Failure Analysis of Bank-Wall Side Boiler Tube in a Petrochemical Plant
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Site Inspection of Boiler Tube
3.2. Boiler Tube Materials Verification
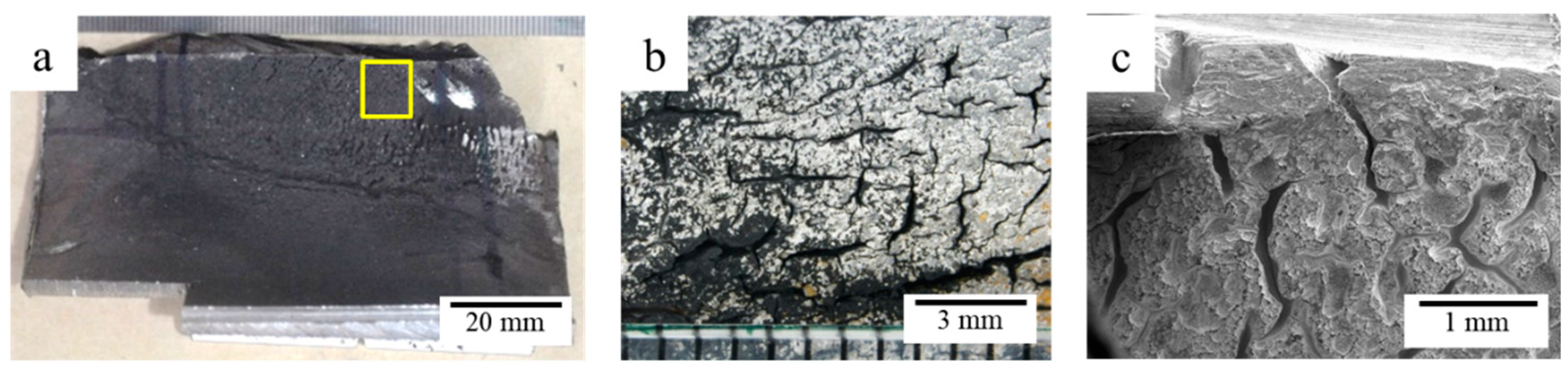
3.3. Visual Examination
3.4. Fractography Analysis
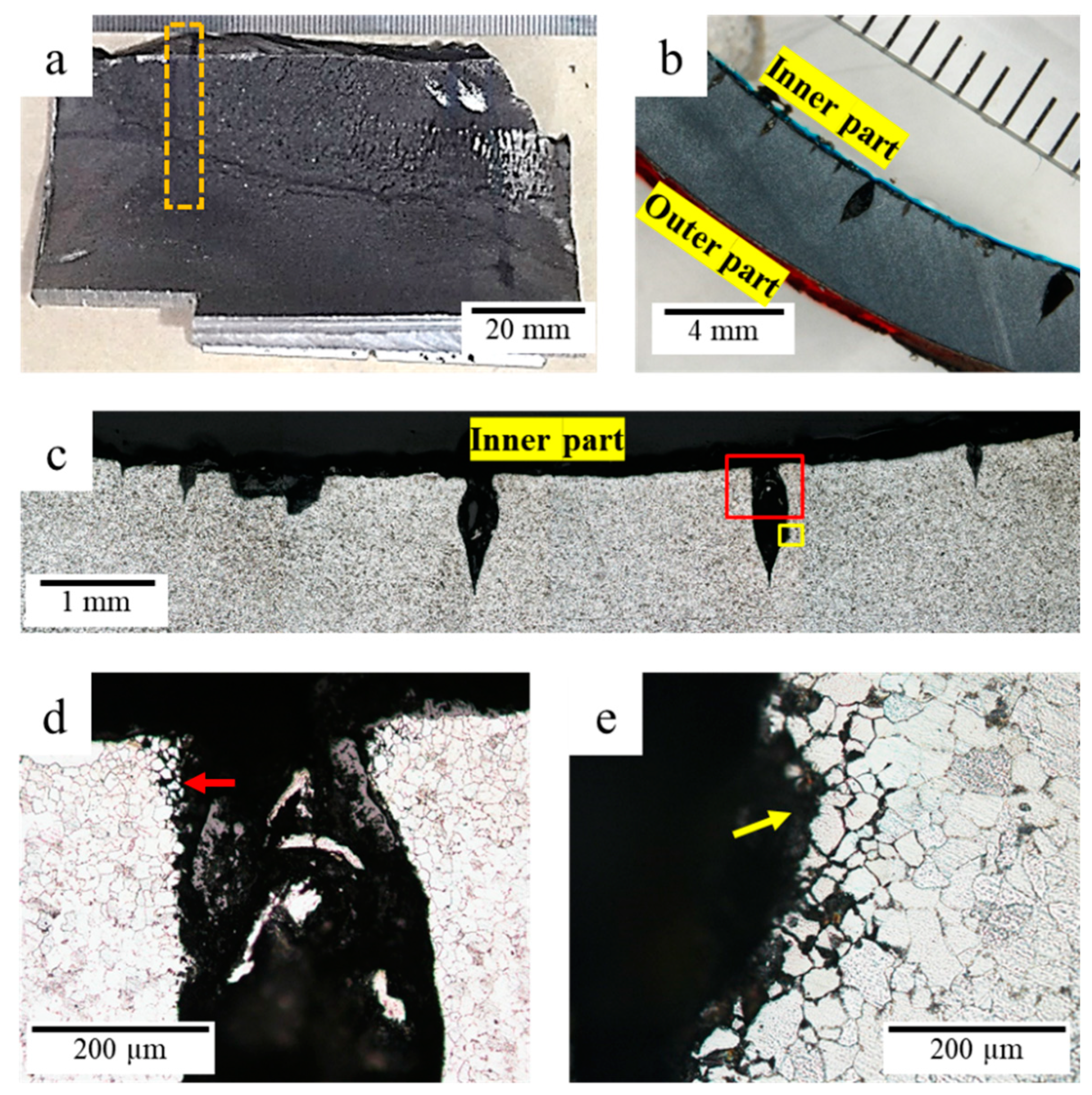
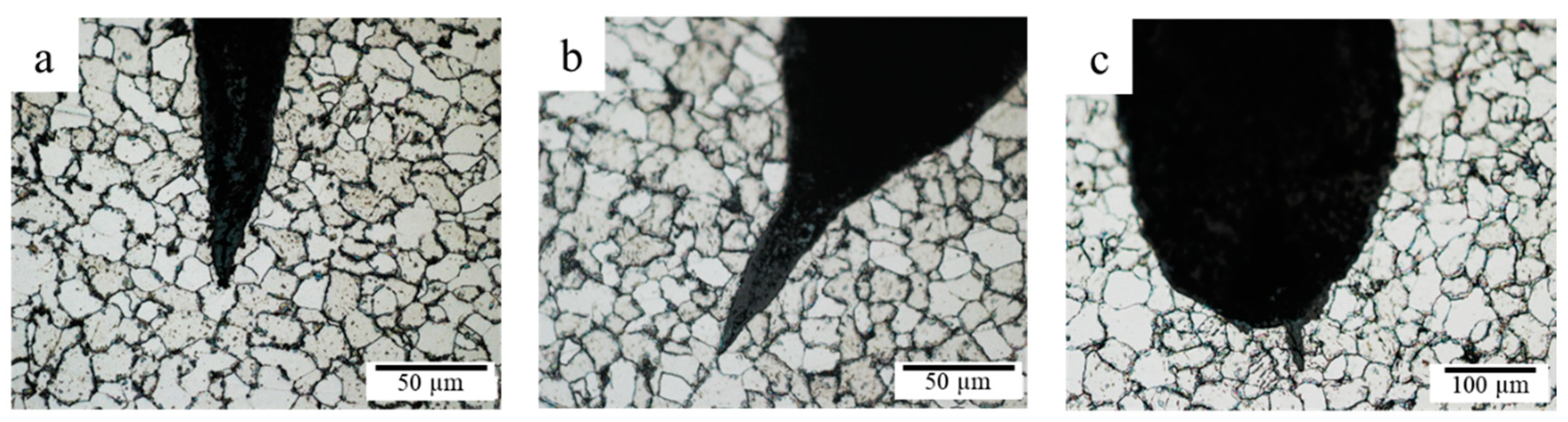
3.5. Microstructure Analysis
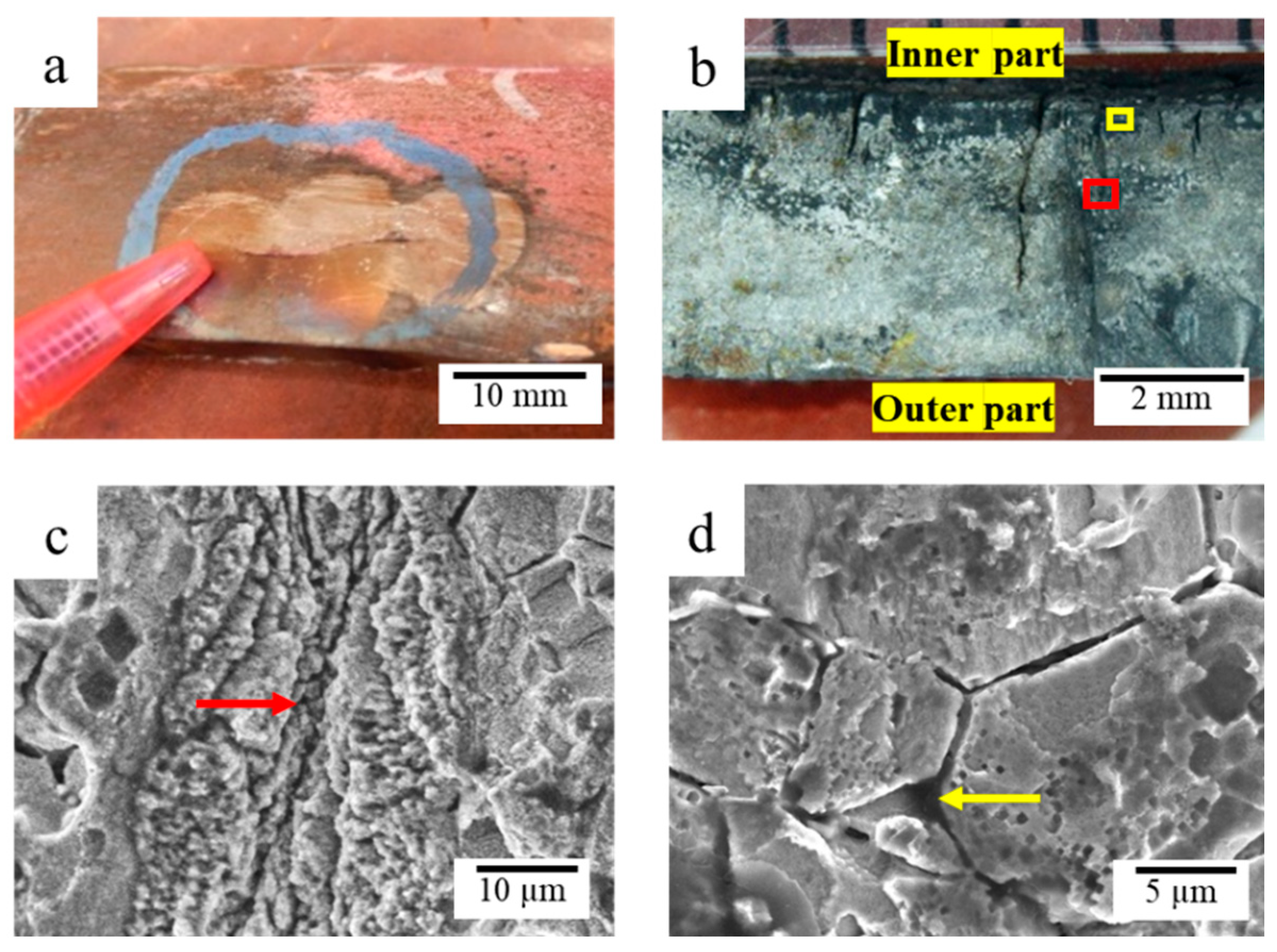
3.5.1. Microstructure Analysis of Corroded Surface
3.5.2. Microstructure Analysis of Leak Interface
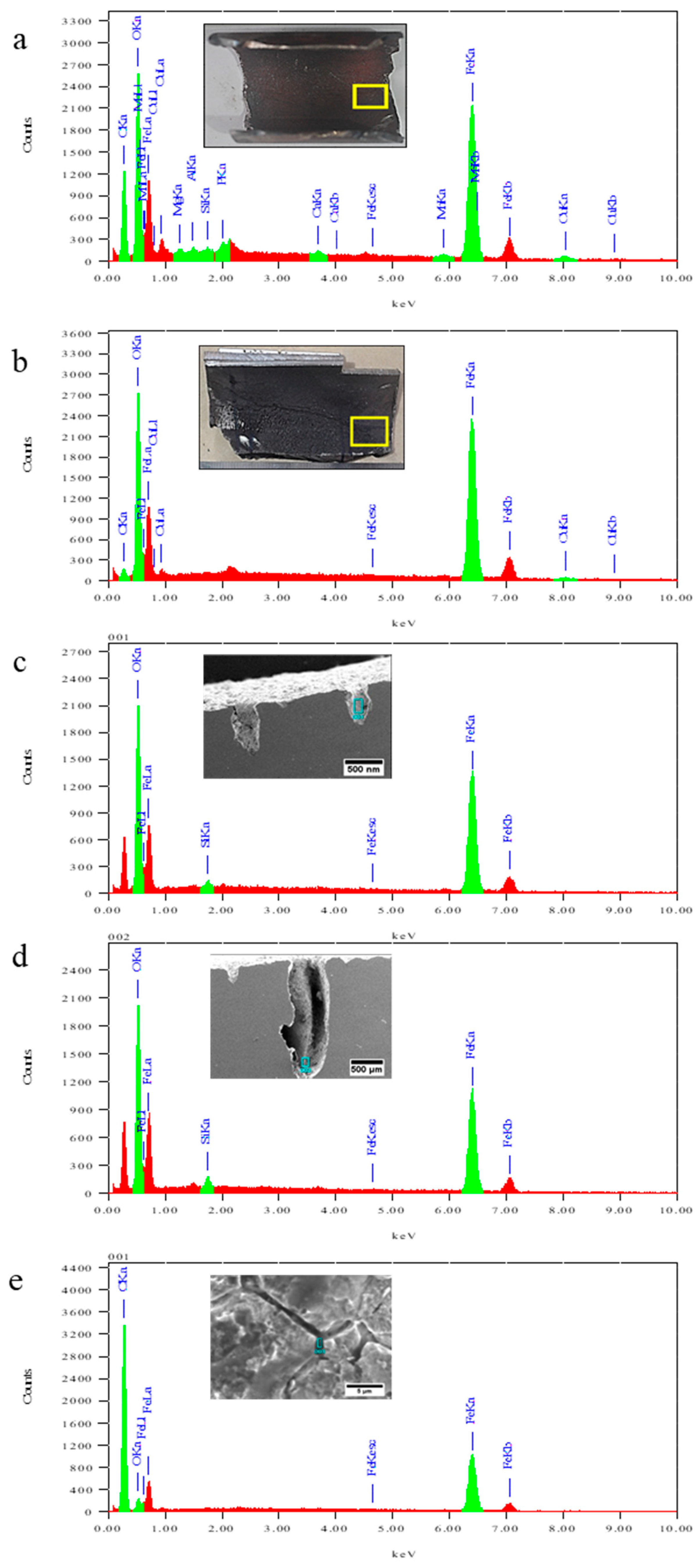
3.6. Analysis of Deposit
4. Discussion
4.1. Deposit Formation Mechanism
4.2. Crack Propagation Mechanism
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Addel-Geliel, M.; Zakzouk, S.; Sengaby, M.E. Application of model based fault detection for an industrial boiler. In Proceedings of the 2012 20th Mediterranean Conference on Control & Automation (MED), Barcelona, Spain, 3–6 July 2012; pp. 98–103. [Google Scholar]
- Skiles, D. Improve the Performance of Your Boiler System. Chem. Eng. Prog. 2014, 11, 31. [Google Scholar]
- Sathish, T.; Mohanavel, V.; Afzal, A.; Arunkumar, M.; Ravichandran, M.; Khan, S.A.; Rajendran, P.; Asif, M. Advancement of steam generation process in water tube boiler using Taguchi design of experiments. Case Stud. Therm. Eng. 2021, 27, 101247. [Google Scholar] [CrossRef]
- Amir, M.K.; Samad, M.; Behzad, S.M.; Mehdi, S.M. Corrosion prevention in boilers by using energy audit consideration. In Proceedings of the Applied Mechanics and Materials, Zurich, Switzerland, 7–8 January 2014; pp. 307–310. [Google Scholar]
- Hong, M.; Chae, H.; Kim, W.C.; Kim, J.-G.; Kim, H.; Lee, S.Y. Failure Analysis of a Water Wall Boiler Tube for Power Generation in a District Heating System. Met. Mater. Int. 2019, 25, 1191–1201. [Google Scholar] [CrossRef]
- Ardy, H.; Putra, Y.P.; Anggoro, A.D.; Wibowo, A. Failure analysis of primary waste heat boiler tube in ammonia plant. Heliyon 2021, 7, e06151. [Google Scholar] [CrossRef]
- Ardy, H.; Nurimam, A.; Hamdani, M.; Abednego, T.H.; Helmy, R.K.; Bangun, D.A.; Setiawan, A.R.; Wibowo, A.; Sumboja, A. Failure analysis of admiralty brass tubes in a surface condenser: A case study at the petrochemical industry. Mater. High Temp. 2021, 38, 177–185. [Google Scholar] [CrossRef]
- Duarte, C.A.; Espejo, E.; Martinez, J.C. Failure analysis of the wall tubes of a water-tube boiler. Eng. Fail. Anal. 2017, 79, 704–713. [Google Scholar] [CrossRef]
- Liu, S.W.; Wang, W.Z.; Liu, C.J. Failure analysis of the boiler water-wall tube. Case Stud. Eng. Fail. Anal. 2017, 9, 35–39. [Google Scholar] [CrossRef]
- Asnavandi, M.; Kahram, M.; Rezaei, M.; Rezakhani, D. Fire-Side Corrosion: A Case Study of Failed Tubes of a Fossil Fuel Boiler. Int. J. Corros. 2017, 2017, 7367046. [Google Scholar] [CrossRef]
- Djukic, M.B.; Sijacki Zeravcic, V.; Bakic, G.M.; Sedmak, A.; Rajicic, B. Hydrogen damage of steels: A case study and hydrogen embrittlement model. Eng. Fail. Anal. 2015, 58, 485–498. [Google Scholar] [CrossRef]
- Dooley, R.B.; Bursik, A. Hydrogen damage. PowerPlant Chem. 2010, 12, 122. [Google Scholar]
- McCarroll, I.E.; Lin, Y.-C.; Rosenthal, A.; Yen, H.-W.; Cairney, J.M. Hydrogen trapping at dislocations, carbides, copper precipitates and grain boundaries in a dual precipitating low-carbon martensitic steel. Scr. Mater. 2022, 221, 114934. [Google Scholar] [CrossRef]
- Bose, S.; Reddy, S.; Singh, K. Interdependence of on-load corrosion, creep-rupture, and copper deposit in augmenting failure processes of boiler tubes. Corrosion 2000, 56, 1158–1169. [Google Scholar] [CrossRef]
- Daneshvar-Fatah, F.; Mostafaei, A.; Hosseinzadeh-Taghani, R.; Nasirpouri, F. Caustic corrosion in a boiler waterside tube: Root cause and mechanism. Eng. Fail. Anal. 2013, 28, 69–77. [Google Scholar] [CrossRef]
- ASTM A178-19; Standard Specification for Electric-Resistance-Welded Carbon Steel and Carbon-Manganese Steel Boiler and Superheater Tubes. ASTM International: West Conshohocken, PA, USA, 2019.
- ASTM E8/E8M-13a; Standard Test Methods for Tension Testing of Metallic Materials. ASTM International: West Conshohocken, PA, USA, 2001.
- ASTM E3-11; Standard Guide for Preparation of Metallographic Specimens. ASTM International: West Conshohocken, PA, USA, 2017.
- ASTM G131-96; Standard Practice for Cleaning of Materials and Components by Ultrasonic Techniques. ASTM International: West Conshohocken, PA, USA, 2016.
- Cai, G.; Li, C.; Wang, D.; Zhou, Y. Investigation of annealing temperature on microstructure and texture of Fe-19Cr-2Mo-Nb-Ti ferritic stainless steel. Mater. Charact. 2018, 141, 169–176. [Google Scholar] [CrossRef]
- Klobčar, D.; Kosec, L.; Kosec, B.; Tušek, J. Thermo fatigue cracking of die casting dies. Eng. Fail. Anal. 2012, 20, 43–53. [Google Scholar] [CrossRef]
- Klobčar, D.; Tušek, J.; Taljat, B. Thermal fatigue of materials for die-casting tooling. Mater. Sci. Eng. A 2008, 472, 198–207. [Google Scholar] [CrossRef]
- Zhan, J.; Li, M.; Huang, J.; Bi, H.; Li, Q.; Gu, H. Thermal Fatigue Characteristics of Type 309 Austenitic Stainless Steel for Automotive Manifolds. Metals 2019, 9, 129. [Google Scholar] [CrossRef] [Green Version]
- Bahrami, A.; Taheri, P. Creep Failure of Reformer Tubes in a Petrochemical Plant. Metals 2019, 9, 1026. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.S.; Kim, S.S.; Choe, B.H. The Role of Hydrogen in Hydrogen Embrittlement of Metals: The Case of Stainless Steel. Metals 2019, 9, 406. [Google Scholar] [CrossRef] [Green Version]
- Khajavi, M.R.; Abdolmaleki, A.R.; Adibi, N.; Mirfendereski, S. Failure analysis of bank front boiler tubes. Eng. Fail. Anal. 2007, 14, 731–738. [Google Scholar] [CrossRef]
- Howell, A.G. Under Deposit Corrosion Mechanisms in Boilers. In Proceedings of the CORROSION 2006, San Diego, CA, USA, 12–16 March 2006. [Google Scholar]
- DeWitt-Dick, D.B.; Beardwood, E.S. What Happens When Real World Operation of Steam Generators Deviates From Design Conditions. In Proceedings of the CORROSION 2008, New Orleans, LA, USA, 16–20 March 2008. [Google Scholar]
- Li, P.; Chen, Y.; Li, X.; Yan, B.; Chen, D.; Guo, H. Hydrogen generation characteristics of steel slag-steam high temperature reaction in terms of particle size. Int. J. Hydrogen Energy 2020, 45, 17140–17152. [Google Scholar] [CrossRef]
- Monazam, E.R.; Breault, R.W.; Siriwardane, R. Kinetics of Magnetite (Fe3O4) Oxidation to Hematite (Fe2O3) in Air for Chemical Looping Combustion. Ind. Eng. Chem. Res. 2014, 53, 13320–13328. [Google Scholar] [CrossRef]
- Yule, L.C.; Shkirskiy, V.; Aarons, J.; West, G.; Bentley, C.L.; Shollock, B.A.; Unwin, P.R. Nanoscale Active Sites for the Hydrogen Evolution Reaction on Low Carbon Steel. J. Phys. Chem. C 2019, 123, 24146–24155. [Google Scholar] [CrossRef] [Green Version]
- Cetinkaya, S.; Eroglu, S. Effect of the extent of pre-reduction of γ-Fe2O3 by H2 on the synthesis of Fe3C powder from CH4. Int. J. Miner. Process. 2016, 152, 46–52. [Google Scholar] [CrossRef]
- Martin, M.L.; Dadfarnia, M.; Orwig, S.; Moore, D.; Sofronis, P. A microstructure-based mechanism of cracking in high temperature hydrogen attack. Acta Mater. 2017, 140, 300–304. [Google Scholar] [CrossRef]
- Hertzberg, R.W.; Vincy, R.P.; Hertzberg, J.L. Deformation and Fracture Mechanics of Engineering Materials, 5th ed.; Wiley: Hoboken, NJ, USA, 2013. [Google Scholar]





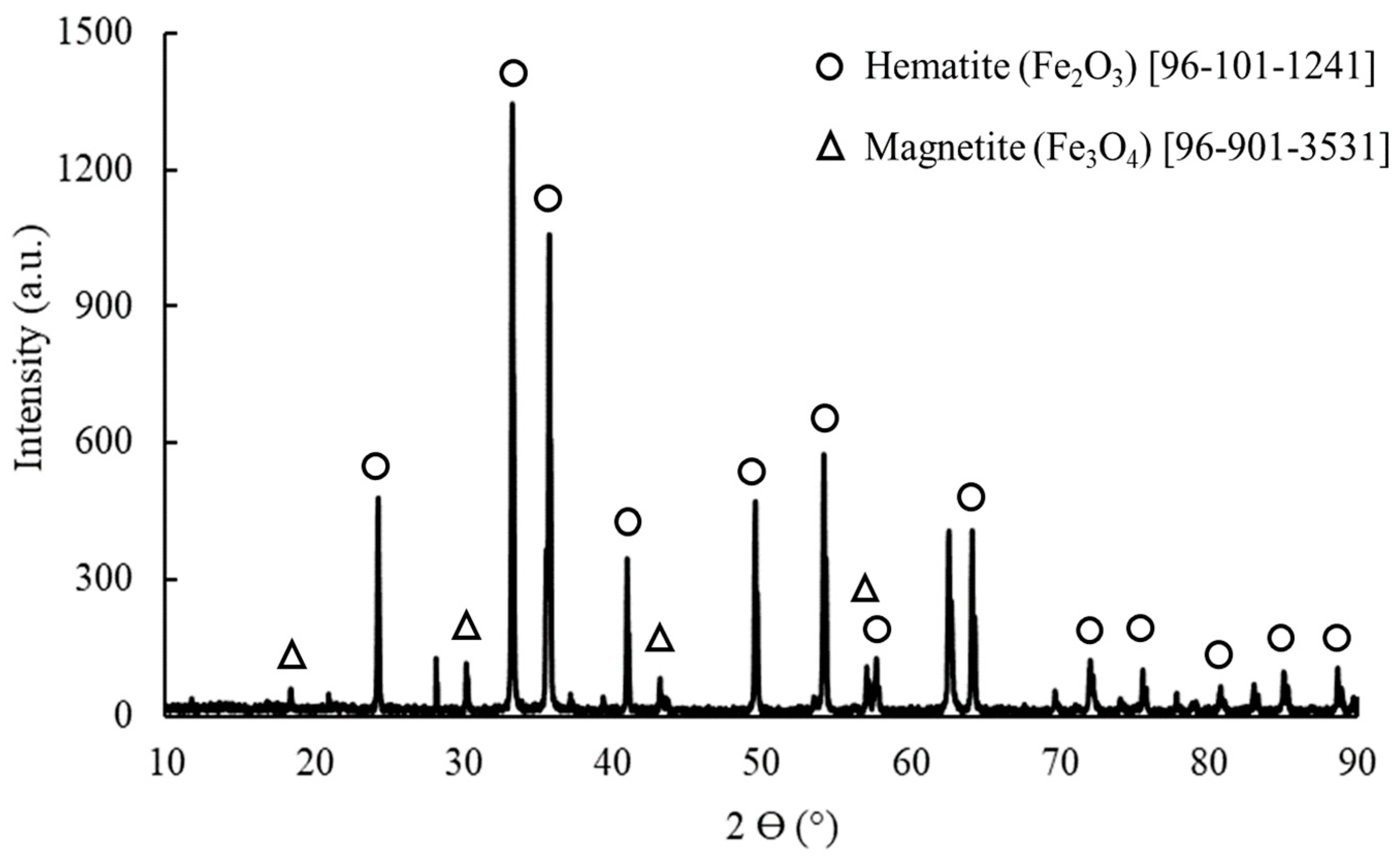
| Parameters | Values | Unit | |
|---|---|---|---|
| Operating condition | Maximum allowable working temperature | 371 | °C |
| Maximum allowable working pressure | 5.5 | MPa | |
| Feedwater temperature at boiler inlet | 147 | °C | |
| Water velocity | 1.0 | m/s | |
| The tube specification | Water-tube material | ASTM A178 Gr. A | |
| Outside diameter | 76.2 | mm | |
| Thickness | 4.0 | mm | |
| Element | ||||||
|---|---|---|---|---|---|---|
| C | Si | S | P | Mn | Fe | |
| Sample | 0.067 | 0.121 | 0.006 | 0.026 | 0.495 | Balance |
| ASTM A178 Gr A [16] | 0.06–0.18 | ….. | ≤0.035 | ≤0.035 | 0.27–0.63 | Balance |
| Sample | ASTM A178 Grade A [16] | |
|---|---|---|
| Tensile Strength, MPa | 380 | ≥325 |
| Yield Strength, MPa | 273 | ≥180 |
| Elongation, % | 42 | ≥35 |
| Location | Elements | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C | O | Mg | Al | Si | P | Ca | Mn | Fe | Cu | |
| Bottom | 31.45 | 23.52 | 0.25 | 0.16 | 0.1 | 0.14 | 0.27 | 0.69 | 41.26 | 2.16 |
| Top | 5.06 | 24.97 | 68.41 | 1.56 | ||||||
| Short Crack | 29.9 | 0.55 | 69.54 | |||||||
| Long Crack | 33.44 | 1.09 | 65.47 | |||||||
| Grain boundary | 72.76 | 3.54 | 23.70 | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ardy, H.; Nurimam, A.; Hamdani, M.; Firmansyah, D.; Aditya, D.M.; Setiawan, A.R.; Wibowo, A. Failure Analysis of Bank-Wall Side Boiler Tube in a Petrochemical Plant. Metals 2022, 12, 2064. https://doi.org/10.3390/met12122064
Ardy H, Nurimam A, Hamdani M, Firmansyah D, Aditya DM, Setiawan AR, Wibowo A. Failure Analysis of Bank-Wall Side Boiler Tube in a Petrochemical Plant. Metals. 2022; 12(12):2064. https://doi.org/10.3390/met12122064
Chicago/Turabian StyleArdy, Husaini, Asep Nurimam, Mohammad Hamdani, Deny Firmansyah, Dominico Michael Aditya, Asep Ridwan Setiawan, and Arie Wibowo. 2022. "Failure Analysis of Bank-Wall Side Boiler Tube in a Petrochemical Plant" Metals 12, no. 12: 2064. https://doi.org/10.3390/met12122064
APA StyleArdy, H., Nurimam, A., Hamdani, M., Firmansyah, D., Aditya, D. M., Setiawan, A. R., & Wibowo, A. (2022). Failure Analysis of Bank-Wall Side Boiler Tube in a Petrochemical Plant. Metals, 12(12), 2064. https://doi.org/10.3390/met12122064






