CD36—A Host Receptor Necessary for Malaria Parasites to Establish and Maintain Infection
Abstract
1. Introduction
2. The PfEMP1 Family
3. Knobs—Anchor Point for PfEMP1s
4. Endothelial Cell Receptors (ECRs)
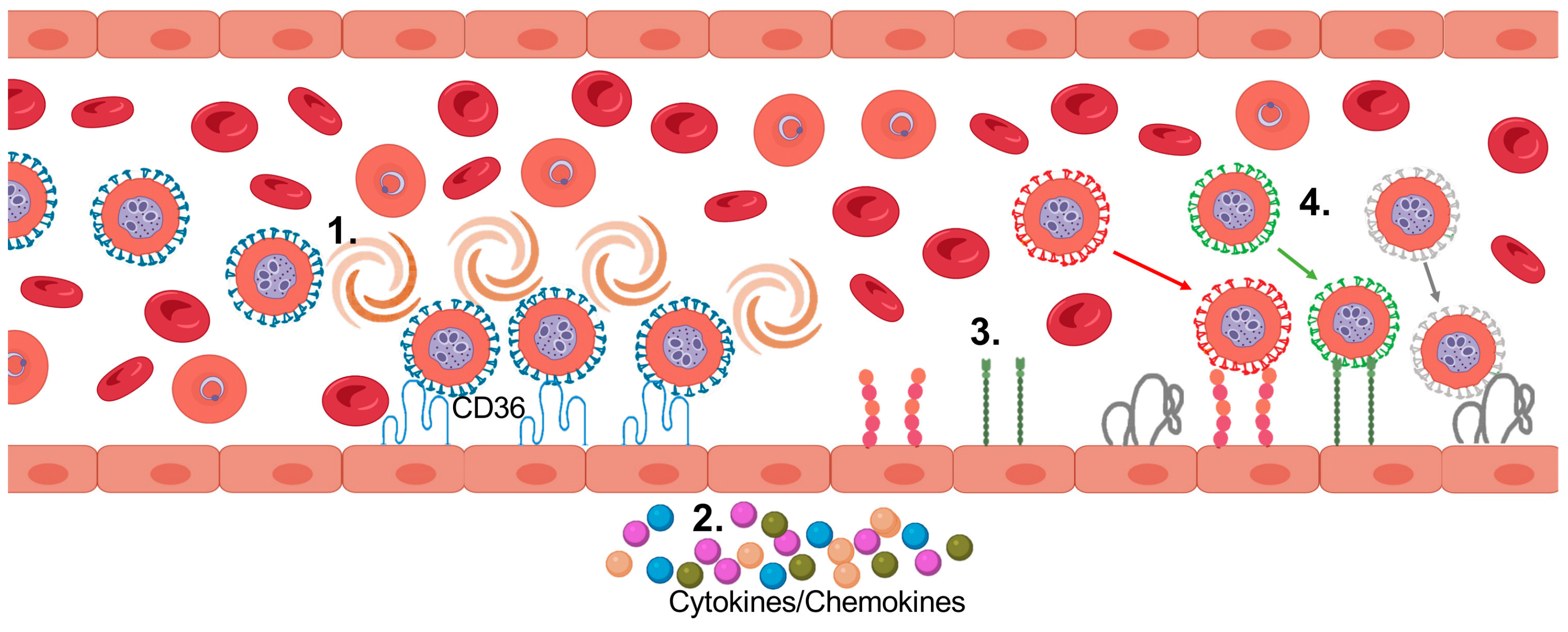
5. Cytoadhesion of PfIEs
6. Pathology Induced by Cytoadhesion
7. ECR-Specific Expression in Relation to the Origin of the Endothelial Cells
8. Hierarchy of var Expression during the Human Blood Phase
9. P. falciparum and CD36
10. CD36
11. CD36 Binding PfEMP1 Variants—Benefits for Parasite and Host
- The parasite targets a region of CD36 that is essential for its physiological role in fatty acid uptake because mutation of F153 disrupts the interaction of CD36 with CIDRα2–6 but also abolishes the binding of CD36 to oxidized LDL particles. This reduces the likelihood that the human host can escape from PfEMP1 binding by altering its CD36 [28].
- In contrast to the EPCR binding surface of CIDRα1 domains, which protrudes and is a structure that is likely to be well recognized by antibodies, the CD36 binding site is concave, and the conserved hydrophobic residues are hidden in a pocket, so maybe they are less easily recognized. In addition, the binding site is surrounded by a sequence-diverse protein surface containing a flexible loop that may make antibody recognition less likely. This unique interaction site of the parasite with CD36, which protects essential residues from exposure to the immune system, appears to allow the parasite to utilize an antigenically diverse set of CIDRα2–6 for cytoadhesion to CD36 to be protected from splenic clearance [28].
- CD36 is found in cells of the innate and adaptive immune system [104,105,106,107,108]. It has been shown that PfIEs can adhere to dendritic cells (DCs). This attachment inhibits maturation of these cells and their ability to stimulate T cells. Thus, the parasite can trigger dysregulation of the immune system. This favors the development of the parasite by impairing the host immune system’s ability to clear the infection [108,111,112,113,114]. However, there is also an observation that the mechanism of DC inhibition by PfIEs may be independent of PfEMP1 and CD36 [115].
- The previously determined hierarchy of var expression upon parasite entry into human blood begins with group B and suggests that most parasites bind to CD36, as they all encode a CD36-binding phenotype. Most infected individuals, including those who are not immune, do not develop severe malaria, and cytoadhesion of PfIEs occurs in extensive microvascular beds in tissues other than the brain (skin, muscle, adipose tissue). Therefore, cytoadhesion in such non-vital tissues could promote survival and transmission of the parasite while minimizing host damage and death [87,88,89,90].
- Antibody-induced selective binding and internalization of CD36 do not result in proinflammatory cytokine production by human macrophages. Interestingly, CD36-mediated phagocytosis of PfIEs also did not result in cytokine secretion by primary macrophages [116]. However, CD36-mediated binding of PfIEs increases the likelihood of phagocytosis by macrophages. This can lead to a reduction in parasitemia, but also allows the parasite to maintain a viable infection without causing too much damage to the host through high parasitemia [108,114,117,118].
- DCs react to P. falciparum very early during infection and can, thus, influence the development of immunity. Internalization of PfIEs by DCs and subsequent pro-inflammatory cytokine production of DCs, NK, and T cells depends on CD36. Notably, plasmacytoid DCs regulate innate and adaptive immunity to malaria via the production of proinflammatory cytokines. As this effect is particularly evident at low levels of parasitemia, the role of CD36 for malaria immunity appears to take place early during infection and to promote the development of protective immunity against malaria [118,119].
12. Binding Phenotypes of PfIEs
13. Importance of Knobs for Cytoadhesion
14. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- WHO. World Malaria Report 2021; WHO: Geneva, Switzerland, 2021. [Google Scholar]
- Saul, A. The role of variant surface antigens on malaria-infected red blood cells. Parasitol. Today 1999, 15, 455–457. [Google Scholar] [CrossRef] [PubMed]
- Phillips, M.A.; Burrows, J.N.; Manyando, C.; van Huijsduijnen, R.H.; Van Voorhis, W.C.; Wells, T.N.C. Malaria. Nat. Rev. Dis. Prim. 2017, 3, 17050. [Google Scholar] [CrossRef] [PubMed]
- Craig, A.G.; Khairul, M.F.; Patil, P.R. Cytoadherence and severe malaria. Malays. J. Med. Sci. 2012, 19, 5–18. [Google Scholar] [PubMed]
- Newbold, C.; Craig, A.; Kyes, S.; Rowe, A.; Fernandez-Reyes, D.; Fagan, T. Cytoadherence, pathogenesis and the infected red cell surface in Plasmodium falciparum. Int. J. Parasitol. 1999, 29, 927–937. [Google Scholar] [CrossRef] [PubMed]
- Rowe, J.A.; Claessens, A.; Corrigan, R.A.; Arman, M. Adhesion of Plasmodium falciparum-infected erythrocytes to human cells: Molecular mechanisms and therapeutic implications. Expert Rev. Mol. Med. 2009, 11, e16. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.A.; Howell, K.B.; Reiling, L.; Ataide, R.; Mackintosh, C.L.; Fowkes, F.J.; Petter, M.; Chesson, J.M.; Langer, C.; Warimwe, G.M.; et al. Targets of antibodies against Plasmodium falciparum-infected erythrocytes in malaria immunity. J. Clin. Investig. 2012, 122, 3227–3238. [Google Scholar] [CrossRef]
- Miller, L.H.; Good, M.F.; Milon, G. Malaria pathogenesis. Science 1994, 264, 1878–1883. [Google Scholar] [CrossRef]
- Nyarko, P.B.; Claessens, A. Understanding Host-Pathogen-Vector Interactions with Chronic Asymptomatic Malaria Infections. Trends Parasitol. 2021, 37, 195–204. [Google Scholar] [CrossRef]
- Deitsch, K.W.; Dzikowski, R. Variant Gene Expression and Antigenic Variation by Malaria Parasites. Annu. Rev. Microbiol. 2017, 71, 625–641. [Google Scholar] [CrossRef] [PubMed]
- Petter, M.; Duffy, M.F. Antigenic Variation in Plasmodium falciparum. Results Probl. Cell Differ. 2015, 57, 47–90. [Google Scholar] [CrossRef] [PubMed]
- Otto, T.D.; Assefa, S.A.; Bohme, U.; Sanders, M.J.; Kwiatkowski, D.; Pf3k, c.; Berriman, M.; Newbold, C. Evolutionary analysis of the most polymorphic gene family in falciparum malaria. Wellcome Open Res. 2019, 4, 193. [Google Scholar] [CrossRef] [PubMed]
- Voss, T.S.; Healer, J.; Marty, A.J.; Duffy, M.F.; Thompson, J.K.; Beeson, J.G.; Reeder, J.C.; Crabb, B.S.; Cowman, A.F. A var gene promoter controls allelic exclusion of virulence genes in Plasmodium falciparum malaria. Nature 2006, 439, 1004–1008. [Google Scholar] [CrossRef] [PubMed]
- Kyes, S.A.; Kraemer, S.M.; Smith, J.D. Antigenic variation in Plasmodium falciparum: Gene organization and regulation of the var multigene family. Eukaryot. Cell 2007, 6, 1511–1520. [Google Scholar] [CrossRef] [PubMed]
- Pasternak, N.D.; Dzikowski, R. PfEMP1: An antigen that plays a key role in the pathogenicity and immune evasion of the malaria parasite Plasmodium falciparum. Int. J. Biochem. Cell Biol. 2009, 41, 1463–1466. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, S.M.; Smith, J.D. A family affair: Var genes, PfEMP1 binding, and malaria disease. Curr. Opin. Microbiol. 2006, 9, 374–380. [Google Scholar] [CrossRef]
- Rask, T.S.; Hansen, D.A.; Theander, T.G.; Gorm Pedersen, A.; Lavstsen, T. Plasmodium falciparum erythrocyte membrane protein 1 diversity in seven genomes—Divide and conquer. PLoS Comput. Biol. 2010, 6, e1000933. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.D.; Craig, A.G.; Kriek, N.; Hudson-Taylor, D.; Kyes, S.; Fagan, T.; Pinches, R.; Baruch, D.I.; Newbold, C.I.; Miller, L.H. Identification of a Plasmodium falciparum intercellular adhesion molecule-1 binding domain: A parasite adhesion trait implicated in cerebral malaria. Proc. Natl. Acad. Sci. USA 2000, 97, 1766–1771. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.D.; Subramanian, G.; Gamain, B.; Baruch, D.I.; Miller, L.H. Classification of adhesive domains in the Plasmodium falciparum erythrocyte membrane protein 1 family. Mol. Biochem. Parasitol. 2000, 110, 293–310. [Google Scholar] [CrossRef] [PubMed]
- Gardner, M.J.; Hall, N.; Fung, E.; White, O.; Berriman, M.; Hyman, R.W.; Carlton, J.M.; Pain, A.; Nelson, K.E.; Bowman, S.; et al. Genome sequence of the human malaria parasite Plasmodium falciparum. Nature 2002, 419, 498–511. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, S.M.; Smith, J.D. Evidence for the importance of genetic structuring to the structural and functional specialization of the Plasmodium falciparum var gene family. Mol. Microbiol. 2003, 50, 1527–1538. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, S.M.; Kyes, S.A.; Aggarwal, G.; Springer, A.L.; Nelson, S.O.; Christodoulou, Z.; Smith, L.M.; Wang, W.; Levin, E.; Newbold, C.I.; et al. Patterns of gene recombination shape var gene repertoires in Plasmodium falciparum: Comparisons of geographically diverse isolates. BMC Genom. 2007, 8, 45. [Google Scholar] [CrossRef]
- Lavstsen, T.; Salanti, A.; Jensen, A.T.; Arnot, D.E.; Theander, T.G. Sub-grouping of Plasmodium falciparum 3D7 var genes based on sequence analysis of coding and non-coding regions. Malar. J. 2003, 2, 27. [Google Scholar] [CrossRef]
- Kyes, S.A.; Christodoulou, Z.; Raza, A.; Horrocks, P.; Pinches, R.; Rowe, J.A.; Newbold, C.I. A well-conserved Plasmodium falciparum var gene shows an unusual stage-specific transcript pattern. Mol. Microbiol. 2003, 48, 1339–1348. [Google Scholar] [CrossRef]
- Rowe, J.A.; Kyes, S.A. The role of Plasmodium falciparum var genes in malaria in pregnancy. Mol. Microbiol. 2004, 53, 1011–1019. [Google Scholar] [CrossRef]
- Wang, C.W.; Lavstsen, T.; Bengtsson, D.C.; Magistrado, P.A.; Berger, S.S.; Marquard, A.M.; Alifrangis, M.; Lusingu, J.P.; Theander, T.G.; Turner, L. Evidence for in vitro and in vivo expression of the conserved VAR3 (type 3) Plasmodium falciparum erythrocyte membrane protein 1. Malar. J. 2012, 11, 129. [Google Scholar] [CrossRef]
- Robinson, B.A.; Welch, T.L.; Smith, J.D. Widespread functional specialization of Plasmodium falciparum erythrocyte membrane protein 1 family members to bind CD36 analysed across a parasite genome. Mol. Microbiol. 2003, 47, 1265–1278. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, F.L.; Turner, L.; Bolla, J.R.; Robinson, C.V.; Lavstsen, T.; Higgins, M.K. The structural basis for CD36 binding by the malaria parasite. Nat. Commun. 2016, 7, 12837. [Google Scholar] [CrossRef] [PubMed]
- Mo, M.; Lee, H.C.; Kotaka, M.; Niang, M.; Gao, X.; Iyer, J.K.; Lescar, J.; Preiser, P. The C-terminal segment of the cysteine-rich interdomain of Plasmodium falciparum erythrocyte membrane protein 1 determines CD36 binding and elicits antibodies that inhibit adhesion of parasite-infected erythrocytes. Infect. Immun. 2008, 76, 1837–1847. [Google Scholar] [CrossRef]
- Miller, L.H.; Hudson-Taylor, D.; Gamain, B.; Saul, A.J. Definition of the minimal domain of CIDR1alpha of Plasmodium falciparum PfEMP1 for binding CD36. Mol. Biochem. Parasitol. 2002, 120, 321–323. [Google Scholar] [CrossRef]
- Smith, J.D.; Rowe, J.A.; Higgins, M.K.; Lavstsen, T. Malaria’s deadly grip: Cytoadhesion of Plasmodium falciparum-infected erythrocytes. Cell Microbiol. 2013, 15, 1976–1983. [Google Scholar] [CrossRef] [PubMed]
- Lau, C.K.; Turner, L.; Jespersen, J.S.; Lowe, E.D.; Petersen, B.; Wang, C.W.; Petersen, J.E.; Lusingu, J.; Theander, T.G.; Lavstsen, T.; et al. Structural conservation despite huge sequence diversity allows EPCR binding by the PfEMP1 family implicated in severe childhood malaria. Cell Host Microbe 2015, 17, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Turner, L.; Lavstsen, T.; Berger, S.S.; Wang, C.W.; Petersen, J.E.; Avril, M.; Brazier, A.J.; Freeth, J.; Jespersen, J.S.; Nielsen, M.A.; et al. Severe malaria is associated with parasite binding to endothelial protein C receptor. Nature 2013, 498, 502–505. [Google Scholar] [CrossRef] [PubMed]
- Baruch, D.I.; Ma, X.C.; Singh, H.B.; Bi, X.; Pasloske, B.L.; Howard, R.J. Identification of a region of PfEMP1 that mediates adherence of Plasmodium falciparum infected erythrocytes to CD36: Conserved function with variant sequence. Blood 1997, 90, 3766–3775. [Google Scholar] [CrossRef]
- Mkumbaye, S.I.; Wang, C.W.; Lyimo, E.; Jespersen, J.S.; Manjurano, A.; Mosha, J.; Kavishe, R.A.; Mwakalinga, S.B.; Minja, D.T.R.; Lusingu, J.P.; et al. The Severity of Plasmodium falciparum Infection Is Associated with Transcript Levels of var Genes Encoding Endothelial Protein C Receptor-Binding P. falciparum Erythrocyte Membrane Protein 1. Infect. Immun. 2017, 85. [Google Scholar] [CrossRef]
- Lennartz, F.; Adams, Y.; Bengtsson, A.; Olsen, R.W.; Turner, L.; Ndam, N.T.; Ecklu-Mensah, G.; Moussiliou, A.; Ofori, M.F.; Gamain, B.; et al. Structure-Guided Identification of a Family of Dual Receptor-Binding PfEMP1 that Is Associated with Cerebral Malaria. Cell Host Microbe 2017, 21, 403–414. [Google Scholar] [CrossRef]
- Janes, J.H.; Wang, C.P.; Levin-Edens, E.; Vigan-Womas, I.; Guillotte, M.; Melcher, M.; Mercereau-Puijalon, O.; Smith, J.D. Investigating the host binding signature on the Plasmodium falciparum PfEMP1 protein family. PLoS Pathog. 2011, 7, e1002032. [Google Scholar] [CrossRef] [PubMed]
- Magallon-Tejada, A.; Machevo, S.; Cistero, P.; Lavstsen, T.; Aide, P.; Rubio, M.; Jimenez, A.; Turner, L.; Valmaseda, A.; Gupta, H.; et al. Cytoadhesion to gC1qR through Plasmodium falciparum Erythrocyte Membrane Protein 1 in Severe Malaria. PLoS Pathog. 2016, 12, e1006011. [Google Scholar] [CrossRef] [PubMed]
- Quadt, K.A.; Barfod, L.; Andersen, D.; Bruun, J.; Gyan, B.; Hassenkam, T.; Ofori, M.F.; Hviid, L. The density of knobs on Plasmodium falciparum-infected erythrocytes depends on developmental age and varies among isolates. PLoS ONE 2012, 7, e45658. [Google Scholar] [CrossRef]
- Tilly, A.K.; Thiede, J.; Metwally, N.; Lubiana, P.; Bachmann, A.; Roeder, T.; Rockliffe, N.; Lorenzen, S.; Tannich, E.; Gutsmann, T.; et al. Type of in vitro cultivation influences cytoadhesion, knob structure, protein localization and transcriptome profile of Plasmodium falciparum. Sci. Rep. 2015, 5, 16766. [Google Scholar] [CrossRef]
- Alampalli, S.V.; Grover, M.; Chandran, S.; Tatu, U.; Acharya, P. Proteome and Structural Organization of the Knob Complex on the Surface of the Plasmodium Infected Red Blood Cell. Proteom. Clin. Appl. 2018, 12, e1600177. [Google Scholar] [CrossRef]
- Maier, A.G.; Cooke, B.M.; Cowman, A.F.; Tilley, L. Malaria parasite proteins that remodel the host erythrocyte. Nat. Rev. Microbiol. 2009, 7, 341–354. [Google Scholar] [CrossRef]
- Watermeyer, J.M.; Hale, V.L.; Hackett, F.; Clare, D.K.; Cutts, E.E.; Vakonakis, I.; Fleck, R.A.; Blackman, M.J.; Saibil, H.R. A spiral scaffold underlies cytoadherent knobs in Plasmodium falciparum-infected erythrocytes. Blood 2016, 127, 343–351. [Google Scholar] [CrossRef]
- Gruenberg, J.; Allred, D.R.; Sherman, I.W. Scanning electron microscope-analysis of the protrusions (knobs) present on the surface of Plasmodium falciparum-infected erythrocytes. J. Cell Biol. 1983, 97, 795–802. [Google Scholar] [CrossRef]
- Cutts, E.E.; Laasch, N.; Reiter, D.M.; Trenker, R.; Slater, L.M.; Stansfeld, P.J.; Vakonakis, I. Structural analysis of P. falciparum KAHRP and PfEMP1 complexes with host erythrocyte spectrin suggests a model for cytoadherent knob protrusions. PLoS Pathog. 2017, 13, e1006552. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, C.P.; Karathanasis, C.; Sanchez, R.; Cyrklaff, M.; Jager, J.; Buchholz, B.; Schwarz, U.S.; Heilemann, M.; Lanzer, M. Single-molecule imaging and quantification of the immune-variant adhesin VAR2CSA on knobs of Plasmodium falciparum-infected erythrocytes. Commun. Biol. 2019, 2, 172. [Google Scholar] [CrossRef] [PubMed]
- Crabb, B.S.; Cooke, B.M.; Reeder, J.C.; Waller, R.F.; Caruana, S.R.; Davern, K.M.; Wickham, M.E.; Brown, G.V.; Coppel, R.L.; Cowman, A.F. Targeted gene disruption shows that knobs enable malaria-infected red cells to cytoadhere under physiological shear stress. Cell 1997, 89, 287–296. [Google Scholar] [CrossRef] [PubMed]
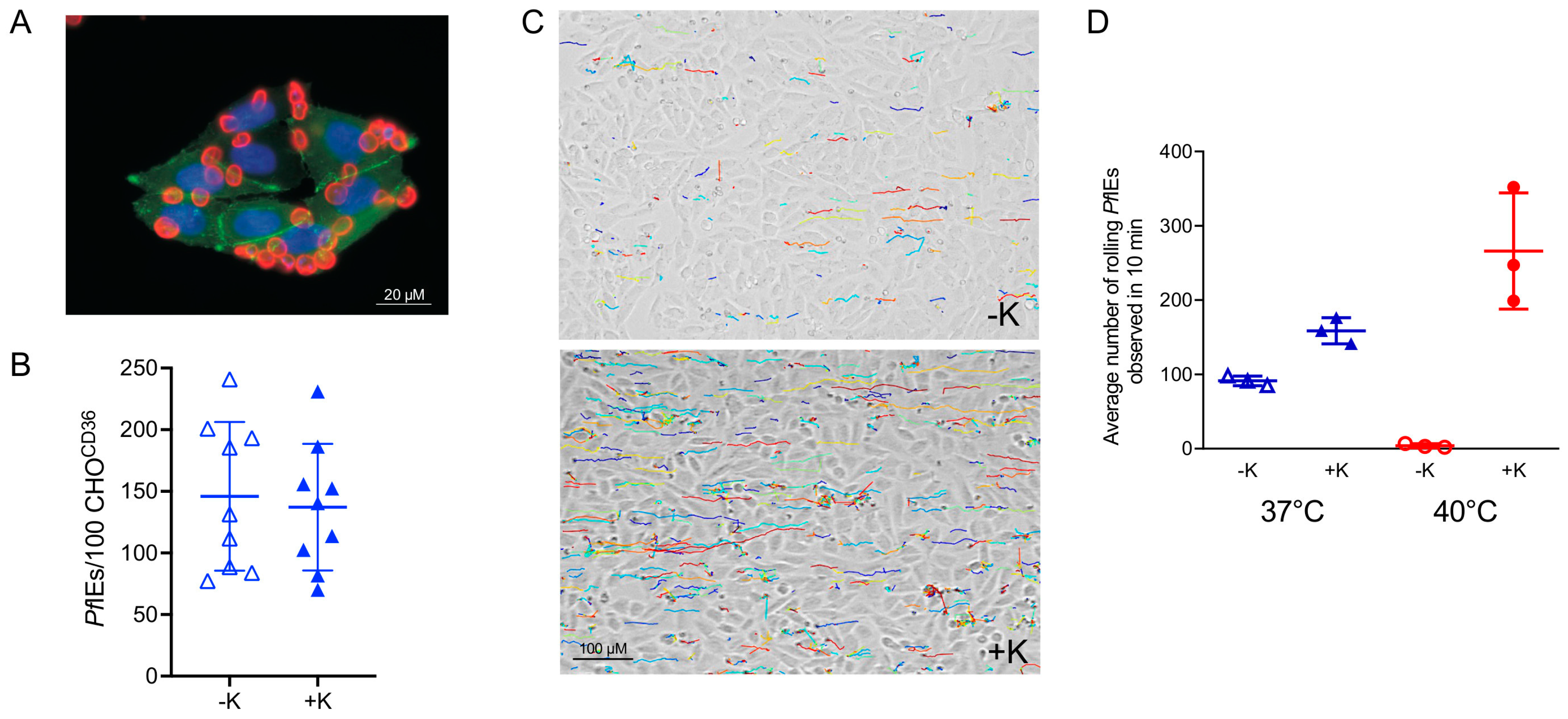
- Dorpinghaus, M.; Furstenwerth, F.; Roth, L.K.; Bouws, P.; Rakotonirinalalao, M.; Jordan, V.; Sauer, M.; Rehn, T.; Pansegrau, E.; Hohn, K.; et al. Stringent Selection of Knobby Plasmodium falciparum-Infected Erythrocytes during Cytoadhesion at Febrile Temperature. Microorganisms 2020, 8, 174. [Google Scholar] [CrossRef] [PubMed]
- Boeuf, P.; Hasang, W.; Hanssen, E.; Glazier, J.D.; Rogerson, S.J. Relevant assay to study the adhesion of Plasmodium falciparum-infected erythrocytes to the placental epithelium. PLoS ONE 2011, 6, e21126. [Google Scholar] [CrossRef] [PubMed]
- Esser, C.; Bachmann, A.; Kuhn, D.; Schuldt, K.; Forster, B.; Thiel, M.; May, J.; Koch-Nolte, F.; Yanez-Mo, M.; Sanchez-Madrid, F.; et al. Evidence of promiscuous endothelial binding by Plasmodium falciparum-infected erythrocytes. Cell Microbiol. 2014, 16, 701–708. [Google Scholar] [CrossRef]
- Chesnokov, O.; Merritt, J.; Tcherniuk, S.O.; Milman, N.; Oleinikov, A.V. Plasmodium falciparum infected erythrocytes can bind to host receptors integrins alphaVbeta3 and alphaVbeta6 through DBLdelta1_D4 domain of PFL2665c PfEMP1 protein. Sci. Rep. 2018, 8, 17871. [Google Scholar] [CrossRef] [PubMed]
- Siano, J.P.; Grady, K.K.; Millet, P.; Wick, T.M. Short report: Plasmodium falciparum: Cytoadherence to alpha(v)beta3 on human microvascular endothelial cells. Am. J. Trop Med. Hyg. 1998, 59, 77–79. [Google Scholar] [CrossRef] [PubMed]
- Metwally, N.G.; Tilly, A.K.; Lubiana, P.; Roth, L.K.; Dorpinghaus, M.; Lorenzen, S.; Schuldt, K.; Witt, S.; Bachmann, A.; Tidow, H.; et al. Characterisation of Plasmodium falciparum populations selected on the human endothelial receptors P-selectin, E-selectin, CD9 and CD151. Sci. Rep. 2017, 7, 4069. [Google Scholar] [CrossRef] [PubMed]
- Bengtsson, A.; Joergensen, L.; Rask, T.S.; Olsen, R.W.; Andersen, M.A.; Turner, L.; Theander, T.G.; Hviid, L.; Higgins, M.K.; Craig, A.; et al. A novel domain cassette identifies Plasmodium falciparum PfEMP1 proteins binding ICAM-1 and is a target of cross-reactive, adhesion-inhibitory antibodies. J. Immunol. 2013, 190, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Milner, D.A., Jr.; Valim, C.; Carr, R.A.; Chandak, P.B.; Fosiko, N.G.; Whitten, R.; Playforth, K.B.; Seydel, K.B.; Kamiza, S.; Molyneux, M.E.; et al. A histological method for quantifying Plasmodium falciparum in the brain in fatal paediatric cerebral malaria. Malar. J. 2013, 12, 191. [Google Scholar] [CrossRef] [PubMed]
- Milner, D.A., Jr.; Lee, J.J.; Frantzreb, C.; Whitten, R.O.; Kamiza, S.; Carr, R.A.; Pradham, A.; Factor, R.E.; Playforth, K.; Liomba, G.; et al. Quantitative Assessment of Multiorgan Sequestration of Parasites in Fatal Pediatric Cerebral Malaria. J. Infect. Dis. 2015, 212, 1317–1321. [Google Scholar] [CrossRef]
- Taylor, T.E.; Fu, W.J.; Carr, R.A.; Whitten, R.O.; Mueller, J.S.; Fosiko, N.G.; Lewallen, S.; Liomba, N.G.; Molyneux, M.E. Differentiating the pathologies of cerebral malaria by postmortem parasite counts. Nat. Med. 2004, 10, 143–145. [Google Scholar] [CrossRef]
- Lyke, K.E.; Burges, R.; Cissoko, Y.; Sangare, L.; Dao, M.; Diarra, I.; Kone, A.; Harley, R.; Plowe, C.V.; Doumbo, O.K.; et al. Serum levels of the proinflammatory cytokines interleukin-1 beta (IL-1beta), IL-6, IL-8, IL-10, tumor necrosis factor alpha, and IL-12(p70) in Malian children with severe Plasmodium falciparum malaria and matched uncomplicated malaria or healthy controls. Infect. Immun. 2004, 72, 5630–5637. [Google Scholar] [CrossRef] [PubMed]
- Raacke, M.; Kerr, A.; Dorpinghaus, M.; Brehmer, J.; Wu, Y.; Lorenzen, S.; Fink, C.; Jacobs, T.; Roeder, T.; Sellau, J.; et al. Altered Cytokine Response of Human Brain Endothelial Cells after Stimulation with Malaria Patient Plasma. Cells 2021, 10, 1656. [Google Scholar] [CrossRef] [PubMed]
- Hasday, J.D.; Bannerman, D.; Sakarya, S.; Cross, A.S.; Singh, I.S.; Howard, D.; Drysdale, B.E.; Goldblum, S.E. Exposure to febrile temperature modifies endothelial cell response to tumor necrosis factor-alpha. J. Appl. Physiol. 2001, 90, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Oakley, M.S.; Gerald, N.; McCutchan, T.F.; Aravind, L.; Kumar, S. Clinical and molecular aspects of malaria fever. Trends Parasitol. 2011, 27, 442–449. [Google Scholar] [CrossRef]
- Cunnington, A.J.; Riley, E.M.; Walther, M. Microvascular dysfunction in severe Plasmodium falciparum Malaria. J. Infect. Dis. 2013, 207, 369–370. [Google Scholar] [CrossRef] [PubMed]
- Gazzinelli, R.T.; Kalantari, P.; Fitzgerald, K.A.; Golenbock, D.T. Innate sensing of malaria parasites. Nat. Rev. Immunol. 2014, 14, 744–757. [Google Scholar] [CrossRef]
- Dondorp, A.M.; Lee, S.J.; Faiz, M.A.; Mishra, S.; Price, R.; Tjitra, E.; Than, M.; Htut, Y.; Mohanty, S.; Yunus, E.B.; et al. The relationship between age and the manifestations of and mortality associated with severe malaria. Clin. Infect. Dis. 2008, 47, 151–157. [Google Scholar] [CrossRef]
- Jensen, A.T.; Magistrado, P.; Sharp, S.; Joergensen, L.; Lavstsen, T.; Chiucchiuini, A.; Salanti, A.; Vestergaard, L.S.; Lusingu, J.P.; Hermsen, R.; et al. Plasmodium falciparum associated with severe childhood malaria preferentially expresses PfEMP1 encoded by group A var genes. J. Exp. Med. 2004, 199, 1179–1190. [Google Scholar] [CrossRef] [PubMed]
- Avril, M.; Tripathi, A.K.; Brazier, A.J.; Andisi, C.; Janes, J.H.; Soma, V.L.; Sullivan, D.J., Jr.; Bull, P.C.; Stins, M.F.; Smith, J.D. A restricted subset of var genes mediates adherence of Plasmodium falciparum-infected erythrocytes to brain endothelial cells. Proc. Natl. Acad. Sci. USA 2012, 109, E1782–E1790. [Google Scholar] [CrossRef] [PubMed]
- Claessens, A.; Adams, Y.; Ghumra, A.; Lindergard, G.; Buchan, C.C.; Andisi, C.; Bull, P.C.; Mok, S.; Gupta, A.P.; Wang, C.W.; et al. A subset of group A-like var genes encodes the malaria parasite ligands for binding to human brain endothelial cells. Proc. Natl. Acad. Sci. USA 2012, 109, E1772–E1781. [Google Scholar] [CrossRef] [PubMed]
- Lavstsen, T.; Turner, L.; Saguti, F.; Magistrado, P.; Rask, T.S.; Jespersen, J.S.; Wang, C.W.; Berger, S.S.; Baraka, V.; Marquard, A.M.; et al. Plasmodium falciparum erythrocyte membrane protein 1 domain cassettes 8 and 13 are associated with severe malaria in children. Proc. Natl. Acad. Sci. USA 2012, 109, E1791–E1800. [Google Scholar] [CrossRef]
- Duffy, F.; Bernabeu, M.; Babar, P.H.; Kessler, A.; Wang, C.W.; Vaz, M.; Chery, L.; Mandala, W.L.; Rogerson, S.J.; Taylor, T.E.; et al. Meta-analysis of Plasmodium falciparum var Signatures Contributing to Severe Malaria in African Children and Indian Adults. mBio 2019, 10, e00217-19. [Google Scholar] [CrossRef]
- Jespersen, J.S.; Wang, C.W.; Mkumbaye, S.I.; Minja, D.T.; Petersen, B.; Turner, L.; Petersen, J.E.; Lusingu, J.P.; Theander, T.G.; Lavstsen, T. Plasmodium falciparum var genes expressed in children with severe malaria encode CIDRalpha1 domains. EMBO Mol. Med. 2016, 8, 839–850. [Google Scholar] [CrossRef] [PubMed]
- Bertin, G.I.; Lavstsen, T.; Guillonneau, F.; Doritchamou, J.; Wang, C.W.; Jespersen, J.S.; Ezimegnon, S.; Fievet, N.; Alao, M.J.; Lalya, F.; et al. Expression of the domain cassette 8 Plasmodium falciparum erythrocyte membrane protein 1 is associated with cerebral malaria in Benin. PLoS ONE 2013, 8, e68368. [Google Scholar] [CrossRef] [PubMed]
- Bernabeu, M.; Danziger, S.A.; Avril, M.; Vaz, M.; Babar, P.H.; Brazier, A.J.; Herricks, T.; Maki, J.N.; Pereira, L.; Mascarenhas, A.; et al. Severe adult malaria is associated with specific PfEMP1 adhesion types and high parasite biomass. Proc. Natl. Acad. Sci. USA 2016, 113, E3270–E3279. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Heddini, A.; Barragan, A.; Fernandez, V.; Pearce, S.F.; Wahlgren, M. The semiconserved head structure of Plasmodium falciparum erythrocyte membrane protein 1 mediates binding to multiple independent host receptors. J. Exp. Med. 2000, 192, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Avril, M.; Bernabeu, M.; Benjamin, M.; Brazier, A.J.; Smith, J.D. Interaction between Endothelial Protein C Receptor and Intercellular Adhesion Molecule 1 to Mediate Binding of Plasmodium falciparum-Infected Erythrocytes to Endothelial Cells. mBio 2016, 7, e00615-16. [Google Scholar] [CrossRef]
- Bernabeu, M.; Gunnarsson, C.; Vishnyakova, M.; Howard, C.C.; Nagao, R.J.; Avril, M.; Taylor, T.E.; Seydel, K.B.; Zheng, Y.; Smith, J.D. Binding Heterogeneity of Plasmodium falciparum to Engineered 3D Brain Microvessels Is Mediated by EPCR and ICAM-1. mBio 2019, 10, e00420-19. [Google Scholar] [CrossRef] [PubMed]
- Adams, Y.; Olsen, R.W.; Bengtsson, A.; Dalgaard, N.; Zdioruk, M.; Satpathi, S.; Behera, P.K.; Sahu, P.K.; Lawler, S.E.; Qvortrup, K.; et al. Plasmodium falciparum erythrocyte membrane protein 1 variants induce cell swelling and disrupt the blood-brain barrier in cerebral malaria. J. Exp. Med. 2021, 218, e20201266. [Google Scholar] [CrossRef] [PubMed]
- McCormick, C.J.; Craig, A.; Roberts, D.; Newbold, C.I.; Berendt, A.R. Intercellular adhesion molecule-1 and CD36 synergize to mediate adherence of Plasmodium falciparum-infected erythrocytes to cultured human microvascular endothelial cells. J. Clin. Investig. 1997, 100, 2521–2529. [Google Scholar] [CrossRef]
- Gray, C.; McCormick, C.; Turner, G.; Craig, A. ICAM-1 can play a major role in mediating P. falciparum adhesion to endothelium under flow. Mol. Biochem. Parasitol. 2003, 128, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Storm, J.; Jespersen, J.S.; Seydel, K.B.; Szestak, T.; Mbewe, M.; Chisala, N.V.; Phula, P.; Wang, C.W.; Taylor, T.E.; Moxon, C.A.; et al. Cerebral malaria is associated with differential cytoadherence to brain endothelial cells. EMBO Mol. Med. 2019, 11, e9164. [Google Scholar] [CrossRef] [PubMed]
- Ortolan, L.S.; Avril, M.; Xue, J.; Seydel, K.B.; Zheng, Y.; Smith, J.D. Plasmodium falciparum Parasite Lines Expressing DC8 and Group A PfEMP1 Bind to Brain, Intestinal, and Kidney Endothelial Cells. Front. Cell Infect. Microbiol. 2022, 12, 813011. [Google Scholar] [CrossRef]
- Swerlick, R.A.; Lee, K.H.; Wick, T.M.; Lawley, T.J. Human dermal microvascular endothelial but not human umbilical vein endothelial cells express CD36 in vivo and in vitro. J. Immunol. 1992, 148, 78–83. [Google Scholar]
- Turner, G.D.; Morrison, H.; Jones, M.; Davis, T.M.; Looareesuwan, S.; Buley, I.D.; Gatter, K.C.; Newbold, C.I.; Pukritayakamee, S.; Nagachinta, B.; et al. An immunohistochemical study of the pathology of fatal malaria. Evidence for widespread endothelial activation and a potential role for intercellular adhesion molecule-1 in cerebral sequestration. Am. J. Pathol. 1994, 145, 1057–1069. [Google Scholar]
- Kessler, A.; Dankwa, S.; Bernabeu, M.; Harawa, V.; Danziger, S.A.; Duffy, F.; Kampondeni, S.D.; Potchen, M.J.; Dambrauskas, N.; Vigdorovich, V.; et al. Linking EPCR-Binding PfEMP1 to Brain Swelling in Pediatric Cerebral Malaria. Cell Host. Microbe 2017, 22, 601–614.e605. [Google Scholar] [CrossRef] [PubMed]
- Wichers, J.S.; Tonkin-Hill, G.; Thye, T.; Krumkamp, R.; Kreuels, B.; Strauss, J.; von Thien, H.; Scholz, J.A.; Smedegaard Hansson, H.; Weisel Jensen, R.; et al. Common virulence gene expression in adult first-time infected malaria patients and severe cases. eLife 2021, 10, e69040. [Google Scholar] [CrossRef]
- Tuikue Ndam, N.; Moussiliou, A.; Lavstsen, T.; Kamaliddin, C.; Jensen, A.T.R.; Mama, A.; Tahar, R.; Wang, C.W.; Jespersen, J.S.; Alao, J.M.; et al. Parasites Causing Cerebral Falciparum Malaria Bind Multiple Endothelial Receptors and Express EPCR and ICAM-1-Binding PfEMP1. J. Infect. Dis 2017, 215, 1918–1925. [Google Scholar] [CrossRef] [PubMed]
- Ochola, L.B.; Siddondo, B.R.; Ocholla, H.; Nkya, S.; Kimani, E.N.; Williams, T.N.; Makale, J.O.; Liljander, A.; Urban, B.C.; Bull, P.C.; et al. Specific receptor usage in Plasmodium falciparum cytoadherence is associated with disease outcome. PLoS ONE 2011, 6, e14741. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, A.; Petter, M.; Krumkamp, R.; Esen, M.; Held, J.; Scholz, J.A.; Li, T.; Sim, B.K.; Hoffman, S.L.; Kremsner, P.G.; et al. Mosquito Passage Dramatically Changes var Gene Expression in Controlled Human Plasmodium falciparum Infections. PLoS Pathog. 2016, 12, e1005538. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.W.; Hermsen, C.C.; Sauerwein, R.W.; Arnot, D.E.; Theander, T.G.; Lavstsen, T. The Plasmodium falciparum var gene transcription strategy at the onset of blood stage infection in a human volunteer. Parasitol. Int. 2009, 58, 478–480. [Google Scholar] [CrossRef]
- Milne, K.; Ivens, A.; Reid, A.J.; Lotkowska, M.E.; O’Toole, A.; Sankaranarayanan, G.; Munoz Sandoval, D.; Nahrendorf, W.; Regnault, C.; Edwards, N.J.; et al. Mapping immune variation and var gene switching in naive hosts infected with Plasmodium falciparum. eLife 2021, 10, e62800. [Google Scholar] [CrossRef]
- Pickford, A.K.; Michel-Todo, L.; Dupuy, F.; Mayor, A.; Alonso, P.L.; Lavazec, C.; Cortes, A. Expression Patterns of Plasmodium falciparum Clonally Variant Genes at the Onset of a Blood Infection in Malaria-Naive Humans. mBio 2021, 12, e0163621. [Google Scholar] [CrossRef]
- Bachmann, A.; Bruske, E.; Krumkamp, R.; Turner, L.; Wichers, J.S.; Petter, M.; Held, J.; Duffy, M.F.; Sim, B.K.L.; Hoffman, S.L.; et al. Controlled human malaria infection with Plasmodium falciparum demonstrates impact of naturally acquired immunity on virulence gene expression. PLoS Pathog. 2019, 15, e1007906. [Google Scholar] [CrossRef]
- Kyriacou, H.M.; Stone, G.N.; Challis, R.J.; Raza, A.; Lyke, K.E.; Thera, M.A.; Kone, A.K.; Doumbo, O.K.; Plowe, C.V.; Rowe, J.A. Differential var gene transcription in Plasmodium falciparum isolates from patients with cerebral malaria compared to hyperparasitaemia. Mol. Biochem. Parasitol. 2006, 150, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Warimwe, G.M.; Keane, T.M.; Fegan, G.; Musyoki, J.N.; Newton, C.R.; Pain, A.; Berriman, M.; Marsh, K.; Bull, P.C. Plasmodium falciparum var gene expression is modified by host immunity. Proc. Natl. Acad. Sci. USA 2009, 106, 21801–21806. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, A.; Predehl, S.; May, J.; Harder, S.; Burchard, G.D.; Gilberger, T.W.; Tannich, E.; Bruchhaus, I. Highly co-ordinated var gene expression and switching in clinical Plasmodium falciparum isolates from non-immune malaria patients. Cell Microbiol. 2011, 13, 1397–1409. [Google Scholar] [CrossRef] [PubMed]
- Cham, G.K.; Turner, L.; Lusingu, J.; Vestergaard, L.; Mmbando, B.P.; Kurtis, J.D.; Jensen, A.T.; Salanti, A.; Lavstsen, T.; Theander, T.G. Sequential, ordered acquisition of antibodies to Plasmodium falciparum erythrocyte membrane protein 1 domains. J. Immunol. 2009, 183, 3356–3363. [Google Scholar] [CrossRef] [PubMed]
- Obeng-Adjei, N.; Larremore, D.B.; Turner, L.; Ongoiba, A.; Li, S.; Doumbo, S.; Yazew, T.B.; Kayentao, K.; Miller, L.H.; Traore, B.; et al. Longitudinal analysis of naturally acquired PfEMP1 CIDR domain variant antibodies identifies associations with malaria protection. JCI Insight 2020, 5. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, A.; Neculai, D.; Kain, K.C. CD36 and malaria: Friends or foes? A decade of data provides some answers. Trends Parasitol. 2014, 30, 436–444. [Google Scholar] [CrossRef]
- Turner, L.; Lavstsen, T.; Mmbando, B.P.; Wang, C.W.; Magistrado, P.A.; Vestergaard, L.S.; Ishengoma, D.S.; Minja, D.T.; Lusingu, J.P.; Theander, T.G. IgG antibodies to endothelial protein C receptor-binding cysteine-rich interdomain region domains of Plasmodium falciparum erythrocyte membrane protein 1 are acquired early in life in individuals exposed to malaria. Infect. Immun. 2015, 83, 3096–3103. [Google Scholar] [CrossRef]
- Andrade, C.M.; Fleckenstein, H.; Thomson-Luque, R.; Doumbo, S.; Lima, N.F.; Anderson, C.; Hibbert, J.; Hopp, C.S.; Tran, T.M.; Li, S.; et al. Increased circulation time of Plasmodium falciparum underlies persistent asymptomatic infection in the dry season. Nat. Med. 2020, 26, 1929–1940. [Google Scholar] [CrossRef]
- Frank, M.; Dzikowski, R.; Amulic, B.; Deitsch, K. Variable switching rates of malaria virulence genes are associated with chromosomal position. Mol. Microbiol. 2007, 64, 1486–1498. [Google Scholar] [CrossRef]
- Paget-McNicol, S.; Gatton, M.; Hastings, I.; Saul, A. The Plasmodium falciparum var gene switching rate, switching mechanism and patterns of parasite recrudescence described by mathematical modelling. Parasitology 2002, 124, 225–235. [Google Scholar] [CrossRef]
- Gatton, M.L.; Cheng, Q. Investigating antigenic variation and other parasite-host interactions in Plasmodium falciparum infections in naive hosts. Parasitology 2004, 128, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Otto, T.D.; Gilabert, A.; Crellen, T.; Bohme, U.; Arnathau, C.; Sanders, M.; Oyola, S.O.; Okouga, A.P.; Boundenga, L.; Willaume, E.; et al. Genomes of all known members of a Plasmodium subgenus reveal paths to virulent human malaria. Nat. Microbiol. 2018, 3, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Pepino, M.Y.; Kuda, O.; Samovski, D.; Abumrad, N.A. Structure-function of CD36 and importance of fatty acid signal transduction in fat metabolism. Annu. Rev. Nutr. 2014, 34, 281–303. [Google Scholar] [CrossRef] [PubMed]
- Silverstein, R.L.; Febbraio, M. CD36, a scavenger receptor involved in immunity, metabolism, angiogenesis, and behavior. Sci. Signal. 2009, 2, re3. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, J.; Cui, W.; Silverstein, R.L. CD36, a signaling receptor and fatty acid transporter that regulates immune cell metabolism and fate. J. Exp. Med. 2022, 219, e20211314. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Silverstein, R.L. CD36 signaling in vascular redox stress. Free Radic. Biol. Med. 2019, 136, 159–171. [Google Scholar] [CrossRef]
- Serghides, L.; Smith, T.G.; Patel, S.N.; Kain, K.C. CD36 and malaria: Friends or foes? Trends Parasitol. 2003, 19, 461–469. [Google Scholar] [CrossRef]
- Canton, J.; Neculai, D.; Grinstein, S. Scavenger receptors in homeostasis and immunity. Nat. Rev. Immunol. 2013, 13, 621–634. [Google Scholar] [CrossRef]
- Xu, X.; Zheng, X.; Zhu, F. CD36 gene variants and their clinical relevance: A narrative review. Ann. Blood 2021, 6, 34. [Google Scholar] [CrossRef]
- Urban, B.C.; Willcox, N.; Roberts, D.J. A role for CD36 in the regulation of dendritic cell function. Proc. Natl. Acad. Sci. USA 2001, 98, 8750–8755. [Google Scholar] [CrossRef]
- Urban, B.C.; Ferguson, D.J.; Pain, A.; Willcox, N.; Plebanski, M.; Austyn, J.M.; Roberts, D.J. Plasmodium falciparum-infected erythrocytes modulate the maturation of dendritic cells. Nature 1999, 400, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Gowda, N.M.; Gowda, D.C. Plasmodium falciparum: Differential merozoite dose requirements for maximal production of various inflammatory cytokines. Exp. Parasitol. 2011, 127, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.N.; Serghides, L.; Smith, T.G.; Febbraio, M.; Silverstein, R.L.; Kurtz, T.W.; Pravenec, M.; Kain, K.C. CD36 mediates the phagocytosis of Plasmodium falciparum-infected erythrocytes by rodent macrophages. J. Infect. Dis. 2004, 189, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Elliott, S.R.; Spurck, T.P.; Dodin, J.M.; Maier, A.G.; Voss, T.S.; Yosaatmadja, F.; Payne, P.D.; McFadden, G.I.; Cowman, A.F.; Rogerson, S.J.; et al. Inhibition of dendritic cell maturation by malaria is dose dependent and does not require Plasmodium falciparum erythrocyte membrane protein 1. Infect. Immun. 2007, 75, 3621–3632. [Google Scholar] [CrossRef]
- Erdman, L.K.; Cosio, G.; Helmers, A.J.; Gowda, D.C.; Grinstein, S.; Kain, K.C. CD36 and TLR interactions in inflammation and phagocytosis: Implications for malaria. J. Immunol. 2009, 183, 6452–6459. [Google Scholar] [CrossRef]
- McGilvray, I.D.; Serghides, L.; Kapus, A.; Rotstein, O.D.; Kain, K.C. Nonopsonic monocyte/macrophage phagocytosis of Plasmodium falciparum-parasitized erythrocytes: A role for CD36 in malarial clearance. Blood 2000, 96, 3231–3240. [Google Scholar] [CrossRef]
- Gowda, N.M.; Wu, X.; Kumar, S.; Febbraio, M.; Gowda, D.C. CD36 contributes to malaria parasite-induced pro-inflammatory cytokine production and NK and T cell activation by dendritic cells. PLoS ONE 2013, 8, e77604. [Google Scholar] [CrossRef]
- Thylur, R.P.; Wu, X.; Gowda, N.M.; Punnath, K.; Neelgund, S.E.; Febbraio, M.; Gowda, D.C. CD36 receptor regulates malaria-induced immune responses primarily at early blood stage infection contributing to parasitemia control and resistance to mortality. J. Biol. Chem. 2017, 292, 9394–9408. [Google Scholar] [CrossRef]
- Simon, S.I.; Goldsmith, H.L. Leukocyte adhesion dynamics in shear flow. Ann. Biomed. Eng. 2002, 30, 315–332. [Google Scholar] [CrossRef]
- Kunkel, E.J.; Dunne, J.L.; Ley, K. Leukocyte arrest during cytokine-dependent inflammation in vivo. J. Immunol. 2000, 164, 3301–3308. [Google Scholar] [CrossRef]
- Helms, G.; Dasanna, A.K.; Schwarz, U.S.; Lanzer, M. Modeling cytoadhesion of Plasmodium falciparum-infected erythrocytes and leukocytes-common principles and distinctive features. FEBS Lett. 2016, 590, 1955–1971. [Google Scholar] [CrossRef] [PubMed]
- Antia, M.; Herricks, T.; Rathod, P.K. Microfluidic modeling of cell-cell interactions in malaria pathogenesis. PLoS Pathog. 2007, 3, e99. [Google Scholar] [CrossRef]
- Flatt, C.; Mitchell, S.; Yipp, B.; Looareesuwan, S.; Ho, M. Attenuation of cytoadherence of Plasmodium falciparum to microvascular endothelium under flow by hemodilution. Am. J. Trop Med. Hyg. 2005, 72, 660–665. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Lim, T.S.; Shi, H.; Yin, J.; Tan, S.J.; Li, Z.; Low, B.C.; Tan, K.S.; Lim, C.T. Molecular mechanistic insights into the endothelial receptor mediated cytoadherence of Plasmodium falciparum-infected erythrocytes. PLoS ONE 2011, 6, e16929. [Google Scholar] [CrossRef]
- Yipp, B.G.; Hickey, M.J.; Andonegui, G.; Murray, A.G.; Looareesuwan, S.; Kubes, P.; Ho, M. Differential roles of CD36, ICAM-1, and P-selectin in Plasmodium falciparum cytoadherence in vivo. Microcirculation 2007, 14, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Lubiana, P.; Bouws, P.; Roth, L.K.; Dorpinghaus, M.; Rehn, T.; Brehmer, J.; Wichers, J.S.; Bachmann, A.; Hohn, K.; Roeder, T.; et al. Adhesion between P. falciparum infected erythrocytes and human endothelial receptors follows alternative binding dynamics under flow and febrile conditions. Sci. Rep. 2020, 10, 4548. [Google Scholar] [CrossRef] [PubMed]
- Ho, M.; Hoang, H.L.; Lee, K.M.; Liu, N.; MacRae, T.; Montes, L.; Flatt, C.L.; Yipp, B.G.; Berger, B.J.; Looareesuwan, S.; et al. Ectophosphorylation of CD36 regulates cytoadherence of Plasmodium falciparum to microvascular endothelium under flow conditions. Infect. Immun. 2005, 73, 8179–8187. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Herricks, T.; Avril, M.; Janes, J.; Smith, J.D.; Rathod, P.K. Clonal variants of Plasmodium falciparum exhibit a narrow range of rolling velocities to host receptor CD36 under dynamic flow conditions. Eukaryot. Cell 2013, 12, 1490–1498. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dasanna, A.K.; Lansche, C.; Lanzer, M.; Schwarz, U.S. Rolling Adhesion of Schizont Stage Malaria-Infected Red Blood Cells in Shear Flow. Biophys. J. 2017, 112, 1908–1919. [Google Scholar] [CrossRef] [PubMed]
- Yipp, B.G.; Robbins, S.M.; Resek, M.E.; Baruch, D.I.; Looareesuwan, S.; Ho, M. Src-family kinase signaling modulates the adhesion of Plasmodium falciparum on human microvascular endothelium under flow. Blood 2003, 101, 2850–2857. [Google Scholar] [CrossRef] [PubMed]
- Fedosov, D.A.; Caswell, B.; Karniadakis, G.E. Wall shear stress-based model for adhesive dynamics of red blood cells in malaria. Biophys. J. 2011, 100, 2084–2093. [Google Scholar] [CrossRef] [PubMed]
- Fedosov, D.A.; Lei, H.; Caswell, B.; Suresh, S.; Karniadakis, G.E. Multiscale modeling of red blood cell mechanics and blood flow in malaria. PLoS Comput. Biol. 2011, 7, e1002270. [Google Scholar] [CrossRef] [PubMed]
- Davis, S.P.; Amrein, M.; Gillrie, M.R.; Lee, K.; Muruve, D.A.; Ho, M. Plasmodium falciparum-induced CD36 clustering rapidly strengthens cytoadherence via p130CAS-mediated actin cytoskeletal rearrangement. FASEB J. 2012, 26, 1119–1130. [Google Scholar] [CrossRef] [PubMed]
- Horrocks, P.; Pinches, R.A.; Chakravorty, S.J.; Papakrivos, J.; Christodoulou, Z.; Kyes, S.A.; Urban, B.C.; Ferguson, D.J.; Newbold, C.I. PfEMP1 expression is reduced on the surface of knobless Plasmodium falciparum infected erythrocytes. J. Cell Sci. 2005, 118, 2507–2518. [Google Scholar] [CrossRef]
- May, J.; Evans, J.A.; Timmann, C.; Ehmen, C.; Busch, W.; Thye, T.; Agbenyega, T.; Horstmann, R.D. Hemoglobin variants and disease manifestations in severe falciparum malaria. JAMA 2007, 297, 2220–2226. [Google Scholar] [CrossRef] [PubMed]
- Modiano, D.; Luoni, G.; Sirima, B.S.; Simpore, J.; Verra, F.; Konate, A.; Rastrelli, E.; Olivieri, A.; Calissano, C.; Paganotti, G.M.; et al. Haemoglobin C protects against clinical Plasmodium falciparum malaria. Nature 2001, 414, 305–308. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Guindo, A.; Cissoko, Y.; Taylor, J.G.; Coulibaly, D.; Kone, A.; Kayentao, K.; Djimde, A.; Plowe, C.V.; Doumbo, O.; et al. Hemoglobin C associated with protection from severe malaria in the Dogon of Mali, a West African population with a low prevalence of hemoglobin S. Blood 2000, 96, 2358–2363. [Google Scholar] [CrossRef]
- Fairhurst, R.M.; Baruch, D.I.; Brittain, N.J.; Ostera, G.R.; Wallach, J.S.; Hoang, H.L.; Hayton, K.; Guindo, A.; Makobongo, M.O.; Schwartz, O.M.; et al. Abnormal display of PfEMP-1 on erythrocytes carrying haemoglobin C may protect against malaria. Nature 2005, 435, 1117–1121. [Google Scholar] [CrossRef]
- Cholera, R.; Brittain, N.J.; Gillrie, M.R.; Lopera-Mesa, T.M.; Diakite, S.A.; Arie, T.; Krause, M.A.; Guindo, A.; Tubman, A.; Fujioka, H.; et al. Impaired cytoadherence of Plasmodium falciparum-infected erythrocytes containing sickle hemoglobin. Proc. Natl. Acad. Sci. USA 2008, 105, 991–996. [Google Scholar] [CrossRef] [PubMed]
- Arman, M.; Adams, Y.; Lindergard, G.; Rowe, J.A. A method for positive and negative selection of Plasmodium falciparum platelet-mediated clumping parasites and investigation of the role of CD36. PLoS ONE 2013, 8, e55453. [Google Scholar] [CrossRef]
- Xu, X.; Efremov, A.K.; Li, A.; Lai, L.; Dao, M.; Lim, C.T.; Cao, J. Probing the cytoadherence of malaria infected red blood cells under flow. PLoS ONE 2013, 8, e64763. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, P.A.; Diez-Silva, M.; Chen, H.; Dao, M.; Suresh, S. Cytoadherence of erythrocytes invaded by Plasmodium falciparum: Quantitative contact-probing of a human malaria receptor. Acta Biomater. 2013, 9, 6349–6359. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.B.; Thingna, J.; Cao, J.; Lim, C.T. Single molecule and multiple bond characterization of catch bond associated cytoadhesion in malaria. Sci. Rep. 2017, 7, 4208. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bachmann, A.; Metwally, N.G.; Allweier, J.; Cronshagen, J.; del Pilar Martinez Tauler, M.; Murk, A.; Roth, L.K.; Torabi, H.; Wu, Y.; Gutsmann, T.; et al. CD36—A Host Receptor Necessary for Malaria Parasites to Establish and Maintain Infection. Microorganisms 2022, 10, 2356. https://doi.org/10.3390/microorganisms10122356
Bachmann A, Metwally NG, Allweier J, Cronshagen J, del Pilar Martinez Tauler M, Murk A, Roth LK, Torabi H, Wu Y, Gutsmann T, et al. CD36—A Host Receptor Necessary for Malaria Parasites to Establish and Maintain Infection. Microorganisms. 2022; 10(12):2356. https://doi.org/10.3390/microorganisms10122356
Chicago/Turabian StyleBachmann, Anna, Nahla Galal Metwally, Johannes Allweier, Jakob Cronshagen, Maria del Pilar Martinez Tauler, Agnes Murk, Lisa Katharina Roth, Hanifeh Torabi, Yifan Wu, Thomas Gutsmann, and et al. 2022. "CD36—A Host Receptor Necessary for Malaria Parasites to Establish and Maintain Infection" Microorganisms 10, no. 12: 2356. https://doi.org/10.3390/microorganisms10122356
APA StyleBachmann, A., Metwally, N. G., Allweier, J., Cronshagen, J., del Pilar Martinez Tauler, M., Murk, A., Roth, L. K., Torabi, H., Wu, Y., Gutsmann, T., & Bruchhaus, I. (2022). CD36—A Host Receptor Necessary for Malaria Parasites to Establish and Maintain Infection. Microorganisms, 10(12), 2356. https://doi.org/10.3390/microorganisms10122356








