.; Casais, R.; Del Cerro, A.; Espí, A.; García, E.J.; Gortázar, C. Concomitance and interactions of pathogens in the Iberian wolf (Canis lupus). Res. Vet. Sci. 2015, 101, 22–27. [Google Scholar] [CrossRef]
- Rosa, G.M.; Santos, N.; Grøndahl-Rosado, R.; Fonseca, F.P.; Tavares, L.; Neto, I.; Cartaxeiro, C.; Duarte, A. Unveiling patterns of viral pathogen infection in free-ranging carnivores of northern Portugal using a complementary methodological approach. Comp. Immunol. Microbiol. Infect. Dis. 2020, 69, 101432. [Google Scholar]
- Sobrino, R.; Arnal, M.C.; Luco, D.F.; Gortázar, C. Prevalence of antibodies against canine distemper virus and canine parvovirus among foxes and wolves from Spain. Vet. Microbiol. 2008, 126, 251–256. [Google Scholar] [CrossRef]
- Dean, D.J.; Abelseth, M.K.; Atanasiu, P. The fluorescent antibody test. In Laboratory Techniques in Rabies; Meslin, F.X., Kaplan, M.M., Koprowski, H., Eds.; World Health Organisation: Genova, Italy, 1996; pp. 88–95. [Google Scholar]
- Toussaint, J.F.; Sailleau, C.; Breard, E.; Zientara, S.; De Clercq, K. Bluetongue virus detection by two real-time RT-qPCRs targeting two different genomic segments. J. Virol. Methods 2007, 140, 115–123. [Google Scholar] [CrossRef]
- Rima, B.K.; Wishaupt, R.G.A.; Welsh, M.J.; Earle, J.A.P. The evolution of morbilliviruses: A comparison of nucleocapsid gene sequences including a porpoise morbillivirus. Vet. Microbiol. 1995, 44, 127–134. [Google Scholar] [CrossRef]
- Elia, G.; Decaro, N.; Martella, V.; Cirone, F.; Lucente, M.S.; Lorusso, E.; Di Trani, L.; Buonavoglia, C. Detection of canine distemper virus in dogs by real-time RT-PCR. J. Virol. Methods 2006, 136, 171–176. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Blancou, J. Dog distemper: Imported into Europe from South America? Hist. Med. Vet. 2004, 29, 35–41. [Google Scholar]
- Beineke, A.; Baumgärtner, W.; Wohlsein, P. Cross-species transmission of canine distemper virus-an update. One Health 2015, 1, 49–59. [Google Scholar] [CrossRef]
- Bourg, M.; Nobach, D.; Herzog, S.; Lange-Herbst, H.; Nesseler, A.; Hamann, H.P.; Becker, S.; Höper, D.; Hoffmann, B.; Eickmann, M.; et al. Screening red foxes (Vulpes vulpes) for possible viral causes of encephalitis. Virol. J. 2016, 13, 151. [Google Scholar] [CrossRef]
- Loots, A.K.; Mitchell, E.; Dalton, D.L.; Kotzé, A.; Venter, E.H. Advances in canine distemper virus (CDV) pathogenesis research: A wildlife perspective. J. Gen. Virol. 2016, 98, 311–321. [Google Scholar] [CrossRef]
- Nambulli, S.; Sharp, C.R.; Acciardo, A.S.; Drexler, J.F.; Duprex, W.P. Mapping the evolutionary trajectories of morbilliviruses: What, where and whither. Curr. Opin. Virol. 2016, 16, 95–105. [Google Scholar] [CrossRef]
- Sakai, K.; Nagata, N.; Ami, Y.; Seki, F.; Suzaki, Y.; Iwata-Yoshikawa, N.; Suzuki, T.; Fukushi, S.; Mizutani, T.; Yoshikawa, T.; et al. Lethal canine distemper virus outbreak in cynomolgus monkeys in Japan in 2008. J. Virol. 2013, 87, 1105–1114. [Google Scholar] [CrossRef]
- Sun, Z.; Li, A.; Ye, H.; Shi, Y.; Hu, Z.; Zeng, L. Natural infection with canine distemper virus in hand-feeding Rhesus monkeys in China. Vet. Microbiol. 2010, 141, 374–378. [Google Scholar] [CrossRef]
- Kelly, T.R.; Pandit, P.S.; Carion, N.; Dombrowski, D.F.; Rogers, K.H.; McMillin, S.C.; Clifford, D.L.; Riberi, A.; Ziccardi, M.H.; Donnelly-Greenan, E.L.; et al. Early detection of wildlife morbidity and mortality through an event-based surveillance system. Proc. R. Soc. B 2021, 288, 20210974. [Google Scholar] [CrossRef]
- Damien, B.C.; Martina, B.E.; Losch, S.; Mossong, J.; Osterhaus, A.D.; Muller, C.P. Prevalence of antibodies against canine distemper virus among red foxes in Luxembourg. J. Wildl. Dis. 2002, 38, 856–859. [Google Scholar] [CrossRef] [PubMed]
- Demeter, Z.; Lakatos, B.; Palade, E.A.; Kozma, T.; Forgach, P.; Rusvai, M. Genetic diversity of Hungarian canine distemper virus strains. Vet. Microbiol. 2007, 122, 258–269. [Google Scholar] [CrossRef]
- Benetka, V.; Leschnik, M.; Affenzeller, N.; Mostl, K. Phylogenetic analysis of Austrian canine distemper virus strains from clinical samples from dogs and wild carnivores. Vet. Rec. 2011, 168, 377. [Google Scholar] [CrossRef] [PubMed]
- Monne, I.; Fusaro, A.; Valastro, V.; Citterio, C.; Pozza, M.D.; Obber, F.; Trevisiol, K.; Cova, M.; De Benedictis, P.; Bregoli, M.; et al. A distinct CDV genotype causing a major epidemic in Alpine wildlife. Vet. Microbiol. 2011, 150, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Bellan, S.E.; Cizauskas, C.A.; Miyen, J.; Ebersohn, K.; Küsters, M.; Prager, K.C.; Van Vuuren, M.; Sabeta, C.; Getz, W.M. Black-backed jackal exposure to rabies virus, canine distemper virus, and Bacillus anthracis in Etosha National Park, Namibia. J. Wildl. Dis. 2012, 48, 371–381. [Google Scholar] [CrossRef]
- Gowtage-Sequeira, S.; Banyard, A.C.; Barrett, T.; Buczkowski, H.; Funk, S.M.; Cleaveland, S. Epidemiology, pathology, and genetic analysis of a canine distemper epidemic in Namibia. J. Wildl. Dis. 2009, 45, 1008–1020. [Google Scholar] [CrossRef]
- Deem, S.L.; Spelman, L.H.; Yates, R.A.; Montali, R. Canine distemper in terrestrial carnivores: A review. J. Zoo Wildl. Med. 2000, 31, 441–451. [Google Scholar]
- Craft, M.E.; Volz, E.; Packer, C.; Meyers, L.A. Distinguishing epidemic waves from disease spillover in a wildlife population. Proc. R. Soc. B 2009, 276, 1777–1785. [Google Scholar] [CrossRef]
- Bedeković, T.; Lohman Janković, I.; Šimić, I.; Krešić, N.; Lojkić, I.; Sučec, I.; Robardet, E.; Cliquet, F. Control and elimination of rabies in Croatia. PLoS ONE 2018, 13, e0204115. [Google Scholar] [CrossRef]
- Dežđek, D.; Lipej, Z.; Vojta, A.; Mihaljević, Ž.; Keros, T. Investigation of prevalence distemper virus in population red fox (Vulpes vulpes) in northwestern region of Croatia. In Proceedings of the 45th Croatian and 5th International Symposium on Agriculture, Opatija, Croatia, 15–19 February 2010. [Google Scholar]
- Macdonald, D.W.; Buesching, C.D.; Stopka, P.; Henderson, J.; Ellwood, S.A.; Baker, S.E. Encounters between two sympatric carnivores: Red foxes (Vulpes vulpes) and European badgers (Meles meles). J. Zool. 2004, 263, 385–392. [Google Scholar] [CrossRef]
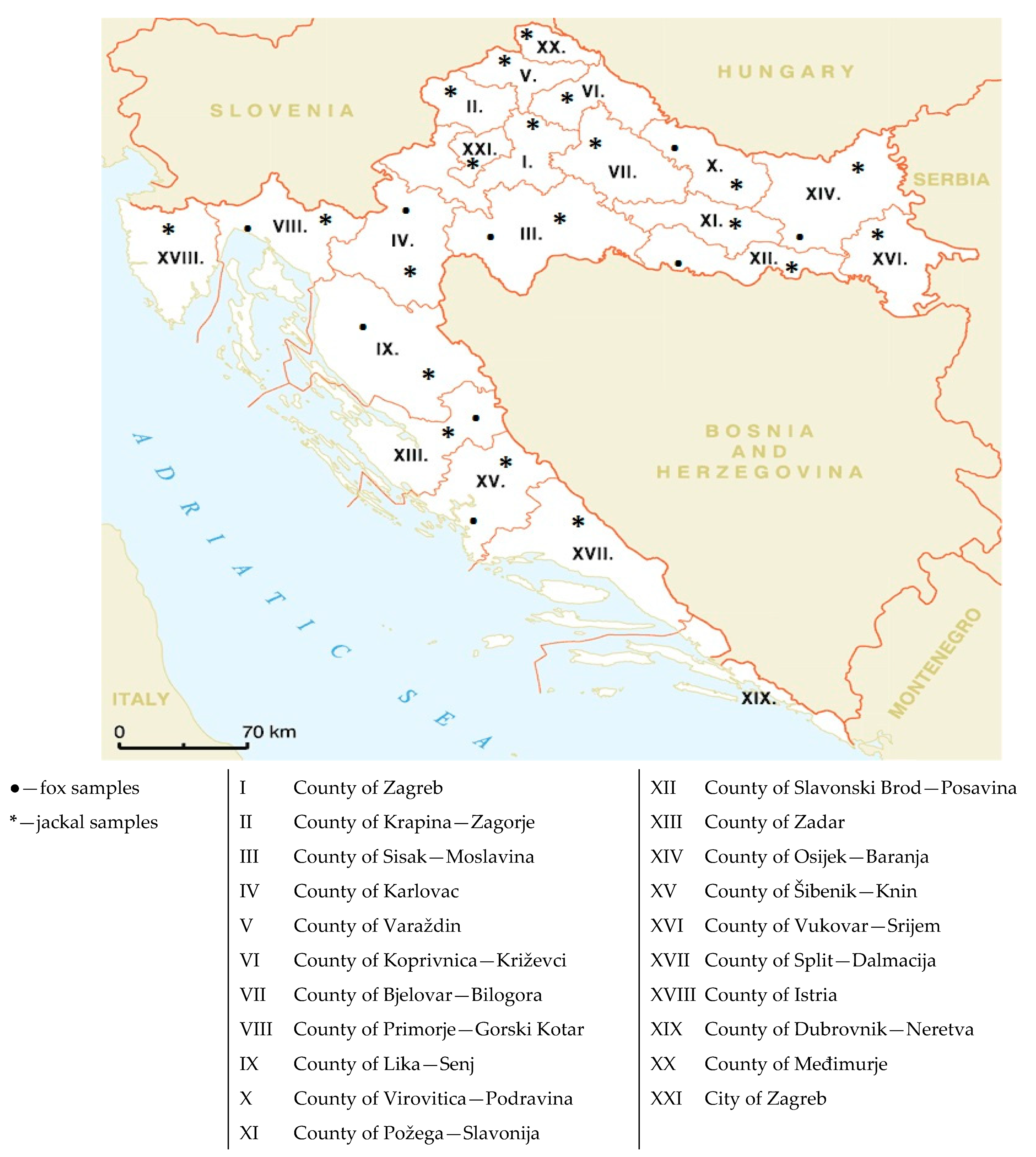

| County | Number of Red Fox Samples | Number of Jackal Samples |
|---|---|---|
| County of Zagreb | 9 | 0 |
| County of Krapina-Zagorje | 7 | 0 |
| County of Sisak-Moslavina | 13 | 6 |
| County of Karlovac | 24 | 1 |
| County of Varaždin | 3 | 0 |
| County of Koprivnica-Križevci | 5 | 0 |
| County of Bjelovar-Bilogora | 14 | 0 |
| County of Primorje-Gorski Kotar | 9 | 1 |
| County of Lika-Senj | 30 | 4 |
| County of Virovitica-Podravina | 8 | 3 |
| County of Požega-Slavonija | 4 | 0 |
| County of Slavonski Brod-Posavina | 15 | 4 |
| County of Zadar | 2 | 1 |
| County of Osijek-Baranja | 7 | 3 |
| County of Šibenik-Knin | 8 | 1 |
| County of Vukovar-Srijem | 6 | 0 |
| County of Split-Dalmacija | 1 | 0 |
| County of Istria | 7 | 0 |
| County of Dubrovnik-Neretva | 0 | 0 |
| County of Međimurje | 2 | 0 |
| City of Zagreb | 2 | 0 |
| Σ | 176 | 24 |
| Σ | Real-Time RT-PCR | RT-PCR | |
|---|---|---|---|
| Red fox samples | 176 | 4 (2.27%) | 2 (1.14%) |
| Jackal samples | 24 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prpić, J.; Lojkić, I.; Keros, T.; Krešić, N.; Jemeršić, L. Canine Distemper Virus Infection in the Free-Living Wild Canines, the Red Fox (Vulpes vulpes) and Jackal (Canis aureus moreoticus), in Croatia. Pathogens 2023, 12, 833. https://doi.org/10.3390/pathogens12060833
Prpić J, Lojkić I, Keros T, Krešić N, Jemeršić L. Canine Distemper Virus Infection in the Free-Living Wild Canines, the Red Fox (Vulpes vulpes) and Jackal (Canis aureus moreoticus), in Croatia. Pathogens. 2023; 12(6):833. https://doi.org/10.3390/pathogens12060833
Chicago/Turabian StylePrpić, Jelena, Ivana Lojkić, Tomislav Keros, Nina Krešić, and Lorena Jemeršić. 2023. "Canine Distemper Virus Infection in the Free-Living Wild Canines, the Red Fox (Vulpes vulpes) and Jackal (Canis aureus moreoticus), in Croatia" Pathogens 12, no. 6: 833. https://doi.org/10.3390/pathogens12060833
APA StylePrpić, J., Lojkić, I., Keros, T., Krešić, N., & Jemeršić, L. (2023). Canine Distemper Virus Infection in the Free-Living Wild Canines, the Red Fox (Vulpes vulpes) and Jackal (Canis aureus moreoticus), in Croatia. Pathogens, 12(6), 833. https://doi.org/10.3390/pathogens12060833








