Physiological and Biochemical Changes in Vegetable and Field Crops under Drought, Salinity and Weeds Stresses: Control Strategies and Management
Abstract
:1. Introduction
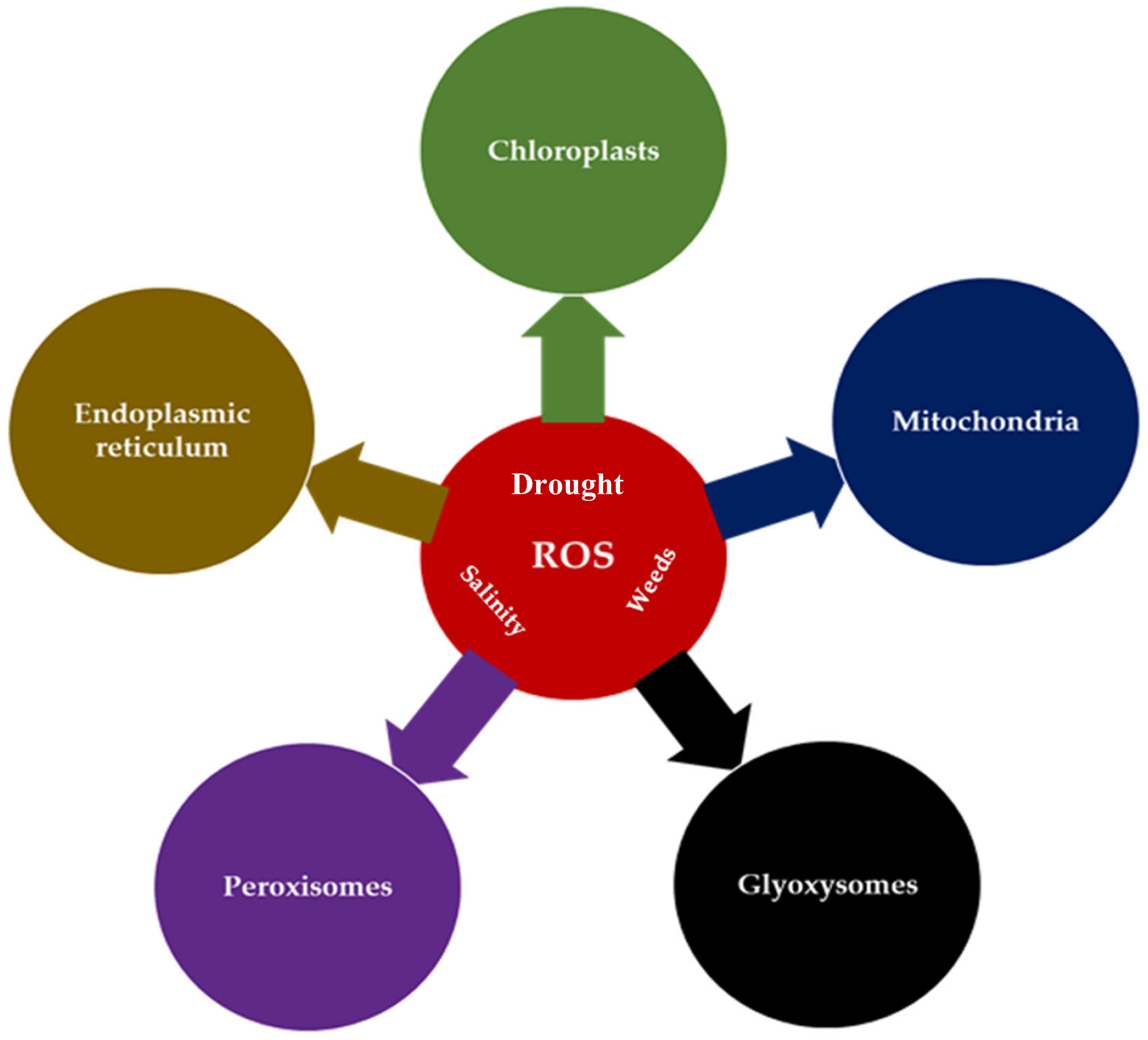
2. Production of ROS as One of the Stress Signals in Plants
2.1. The Localization and Processes of ROS Generation
2.1.1. ROS in Mitochondria
2.1.2. ROS in Chloroplasts
2.1.3. ROS in Peroxisomes
2.1.4. ROS in the Apoplast
2.2. The Downstream Consequences of Oxidative Stress in Plants
2.3. Hormonal Crosstalk in Stress Regulation
3. Induction of Antioxidant Mechanisms under Drought, Salinity and Weeds Stresses in Plants
3.1. Enzymatic Antioxidants
3.1.1. Peroxidases
3.1.2. Glutathione Reductase (GR)
3.1.3. Superoxide Dismutase (SOD)
3.1.4. Peroxiredoxin (Prxs)
3.1.5. Ascorbate Peroxidase (APX)
3.1.6. Catalase (CAT)
3.2. Non-Enzymatic Antioxidants
3.2.1. AsA
3.2.2. Carotenoids
3.2.3. Gamma-Aminobutyric Acid (GABA)
3.2.4. α-Tocopherols (Vitamin E)
3.2.5. Glutathione
3.2.6. Proline
3.2.7. Phenolics and Flavonoids
4. Mechanism of Salt and Drought Tolerance in Vegetables and Field Crops Associated with Improvement of Plant Growth under Stress Conditions
| Osmoprotectants | The Mechanism of Improving Plant Growth and Tolerance to Drought, Salinity and Weed Stress | Stress Type | Plant | References |
|---|---|---|---|---|
| Mannitol | Scavenge super oxide and hydroxyl radical Protect the chloroplasts against photo-oxidative stress Increase drought and salinity tolerance | Water stress | Wheat Arabidopsis thaliana | [177] [178] |
| Trehalose | Reduce photo-oxidative damage to photosystem II Stabilize membranes and proteins against osmotic stress | Salt, drought, and low-temperature | Arabidopsis thaliana Rice | [179] [180] |
| Proline | Protecting plant cells against the increase in ROS production Increase water availability under drought and salinity stress | Salinity and drought | Bean, calendula, sugar beet and barley | [7,8,13,15] |
| α-Tocopherols | Scavenge ROS under salinity stress | Salinity | Tomato | [213] |
| PGPR | Act as osmoregulator under drought stress | Drought | Some plants | [214] |
| Zinc oxide | Act as osmoprotectant under drought stress | Drought | Tomato | [215] |
| Glycine betaine (GB) | Increase membrane stability, osmotic adjustment and enzymes activity Decrease the accumulation of ROS under salinity stress | Salinity | Tomato Tobacco | [184] [193] |
| Abscisic acid (AsA) | Decrease the accumulation of ROS under drought Reduce water loss and regulate the stomatal function | Drought | Maize Wheat | [18] [195] |
| Auxin and GAs | Adjust the oxidative stress in cells and regulate polar auxin transport Increase the activity of DELLA proteins and ROS scavenging capacity | Salinity | Arabidopsis Maize | [202] [125] |
| Salicylic acid (SA) | Improve relative water content under salinity conditions Decrease electrolyte leakage and increase grain yield under drought stress | Salinity | Sweet pepper Barley | [4] [13] |
| Jasmonic acid (JA) | Improve the activity of antioxidant enzymes under salinity stress Reduce ROS levels | Salinity | Brassica oleracea | [210] |
| Ascorbic acid (Vitamin C) | Improve relative water content under drought Protect the plant cells against toxic free radicals | Drought | Wheat Tomato | [1] [109,110] |
| Gamma-aminobutyric acid (GABA) | Improve cellular ion homeostasis Reduce ROS production under salt stress Decrease the levels of lipid peroxidation under drought | Drought | Arabidopsis Tomato Black pepper | [114] [119] [134] |
| α-Tocopherols (vitamin E) | Protect the chloroplasts from photo-oxidation Scavenge ROS and lipid peroxidation and regulate signal transduction under drought stress | Drought | Tobacco Arabidopsis | [135] [136] |
| Proline | Act as osmoprotectant under weed stress | Weeds | Potato | [216] |
5. Control of Weeds in Plants
5.1. Physical and Chemical Control of Weeds
5.2. Control of Weeds with Living Mulches or Allelopathy
5.3. Biological Control of Weeds
5.4. Integrated Weed Management in Plants
5.5. Physiological Changes under Weed Infestation
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abdelaal, K.A.A.; Elafry, M.; Abdel-Latif, I.; Elshamy, R.; Hassan, M.; Hafez, Y. Pivotal role of yeast and ascorbic acid in improvement the morpho-physiological characters of two wheat cultivars under water deficit stress in calcareous soil. Fresenius Environ. Bull. 2021, 30, 2554–2565. [Google Scholar]
- Rashwan, E.; Alsohim, A.S.; El-Gammaal, A.; Hafez, Y.; Abdelaal, K.A.A. Foliar application of nano zink-oxide can alleviate the harmful effects of water deficit on some flax cultivars under drought conditions. Fresenius Environ. Bull. 2020, 29, 8889–8904. [Google Scholar]
- Hafez, Y.M.; Attia, K.A.; Alamery, S.; Ghazy, A.; Al-Dosse, A.; Ibrahim, E.; Rashwan, E.; El-Maghraby, L.; Awad, A.; Abdelaal, K.A.A. Beneficial Effects of Biochar and Chitosan on Antioxidative Capacity, Osmolytes Accumulation, and Anatomical Characters of Water-Stressed Barley Plants. Agronomy 2020, 10, 630. [Google Scholar] [CrossRef]
- Abdelaal, K.; EL-Maghraby, L.M.; Elansary, H.; Hafez, Y.M.; Ibrahim, E.I.; El-Banna, M.; El-Esawi, M.; Elkelish, A. Treatment of Sweet Pepper with Stress Tolerance-Inducing Compounds Alleviates Salinity Stress Oxidative Damage by Mediating the Physio-Biochemical Activities and Antioxidant Systems. Agronomy 2020, 10, 26. [Google Scholar] [CrossRef] [Green Version]
- ALKahtani, M.D.F.; Attia, K.A.; Hafez, Y.M.; Khan, N.; Eid, A.M.; Ali, M.A.M.; Abdelaal, K.A.A. Chlorophyll Fluorescence Parameters and Antioxidant Defense System Can Display Salt Tolerance of Salt Acclimated Sweet Pepper Plants Treated with Chitosan and Plant Growth Promoting Rhizobacteria. Agronomy 2020, 10, 1180. [Google Scholar] [CrossRef]
- Abdelaal, K.A.A.; Mazrou, Y.S.A.; Hafez, Y.M. Silicon Foliar Application Mitigates Salt Stress in Sweet Pepper Plants by Enhancing Water Status, Photosynthesis, Antioxidant Enzyme Activity and Fruit Yield. Plants 2020, 9, 733. [Google Scholar] [CrossRef]
- Abdelaal, K.A.A.; El-Afry, M.; Metwaly, M.; Zidan, M.; Rashwan, E. Salt tolerance activation in faba bean plants using proline and salicylic acid associated with physio-biochemical and yield characters improvement. Fresenius Environ. Bull. 2021, 30, 3175–3186. [Google Scholar]
- El-Shawa, G.M.R.; Rashwan, E.M.; Abdelaal, K.A.A. Mitigating salt stress effects by exogenous application of proline and yeast extract on morphophysiological, biochemical and anatomical characters of calendula plants. Sci. J. Flowers Ornam. Plants 2020, 7, 461–482. [Google Scholar] [CrossRef]
- Hafez, Y.; Elkohby, W.; Mazrou, Y.S.A.; Ghazy, M.; Elgamal, A.; Abdelaal, K.A.A. Alleviating the detrimental impacts of salt stress on morpho-physiological and yield characters of rice plants (Oryza sativa L.) using actosol, Nano-Zn and Nano-Si. Fresenius Environ. Bull. 2020, 29, 6882–6897. [Google Scholar]
- El Sabagh, A.; Hossain, A.; Barutcular, C.; Islam, M.S.; Awan, S.I.; Galal, A.; Iqbal, A.; Sytar, O.; Yildirim, M.; Meena, R.S.; et al. Wheat (Triticum aestivum L.) production under drought and heat stress-adverse effects, mechanisms and mitigation: A review. Appl. Ecol. Environ. Res. 2019, 17, 8307–8332. [Google Scholar] [CrossRef]
- Elkelish, A.; Qari, S.H.; Mazrou, Y.M.; Abdelaal, K.A.A.; Hafez, Y.M.; Abu-Elsaoud, A.M.; Batiha, G.; El-Esawi, M.; El Nahhas, N. Exogenous Ascorbic Acid Induced Chilling Tolerance in Tomato Plants Through Modulating Metabolism, Osmolytes, Antioxidants, and Transcriptional Regulation of Catalase and Heat Shock Proteins. Plants 2020, 9, 431. [Google Scholar] [CrossRef] [Green Version]
- Abdelaal, K.A.A.; Hafez, Y.M.; El-Afry, M.M.; Tantawy, D.S.; Alshaal, T. Effect of some osmoregulators on photosynthesis, lipid peroxidation, antioxidative capacity and productivity of barley (Hordeum vulgare L.) under water deficit stress. Environ. Sci. Pollut. Res. 2018, 25, 30199–30211. [Google Scholar] [CrossRef] [PubMed]
- Abdelaal, K.A.A.; Attia, K.A.; Alamery, S.F.; El-Afry, M.M.; Ghazy, A.I.; Tantawy, D.S.; Al-Doss, A.A.; El-Shawy, E.-S.E.; Abu-Elsaoud, A.M.; Hafez, Y.M. Exogenous Application of Proline and Salicylic Acid can Mitigate the Injurious Impacts of Drought Stress on Barley Plants Associated with Physiological and Histological Characters. Sustainability 2020, 12, 1736. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Gupta, A.; Kaur, N. Differential Responses of antioxidative defense system to long-term field drought in wheat (Triticum aestivum L.) genotypes differing in drought tolerance. J. Agron. Crop. Sci. 2012, 198, 185–195. [Google Scholar] [CrossRef]
- EL Sabagh, A.; Hossain, A.; Barutçular, C.; Abdelaal, K.A.A.; Fahad, S.; Anjorin, F.B.; Islam, M.S.; Ratnasekera, D.; Kizilgeçi, F.; Yadav, S.; et al. Sustainable maize (Zea mays L.) production under drought stress by understanding its adverse effect, Survival mechanism and drought tolerance indices. J. Exp. Biol. Agric. Sci. 2018, 6, 282–295. [Google Scholar] [CrossRef]
- Abdelaal, K.A.A.; Hafez, Y.M.; El Sabagh, A.; Saneoka, H. Ameliorative effects of Abscisic acid and yeast on morpho-physiological and yield characteristics of maize plant (Zea mays L.) under water deficit conditions. Fresenius Environ. Bull. 2017, 26, 7372–7383. [Google Scholar]
- Omar, A.; Zayed, B.; Abdel Salam, A.; Hafez, Y.M.; Abdelaal, K.A.A. Folic acid as foliar application can improve growth and yield characters of rice plants under irrigation with drainage water. Fresenius Environ. Bull. 2020, 29, 9420–9428. [Google Scholar]
- AlKahtani, M.D.F.; Hafez, Y.M.; Attia, K.; Rashwan, E.; Husnain, L.A.; AlGwaiz, H.I.M.; Abdelaal, K.A.A. Evaluation of Silicon and Proline Application on the Oxidative Machinery in Drought-Stressed Sugar Beet. Antioxidants 2021, 10, 398. [Google Scholar] [CrossRef]
- Abdelaal, K.A.A. Effect of Salicylic acid and Abscisic acid on morpho-physiological and anatomical characters of faba bean plants (Vicia faba L.) under drought stress. J. Plant Prod. 2015, 6, 1771–1788. [Google Scholar] [CrossRef] [Green Version]
- El-Banna, M.F.; Abdelaal, K.A.A. Response of Strawberry Plants Grown in the Hydroponic System to Pretreatment with H2O2 Before Exposure to Salinity Stress. J. Plant Prod. 2018, 9, 989–1001. [Google Scholar] [CrossRef]
- Helaly, M.N.; Mohammed, Z.; El-Shaeery, N.I.; Abdelaal, K.A.A.; Nofal, I.E. Cucumber grafting onto pumpkin can represent an interesting tool to minimize salinity stress. Physiological and anatomical studies. Middle East J. Agric. Res. 2017, 6, 953–975. [Google Scholar]
- Hasan, M.K.; El Sabagh, A.; Sikdar, M.S.I.; Alam, M.J.; Ratnasekera, D.; Barutcular, C.; Abdelaal, K.A.A.; Islam, M.S. Comparative adaptable agronomic traits of Blackgram and mungbean for saline lands. Plant Arch. 2017, 17, 589–593. [Google Scholar]
- He, M.; He, C.Q.; Ding, N.Z. Abiotic stresses: General defenses of land plants and chances for engineering multi stress tolerance. Front. Plant Sci. 2018, 9, 1771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hafez, Y.; Mazrou, Y.; Shahin, A.; Nehiar, F.; Eid, M.; Abdelaal, K.A. Yield losses in wheat genotypes caused by stripe rust (Puccinia striifarmis f. sp. tritici) in North Delta, Egypt. Not. Bot. Horti Agrobot. Cluj-Napoca 2022, 50, 12622. [Google Scholar] [CrossRef]
- Hafez, Y.; Ali, G.; Shahin, A.; Badr, M.; Esmaeil, R.; Abdelaal, K.A. Screening of resistance sources to stem rust (Ug99) in international wheat genotypes (CIMMYT)I Egypt. Fresenius Environ. Bull. 2022, 31, 5940–5948. [Google Scholar]
- Shahein, A.M.E.A.; Mazrou, Y.; El-Borhamy, A.M.A.; Abdelaal, K.A.A.; EL Hag, D.A.A. Evaluation of some flax genotypes (Linum ustatissimum L.) for seed yield and quality. Fresenius Environ. Bull. 2021, 30, 9417–9425. [Google Scholar]
- Essawy, M.M.; Keratum, A.Y.; Abdallah, F.; Mohamed, H.M.; Mazrou, Y.; Hafez, Y.; Abdelaal, K.A.A. Susceptibility of some faba bean varieties to infestation with the main insect pests associated with physiological, biochemical and yield characters. Fresenius Environ. Bull. 2020, 29, 6147–6158. [Google Scholar]
- El-Tokhy, A.I.A.; Ali, M.A.M.; Hafez, Y.M.; Abdelaal, K.A.A. Impacts of different insecticides on Tuta absoluta (Meyrick) larvae with their effects on physiological characteristics and fruits yield of stressed tomato plants. J. Plant Prot. Pathol. 2020, 11, 269274. [Google Scholar]
- Abdelaal, K.A.A.; Essawy, M.; Quraytam, A.; Abdallah, F.; Mostafa, H.; Shoueir, K.; Fouad, H.; Fahmy, A.S.H.; Hafez, Y.M. Toxicity of Essential Oils Nanoemulsion against Aphis Craccivora and Their Inhibitory Activity on Insect Enzymes. Processes 2021, 9, 624. [Google Scholar] [CrossRef]
- Taha, Y.N.; El-Zahi, S.E.; Keratum, A.Y.; Ismail, T.; Abdelaal, K.A.A.; Hafez, Y.M. Comparative efficacy of salicylic acid and some systemic insecticides against Bemisia tabaci (Genn.), and their impact on cotton defense response. Fresenius Environ. Bull. 2020, 29, 6707–6716. [Google Scholar]
- Elsakhawy, T.; ALKahtani, M.D.F.; Sharshar, A.A.H.; Attia, K.A.; Hafez, Y.M.; Abdelaal, K.A.A. Efficacy of Mushroom Metabolites (Pleurotus ostreatus) as A Natural Product for the Suppression of Broomrape Growth (Orobanche crenata Forsk) in Faba Bean Plants. Plants 2020, 9, 1265. [Google Scholar] [CrossRef] [PubMed]
- Abdelaal, K.A.A.; El Menofy, E.; Nessem, A.; Elhaak, M. The allelopathy potential and glyphosate influence on anatomical features of Egyptian clover plant (Trifolium alexandrinum L.) infested with dodder weed (Cuscuta campestris L.). Fresenius Environ. Bull. 2019, 28, 1262–1269. [Google Scholar]
- El-Sayed, A.A.; Khaffagy, A.E.; Shaheen, F.E.M.; Hafez, Y.; Abdelaal, K.A.A.; Hassan, F.; Elhag, D.A. Comparative efficiency of new herbicides for weed control on quality, yield and its component in maize (Zea mays). Fresenius Environ. Bull. 2021, 30, 5340–5349. [Google Scholar]
- EL-Sayed, A.A.; Mazrou, Y.; Khaffagy, A.E.; Shaheen, F.E.M.; Hafez, Y.; Abdelaal, K.A.A.; EL-Hag, D.A.A. Impacts of herbicides and some growth characters of maize and associated weeds. Fresenius Environ. Bull. 2021, 30, 9380–9388. [Google Scholar]
- Khaffagy, A.E.; Mazrou, Y.S.A.; Morsy, A.R.; El-Mansoury, M.A.M.; El-Tokhy, A.I.; Hafez, Y.; Abdelaal, K.; Khedr, R.A. Impact of Irrigation Levels and Weed Control Treatments on Annual Weeds, Physiological Traits and Productivity of Soybean under Clay Soil Conditions. Agronomy 2022, 12, 1037. [Google Scholar] [CrossRef]
- Oerke, E.-C. Crop losses to pests. J. Agric. Sci. 2006, 144, 31–43. [Google Scholar] [CrossRef]
- Anonymous. Introduction to Weeds Herbicides; Pennsylvania State Extension; The Pennsylvania State University: State College, PA, USA, 2007. [Google Scholar]
- Zhang, Z.; Liu, Y.; Yuan, L.; Weber, E.; van Kleunen, M. Effect of allelopathy on plant performance. bioRxiv Prepr. 2020. [Google Scholar] [CrossRef]
- Andrews, J.; Sanders, Z.; Cabrera, M.; Hill, N.; Radcliffe, D. Simulated nitrate leaching in annually cover cropped and perennial living mulch corn production systems. J. Soil Water Conserv. 2020, 75, 91–102. [Google Scholar] [CrossRef] [Green Version]
- Bhuyan, M.B.; Hasanuzzaman, M.; Parvin, K.; Mohsin, S.M.; Al Mahmud, J.; Nahar, K.; Fujita, M. Nitric oxide and hydrogen sulfide: Two intimate collaborators regulating plant defense against abiotic stress. Plant Growth Regul. 2020, 90, 409–424. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, J.; Gong, Z.; Zhu, J.K. Abiotic stress responses in plants. Nat. Rev. Genet. 2022, 23, 104–119. [Google Scholar] [CrossRef]
- Galicia-Campos, E.; García-Villaraco Velasco, A.; Montero-Palmero, M.B.; Gutiérrez-Mañero, F.J.; Ramos-Solano, B. Modulation of Photosynthesis and ROS Scavenging Response by Beneficial Bacteria in Olea europaea Plantlets under Salt Stress Conditions. Plants 2022, 11, 2748. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Mur, L.A.J.; Wang, Q.; Hou, X.; Zhao, C.; Chen, Z.; Wu, J.; Guo, Q. ROS scavenging and ion homeostasis is required for the adaptation of halophyte Karelinia caspia to high salinity. Front. Plant Sci. 2022, 13, 979956. [Google Scholar] [CrossRef] [PubMed]
- Saad-Allah, K.M.; Nessem, A.A.; Ebrahim, M.K.; Gad, D. Evaluation of drought tolerance of five maize genotypes by virtue of physiological and molecular responses. Agronomy 2021, 12, 59. [Google Scholar] [CrossRef]
- Moghanm, F.S.; El-Banna, M.; El-Esawi, M.A.; Abdel-Daim, M.; Mosa, A.; Abdelaal, K.A.A. Genotoxic and Anatomical Deteriorations Associated with Potentially Toxic Elements Accumulation in Water Hyacinth Grown in Drainage Water Resources. Sustainability 2020, 12, 2147. [Google Scholar] [CrossRef] [Green Version]
- Abdelaal, K.A.A.; EL-Shawy, E.A.; Hafez, Y.M.; Abdel-Dayem, S.M.; Chidya, R.C.G.; Saneoka, H.; El Sabagh, A. Nano-Silver and non-traditional compounds mitigate the adverse effects of net blotch disease of barley in correlation with up-regulation of antioxidant enzymes. Pak. J. Bot. 2020, 52, 1065–1072. [Google Scholar] [CrossRef]
- El-Nashaar, F.; Hafez, Y.M.; Abdelaal, K.A.A.; Abdelfatah, A.; Badr, M.; El-Kady, S.; Yousef, A. Assessment of host reaction and yield losses of commercial barley cultivars to Drechslera teres the causal agent of net blotch disease in Egypt. Fresenius Environ. Bull. 2020, 29, 2371–2377. [Google Scholar]
- Mittler, R. ROS Are Good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef]
- Miller, G.; Suzuki, N.; Ciftci-Yilmaz, S.; Mittler, R. Reactive oxygen species homeostasis and signaling during drought and salinity stresses. Plant Cell Environ. 2010, 33, 453–467. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Al Mahmud, J.; Fujita, M.; Fotopoulos, V. Reactive Oxygen Species and Antioxidant Defense in Plants under Abiotic Stress: Revisiting the Crucial Role of a Universal Defense Regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef]
- Ahmad, A.; Aslam, Z.; Javed, T.; Hussain, S.; Raza, A.; Shabbir, R.; Tauseef, M. Screening of wheat (Triticum aestivum L.) genotypes for drought tolerance through agronomic and physiological response. Agronomy 2022, 12, 287. [Google Scholar] [CrossRef]
- Shetty, N.P.; Jorgensen, H.J.L.; Jensen, J.D.; Collinge, D.B.; Shetty, H.S. Roles of reactive oxygen species in interactions between plants and pathogens. Eur. J. Plant Pathol. 2008, 121, 267–280. [Google Scholar] [CrossRef]
- Omara, R.I.; Abdelaal, K.A.A. Biochemical, histopathological and genetic analysis associated with leaf rust infection in wheat plants (Triticum aestivum L.). Physiol. Mol. Plant Pathol. 2018, 104, 48–57. [Google Scholar] [CrossRef]
- Omara, R.I.; El-Kot, G.; Fadel, F.M.; Abdelaal, K.A.A.; Saleh, E. Efficacy of certain bioagents on patho-physiological characters of wheat plants under wheat leaf rust stress. Physiol. Mol. Plant Pathol. 2019, 106, 102–108. [Google Scholar] [CrossRef]
- Esmail, S.M.; Omara, R.I.; Abdelaal, K.A.A.; Hafez, Y.M. Histological and biochemical aspects of compatible and incompatible wheat-Puccinia striiformis interactions. Physiol. Mol. Plant Pathol. 2019, 106, 120–128. [Google Scholar] [CrossRef]
- Abdelaal, K.A.A.; Rashed, S.H.; Ragab, A.; Hossian, A.; El Sabagh, A. Yield and quality of two sugar beet (Beta vulgaris L. ssp. vulgaris var. altissima Doll) cultivars are influenced by foliar application of salicylic Acid, irrigation timing, and planting density. Acta Agric. Slov. 2020, 115, 239–248. [Google Scholar] [CrossRef]
- Hafez, Y.M.; Attia, K.A.; Kamel, S.; Alamery, S.; El-Gendy, S.; Al-Dosse, A.; Mehiar, F.; Ghazy, A.; Abdelaal, K.A.A. Bacillus subtilis as a bio-agent combined with nano molecules can control powdery mildew disease through histochemical and physiobiochemical changes in cucumber plants. Physiol. Mol. Plant Pathol. 2020, 111, 101489. [Google Scholar] [CrossRef]
- Mittler, R.; Vanderauwera, S.; Gollery, M.; Van Breusegem, F. Reactive oxygen gene network of plants. Trends Plant Sci. 2004, 9, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Davidson, J.F.; Schiestl, R.H. Mitochondrial respiratory electron carriers are involved in oxidative stress during heat stress in Saccharomyces cerevisiae. Mol. Cell Biol. 2001, 21, 8483–8489. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Serrano, M.; Romero-Puertas, M.C.; Pazmino, D.M.; Testillano, P.S.; Risueno, M.C.; del Rio, L.A.; Sandalio, L.M. Cellular response of pea plants to cadmium toxicity: Cross talk between reactive oxygen species, nitric oxide, and calcium. Plant Physiol. 2009, 150, 229–243. [Google Scholar] [CrossRef] [Green Version]
- Bailly, C.; El-Maarouf-Bouteau, H.; Corbineau, F. From intracellular signaling networks to cell death: The dual role of reactive oxygen species in seed physiology. C. R. Biologies 2008, 331, 806–814. [Google Scholar] [CrossRef]
- McDonald, M.B. Seed deterioration: Physiology, repair and assessment. Seed Sci. Technol. 1990, 27, 177–237. [Google Scholar]
- Sun, K.W.; Leopold, C.A. The Maillard reaction and oxidative stress during aging of soybean seeds. Physiol. Plant. 1995, 94, 94–104. [Google Scholar] [CrossRef]
- Bailly, C. Active oxygen species and antioxidants in seed biology. Seed Sci. Res. 2004, 14, 93–107. [Google Scholar] [CrossRef]
- Noctor, G.; De Paepe, R.; Foyer, C.H. Mitochondrial redox biology and homeostasis in plants. Trends Plant. Sci. 2007, 12, 125–134. [Google Scholar] [CrossRef] [PubMed]
- El-Maarouf-Bouteau, H.; Bailly, C. Oxidative signaling in seed germination and dormancy. Plant Signal. Behav. 2008, 3, 175–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pergo, E.M.; Ishii-Iwamoto, E.L. Changes in energy metabolism and antioxidant defense systems during seed germination of the weed species Ipomoea triloba L. and the responses to allelochemicals. J. Chem. Ecol. 2011, 37, 500–513. [Google Scholar] [CrossRef] [PubMed]
- Rhoads, D.M.; Umbach, A.L.; Subbaiah, C.C.; Siedow, J.N. Mitochondrial reactive oxygen species. Contribution to oxidative stress and interorganellar signaling. Plant Physiol. 2006, 141, 357–366. [Google Scholar] [CrossRef] [Green Version]
- Norman, C.; Howell, K.A.; Millar, A.H.; Whelan, J.M.; Day, D.A. Salicylic acid is an uncoupler and inhibitor of mitochondrial electron transport. Plant Physiol. 2004, 134, 492–501. [Google Scholar] [CrossRef]
- Atkin, O.K.; Macherel, D. The crucial role of plant mitochondria in orchestrating drought tolerance. Ann. Bot. 2009, 103, 581–597. [Google Scholar] [CrossRef] [Green Version]
- Foyer, C.H.; Noctor, G. Redox homeostasis and antioxidant signaling: A metabolic interface between stress perception and physiological responses. Plant Cell 2005, 17, 1866–1875. [Google Scholar] [CrossRef] [Green Version]
- Asada, K. Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol. 2006, 141, 391–396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sano, S.; Tao, S.; Endo, Y.; Inaba, T.; Hossain, M.A.; Miyake, C.; Matsuo, M.; Aoki, H.; Asada, K.; Saito, K. Purification and cDNA cloning of chloroplastic monodehydroascorbate reductase from spinach. Biosci. Biotechnol. Biochem. 2005, 69, 762–772. [Google Scholar] [CrossRef] [PubMed]
- Dietz, K.J.; Jacob, S.; Oelze, M.L.; Laxa, M.; Tognetti, V.; De Miranda, S.M.; Baier, M.; Finkemeier, I. The function of peroxiredoxins in plant organelle redox metabolism. J. Exp. Bot. 2006, 57, 1697–1709. [Google Scholar] [CrossRef]
- Miller, G.; Suzuki, N.; Rizhsky, L.; Hegie, A.; Koussevitzky, S.; Mittler, R. Double mutants deficient in cytosolic and thylakoid ascorbate peroxidase reveal a complex mode of interaction between reactive oxygen species, plant development, and response to abiotic stresses. Plant Physiol. 2007, 144, 1777–1785. [Google Scholar] [CrossRef] [Green Version]
- Triantaphylides, C.; Krischke, M.; Hoeberichts, F.A.; Ksas, B.; Gresser, G.; Havaux, M.; Van Breusegem, F.; Mueller, M.J. Singlet oxygen is the major reactive oxygen species involved in photooxidative damage to plants. Plant Physiol. 2008, 148, 960–968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tseng, M.J.; Liu, C.W.; Yiu, J.C. Enhanced tolerance to sulfur dioxide and salt stress of transgenic Chinese cabbage plants expressing both superoxide dismutase and catalase in chloroplasts. Plant Physiol. Biochem. 2007, 45, 822–833. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Noctor, G.; Veljovic-Jovanovic, S.; Driscoll, S.; Novitskaya, L.; Foyer, C.H. Drought and oxidative load in the leaves of C3 plants: A predominant role for photorespiration? Ann. Bot. 2002, 89, 841–850. [Google Scholar] [CrossRef]
- Del Río, L.A.; Palma, J.M.; Sandalio, L.M.; Corpas, F.J.; Pastori, G.M.; Bueno, P.; Lopez-Huertas, E. Peroxisomes as a source of superoxide and hydrogen peroxide in stressed plants. Biochem. Soc. Trans. 1996, 24, 434–438. [Google Scholar] [CrossRef] [Green Version]
- Mittova, V.; Tal, M.; Volokita, M.; Guy, M. Up-regulation of the leaf mitochondrial and peroxisomal antioxidative systems in response to salt-induced oxidative stress in the wild salt-tolerant tomato species Lycopersicon pennellii. Plant Cell Environ. 2003, 26, 845–856. [Google Scholar] [CrossRef]
- Jubany-Marí, T.; Munné-Bosch, S.; Lopez-Carbonell, M.; Alegre, L. Hydrogen peroxide is involved in the acclimation of the Mediterranean shrub, Cistus albidus L., to summer drought. J. Exp. Bot. 2009, 60, 107–120. [Google Scholar] [CrossRef] [Green Version]
- Moschou, P.N.; Paschalidis, K.A.; Delis, I.D.; Andriopoulou, A.H.; Lagiotis, G.D.; Yakoumakis, D.I.; Roubelakis-Angelakis, K.A. Spermidine exodus and oxidation in the apoplast induced by abiotic stress is responsible for H2O2 signatures that direct tolerance responses in tobacco. Plant Cell 2008, 20, 1708–1724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernandez, J.A.; Ferrer, M.A.; Jiménez, A.; Barcelo, A.R.; Sevilla, F. Antioxidant systems and O2-/H2O2 production in the apoplast of pea leaves. Its relation with salt-induced necrotic lesions in minor veins. Plant Physiol. 2001, 127, 817–831. [Google Scholar] [CrossRef] [PubMed]
- Noctor, G.; Reichheld, J.P.; Foyer, C.H. ROS-related redox regulation and signaling in plants. Semin. Cell Dev. Biol. 2018, 80, 3–12. [Google Scholar] [CrossRef] [Green Version]
- Formentin, E.; Barizza, E.; Stevanato, P.; Falda, M.; Massa, F.; Tarkowskà, D.; Novák, O.; Lo Schiavo, F. Fast Regulation of Hormone Metabolism Contributes to Salt Tolerance in Rice (Oryza sativa spp. Japonica, L.) by Inducing Specific Morpho-Physiological Responses. Plants 2018, 7, 75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pappalardo, H.; Genovese, C.; Puglia, G.D.; Leonardi, C.; Toscano, V.; Raccuia, S.A. Principal mechanism of tolerance to abiotic stresses in Cynara cardunculus L. Acta Hortic. 2020, 1284, 109–116. [Google Scholar] [CrossRef]
- Pappalardo, H.; Genovese, C.; Puglia, G.D.; Martines, L. Influence of abiotic stress on phenolic composition in Cynara cardunculus (L.) var. sylvestris. Acta Hortic. 2020, 1284, 263–270. [Google Scholar] [CrossRef]
- Su, T.; Wang, P.; Li, H.; Zhao, Y.; Lu, Y.; Dai, P.; Ren, T.; Wang, X.; Li, X.; Shao, Q.; et al. The Arabidopsis catalase triple mutant reveals important roles of catalases and peroxisome-derived signaling in plant development. J. Integr. Plant Biol. 2018, 60, 591–607. [Google Scholar] [CrossRef]
- Anjum, N.A.; Sharma, P.; Gill, S.S.; Hasanuzzaman, M.; Khan, E.A.; Kachhap, K.; Mohamed, A.A.; Thangavel, P.; Devi, G.D.; Vasudhevan, P.; et al. Catalase and ascorbate peroxidase—representative H2O2-detoxifying heme enzymes in plants. Environ. Sci. Pollut. Res. 2016, 23, 19002–19029. [Google Scholar] [CrossRef]
- Bartoli, C.G.; Buet, A.; Grozeff, G.G.; Galatro, A.; Simontacchi, M. Ascorbate-glutathione cycle and abiotic stress tolerance in plants. In Ascorbic Acid in Plant Growth, Development and Stress Tolerance; Hossain, M.A., Munne-Bosch, S., Burritt, D.J., Diaz-Vivancos, P., Fujita, M., Lorence, A., Eds.; Springer: Cham, Switzerland, 2017; pp. 177–200. [Google Scholar]
- Rao, A.C.; Reddy, A.R. Glutathione reductase: A putative redox regulatory system in plant cells. In Sulfur Assimilation and Abiotic Stress in Plants; Khan, N.A., Singh, S., Umar, S., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 111–147. [Google Scholar]
- Zandalinas, S.I.; Balfagón, D.; Arbona, V.; Gomez-Cadenas, A. Modulation of antioxidant defense system is associated with combined drought and heat stress tolerance in citrus. Front. Plant Sci. 2017, 8, 953. [Google Scholar] [CrossRef] [Green Version]
- Najafi, S.; Nasi, H.N.; Tuncturk, R.; Tuncturk, M.; Sayyed, R.Z.; Amirnia, R. Biofertilizer Application Enhances Drought Stress Tolerance and Alters the Antioxidant Enzymes in Medicinal Pumpkin (Cucurbita pepo convar. pepo var. Styriaca). Horticulturae 2021, 7, 588. [Google Scholar] [CrossRef]
- Zafra, A.; Castro, A.J.; de Dios Alche, J. Identification of novel superoxide dismutase isoenzymes in the olive (Olea europaea L.) pollen. BMC Plant Biol. 2018, 18, 114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hofmann, B.; Hecht, H.J.; Flohé, L. Peroxiredoxins. Biol Chem. 2002, 383, 347–364. [Google Scholar] [CrossRef]
- Kohli, S.K.; Khanna, K.; Bhardwaj, R.; Abd_Allah, E.F.; Ahmad, P.; Corpas, F.J. Assessment of subcellular ROS and NO metabolism in higher plants: Multifunctional signaling molecules. Antioxidants 2019, 8, 641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dietz, K.J. Peroxiredoxins in plants and cyanobacteria. Antioxid. Redox Sign. 2011, 15, 1129–1159. [Google Scholar] [CrossRef] [Green Version]
- Noguera-Mazon, V.; Krimm, I.; Walker, O.; Lancelin, J.M. Protein-protein interactions within peroxiredoxin systems. Photosynth Res. 2006, 89, 277–290. [Google Scholar] [CrossRef] [PubMed]
- Pena-Ahumada, A.; Kahmann, U.; Dietz, K.J.; Baier, M. Regulation of peroxiredoxin expression versus expression of Halliwell-Asada-Cycle enzymes during early seedling development of Arabidopsis thaliana. Photosynth Res. 2006, 89, 99–112. [Google Scholar] [CrossRef]
- Noctor, G.; Foyer, C.H. Christine H Foyer Ascorbate and Glutathione: Keeping Active Oxygen Under Control. Annu. Rev. Plant Biol. 1998, 49, 249–279. [Google Scholar] [CrossRef]
- Boo, Y.C.; Jung, J. Water Deficit—Induced Oxidative Stress and Antioxidative Defenses in Rice Plants. J. Plant Physiol. 1999, 155, 255–261. [Google Scholar]
- Hafez, Y.M.; Mourad, R.Y.; Nasr, E.-B.; Kotb, A.; Abdelaal, K.A.A.; Ghazy, A.I.; Al-Ateeq, T.K.; Ibrahim, E.I.; Mohammed, A.A. Biochemical and Molecular Characterization of Non-host resistance Keys in food Crops. Saudi J. Biol. Sci. 2020, 27, 1091–1099. [Google Scholar] [CrossRef]
- Upadhyaya, H.; Panda, S.K.; Dutta, B.K. Variation of physiological and antioxidative responses in tea cultivars subjected to elevated water stress followed by rehydration recovery. Acta Physiol. Plant 2008, 30, 457–468. [Google Scholar] [CrossRef]
- AlKahtani, M.D.F.; Hafez, Y.M.; Attia, K.; Al-Ateeq, T.; Ali, M.A.M.; Hasanuzzaman, M.; Abdelaal, K.A.A. Bacillus thuringiensis and Silicon Modulate Antioxidant Metabolism and Improve the Physiological Traits to Confer Salt Tolerance in Lettuce. Plants 2021, 10, 1025. [Google Scholar] [CrossRef] [PubMed]
- Vital, S.A.; Fowler, R.W.; Virgen, A.; Gossett, D.R.; Banks, S.W.; Rodriguez, J. Opposing roles for superoxide and nitric oxide in the NaCl stress-induced upregulation of antioxidant enzyme activity in cotton callus tissue. Environ. Exp. Bot. 2008, 62, 60–68. [Google Scholar] [CrossRef]
- Shikanai, T.; Takeda, T.; Yamauchi, H.; Sano, S.; Tomizawa, K.I.; Yokota, A.; Shigeoka, S. Inhibition of ascorbate peroxidase under oxidative stress in tobacco having bacterial catalase in chloroplasts. FEBS Lett. 1998, 428, 47–51. [Google Scholar] [CrossRef] [Green Version]
- Nahar, K.; Hasanuzzaman, M.; Alam, M. Glutathione-induced drought stress tolerance in mung bean: Coordinated roles of 962 the antioxidant defense and methylglyoxal detoxification systems. AoB Plants 2015, 7, 69–75. [Google Scholar] [CrossRef] [Green Version]
- Horemans, N.; Foyer, C.H.; Asard, H. Transport and action of ascorbate at the plant plasma membrane. Trends Plant Sci. 2000, 5, 263–267. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Oxidant and antioxidant signaling in plants: A reevaluation of the concept of oxidative stress in a physiological context. Plant Cell Environ. 2005, 28, 1056–1071. [Google Scholar] [CrossRef]
- Shalata, A.; Neumann, P.M. Exogenous ascorbic acid (vitamin C) increases resistance to salt stress and reduces lipid peroxidation. J. Exp. Bot. 2001, 52, 2207–2211. [Google Scholar] [CrossRef]
- Jahns, P.; Holzwarth, A.R. The role of the xanthophyll cycle and of lutein in photoprotection of photosystem II. Biochim. Biophys. Acta 2012, 1817, 182–193. [Google Scholar] [CrossRef]
- Johnson, M.; Havaux, M.; Triantaphylides, C.; Ksas, B.; Pascal, A.A.; Robert, B.; Davison, P.A.; Ruban, A.V.; Horton, P. Elevated zeaxanthin bound to oligomeric LHCII enhances the resistance of Arabidopsis to photooxidative stress by a lipid-protective, antioxidant mechanism. J. Biol. Chem. 2007, 282, 22605–22618. [Google Scholar] [CrossRef] [Green Version]
- Michel, H. Carotenoid oxidation products as stress signals in plant. Plant J. 2013, 79, 597–606. [Google Scholar]
- Michaeli, S.; Fromm, H. Closing the loop on the GABA shunt in plants: Are GABA metabolism and signaling entwined? Front. Plant Sci. 2015, 6, 419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munns, R. Comparative physiology of salt and water stress. Plant Cell Environ. 2002, 25, 239–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, Y.-G.; Tominaga, T.; Iijima, Y.; Aoki, K.; Shibata, D.; Ashihara, H.; Nishimura, S.; Ezura, H.; Matsukura, C. Metabolic Alterations in Organic Acids and γ-Aminobutyric Acid in Developing Tomato (Solanum lycopersicum L.) Fruits. Plant Cell Physiol. 2010, 51, 1300–1314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mei, X.; Chen, Y.; Zhang, L.; Fu, X.; Wei, Q.; Grierson, D.; Zhou, Y.; Huang, Y.; Dong, F.; Yang, Z. Dual mechanisms regulating glutamate decarboxylases and accumulation of gamma-aminobutyric acid in tea (Camellia sinensis) leaves exposed to multiple stresses. Sci. Rep. 2016, 6, 23685. [Google Scholar] [CrossRef] [Green Version]
- Akçay, N.; Bor, M.; Karabudak, T.; Özdemir, F.; Türkan, I. Contribution of gamma-amino butyric acid to salt stress responses of Nicotiana sylvestris CMSII mutant and wild type plants. J. Plant Physiol. 2012, 169, 452–458.13. [Google Scholar] [CrossRef]
- Bao, H.; Chen, X.; Lv, S.; Jiang, P.; Feng, J.; Fan, P. Virus-induced gene silencing greveals control of reactive oxygen species accumulation and salt tolerance in tomato by γ-aminobutyric acid metabolic pathway. Plant Cell Environ. 2015, 38, 13. [Google Scholar] [CrossRef]
- Hu, X.; Xu, Z.; Xu, W.; Li, J.; Zhao, N.; Zhou, Y. Application of γ-aminobutyric acid demonstrates a protective role of polyamine and GABA metabolism in muskmelon seedlings under Ca(NO3)2 stress. Plant Physiol. Biochem. 2015, 92, 1–10. [Google Scholar] [CrossRef]
- Li, J.; Tian, Z.; Wu, X.; Lv, G.; Ma, W.; Zhang, Y.; Gao, H. Gamma-Aminobutyric Acid (GABA) Modulates Nitrate Concentrations and Metabolism in the Leaves of Pakchoi (Brassica campestris ssp. chinensis Makino) Treated with a Nitrogen-Rich Solution. Plant Mol. Biol. Rep. 2018, 36, 530–542. [Google Scholar] [CrossRef]
- Vijayakumari, K.; Puthur, J.T. γ-Aminobutyric acid priming enhances the osmotic stress tolerance in Piper nigrum L. plants subjected to PEG-induced stress. Plant Growth Regul. 2016, 78, 57–67. [Google Scholar] [CrossRef]
- Takayama, M.; Koike, S.; Kusano, M.; Matsukura, C.; Saito, K.; Ariizumi, T.; Ezura, H. Tomato glutamate decarboxylase genesSlGAD2andSlGAD3 play key roles in regulating γ-aminobutyric acid levels in tomato (Solanum lycopersicum). Plant Cell Physiol. 2015, 56, 1533–1545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srivalli, B.; Chinnusamy, V.; Khanna-Chopra, R. Antioxidant defense in response to abiotic stresses in plants. J. Plant Biol. 2003, 30, 121–139. [Google Scholar]
- Halliwell, B. Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant Physiol. 2006, 141, 312–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.S.; Tang, K.X. MAP Kinase cascades responding to environmental stress in plants. Acta Bot. Sin. 2004, 46, 127–136. [Google Scholar]
- Igamberdiev, A.U.; Hill, R.D. Nitrate, NO and haemoglobin in plant adaptation to hypoxia: An alternative to classic fermentation pathways. J. Exp. Bot. 2004, 55, 2473–2482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noctor, G. Metabolic signaling in defense and stress: The central roles of soluble redox couples. Plant Cell Environ. 2006, 29, 409–425. [Google Scholar] [CrossRef] [PubMed]
- Shao, H.B.; Chu, L.Y.; Wu, G.; Zhang, J.H.; Lu, Z.H.; Hu, Y.C. Changes of some anti-oxidative physiological indices under soil water deficits among 10 wheat (Triticum aestivum) genotypes at tillering stage. Biointerfaces 2007, 59, 113–119. [Google Scholar] [CrossRef]
- Pourcel, L.; Routaboul, J.M.; Cheynier, V.; Lepiniec, L.; Debeaujon, I. Flavonoid oxidation in plants: From biochemical properties to physiological functions. Trends Plant Sci. 2007, 12, 29–36. [Google Scholar] [CrossRef]
- Abbasi, A.R.; Hajirezaei, M.; Hofius, D.; Sonnewald, U.; Voll, L.M. Specific roles of alpha- and gamma-tocopherol in abiotic stress responses of transgenic tobacco. Plant Physiol. 2007, 143, 1720–1738. [Google Scholar] [CrossRef] [Green Version]
- Havaux, M.; Eymery, F.; Porfirova, S.; Rey, P.; Dörmann, P. Vitamin E protects against photoinhibition and photooxidative stress in Arabidopsis thaliana. Plant Cell 2005, 17, 3451–3469. [Google Scholar] [CrossRef] [Green Version]
- Millar, A.H.; Mittova, V.; Kiddle, G.; Heazlewood, J.L.; Bartoli, C.G.; Theodoulou, F.L.; Foyer, C.H. Control of ascorbate synthesis by respiration and its implications for stress responses. Plant Physiol. 2003, 133, 443–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.P.; Li, Y.L.; Zhang, J.G. Effect of high temperature and excessive-light stress on APX activity in apple peel. Acta Agric. Boreali Sin. 2008, 23, 144–147. [Google Scholar]
- Halliwell, B. Oxidative stress and neurodegeneration: Where are we now? J. Neurochem. 2006, 97, 1634–1658. [Google Scholar] [CrossRef] [PubMed]
- Pietrini, F.; Iannelli, M.A.; Pasqualini, S.; Massacci, A. Interaction of cadmium with glutathione and photosynthesis in developing leaves and chloroplasts of Phragmites australis (Cav.) Trin. Ex Steudel. Plant Physiol. 2003, 133, 829–837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katerova, Z.; Miteva, L. Glutathione and herbicide resistance in plants. In Ascorbate Glutathione Pathway and Stress Tolerance in Plants; Chan, M.T., Umar, S., Anjum, N.A., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 191–207. [Google Scholar]
- Arafa, S.A.; Attia, K.A.; Niedbała, G.; Piekutowska, M.; Alamery, S.; Abdelaal, K.; Alateeq, T.K.; Ali, M.A.M.; Elkelish, A.; Attallah, S.Y. Seed Priming Boost Adaptation in Pea Plants under Drought Stress. Plants 2021, 10, 2201. [Google Scholar] [CrossRef] [PubMed]
- Sgherri, C.; Navari-Izzo, F. Sunflower seedlings subjected to increasing water deficit stress: Oxidative stress and defense mechanisms. Physiol. Plant. 1995, 93, 25–30. [Google Scholar] [CrossRef]
- Jain, M.; Choudhary, D.; Kale, R.K.; Bhalla-Sarin, N. Salt and glyphosate-induced increase in glyoxalase I activity in cell lines of groundnut (Arachis hypogaea). Physiol. Plant. 2002, 114, 499–505. [Google Scholar] [CrossRef]
- Ashraf, M.; Foolad, M.R. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Aubert, S.; Assard, N.; Boutin, J.P.; Frenot, Y.; Dorne, A.J. Carbon metabolism in the subantarctic Kerguelen cabbage Pringlea antiscorbutica R. Br.: Environ mental controls over carbohydrates and proline contents and relation to phenology. Plant Cell Environ. 1999, 22, 243–254. [Google Scholar] [CrossRef]
- Matysik, J.; Ali, B.B.; Mohanty, P. Molecular mechanism of quenching of reactive oxygen species by pro line under water stress in plants. Curr. Sci. 2002, 82, 525–532. [Google Scholar]
- Smirnoff, N.; Cumbes, Q.J. Hydroxyl radical scavenging activity of compatible solutes. Phytochemistry 1989, 28, 1057–1060. [Google Scholar] [CrossRef]
- El-Flaah, R.F.; El-Said, R.A.R.; Nassar, M.A.; Hassan, M.; Abdelaal, K.A.A. Effect of rhizobium, nano silica and ascorbic acid on morpho-physiological characters and gene expression of POX and PPO in faba bean (Vicia faba L.) Under salinity stress conditions. Fresenius Environ. Bull. 2021, 30, 5751–5764. [Google Scholar]
- Elsawy, H.I.A.; Alharbi, K.; Mohamed, A.M.M.; Ueda, A.; AlKahtani, M.; AlHusnain, L.; Attia, K.A.; Abdelaal, K.; Shahein, A.M.E.A. Calcium Lignosulfonate Can Mitigate the Impact of Salt Stress on Growth, Physiological, and Yield Characteristics of Two Barley Cultivars (Hordeum vulgare L.). Agriculture 2022, 12, 1459. [Google Scholar] [CrossRef]
- Hoang, H.L.; de Guzman, C.C.; Cadiz, N.M.; Hoang, T.T.H.; Tran, D.H.; Rehman, H. Salicylic acid and calcium signaling induce physiological and phytochemical changes to improve salinity tolerance in red amaranth (Amaranthus tricolor L.). J. Soil Sci. Plant Nutr. 2020, 20, 1–11. [Google Scholar] [CrossRef]
- Ghorbani, A.; Razavi, S.M.; Omran, V.O.G.; Pirdashti, H. Piriformospora indica alleviates salinity by boosting redox poise and antioxidative potential of tomato. Russ. J. Plant Physiol. 2018, 65, 898–907. [Google Scholar] [CrossRef]
- Janas, K.M.; Cvikrova, M.; Pałagiewicz, A.; Szafranska, K.; Posmyk, M.M. Constitutive elevated accumulation of phenylpropanoids in soybean roots at low temperature. Plant Sci. 2002, 163, 369–373. [Google Scholar] [CrossRef]
- Wrobel, M.; Karmac, M.; Amarowicz, R.; Fraczek, E.; Weidner, S. Metabolism of phenolic compounds in Vitis riparia seeds during stratification and during germination under optimal and low temperature stress conditions. Acta Physiol. Plant. 2005, 27, 313–320. [Google Scholar] [CrossRef]
- Hernandez, I.; Leonor, A.; Sergi, M. Drought-induced changes in flavonoids and other low molecular weight antioxidants in Cistus clusii grown under Mediterranean field conditions. Tree Physiol. 2004, 24, 1303–1311. [Google Scholar] [CrossRef] [Green Version]
- Elhity, M.A.; Omer, A.M.; Zayed, B.A.; Assra, A.A.; Hassan, M.M.; Hafez, Y.M.; Abdelaal, K.A.A. Effect of phosphoric acid spray on rice growth and yield under saline sodic soils. Fresenius Environ. Bull. 2021, 30, 4935–4942. [Google Scholar]
- Abdelaal, K.A.A.; AlKahtani, M.D.F.; Attia, K.; Hafez, Y.; Király, L.; Künstler, A. The pivotal role of plant growth promoting bacteria in alleviating the adverse effects of drought on plants. Biology 2021, 10, 520. [Google Scholar] [CrossRef]
- Dkhil, B.B.; Issa, A.; Denden, M. Germination and seedling emergence of primed okra (Abelmoschus esculentus L.) seeds under salt stress and low temperature. Am. J. Plant Physiol. 2014, 9, 38–45. [Google Scholar] [CrossRef] [Green Version]
- Elkoca, E.; Haliloglu, K.; Esikten, A.; Ercisli, S. Hydro and osmopriming improve chickpea germination. Acta Agric. Scand. Sect. B Soil Plant Sci. 2007, 57, 193–200. [Google Scholar] [CrossRef]
- Kaya, M.D.; Okçu, G.; Atak, M.; Çikili, Y.; Kolsarici, O. Seed treatments to overcome salt and drought stress during germination in sunflower (Helianthus annuus L.). Eur. J. Agron. 2006, 24, 291–295. [Google Scholar] [CrossRef]
- Loreti, E.; van Veen, H.; Perata, P. Plant responses to flooding stress. Curr. Opin. Plant Biol. 2016, 33, 64–71. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, A.A.; Eichler-Lobermann, B.; Schnug, E. Response of crops to salinity under Egyptian conditions: A review. Landbauforsch. Volkenrode 2007, 57, 119. [Google Scholar]
- Ramanjulu, S.; Bartels, D. Drought- and desiccation-induced modulation of gene expression in plants. Plant Cell Environ. 2002, 25, 141–151. [Google Scholar] [CrossRef]
- Ibraheem, F.; Al-Hazmi, N.; El-Morsy, M.; Mosa, A. Ecological Risk Assessment of Potential Toxic Elements in Salt Marshes on the East Coast of the Red Sea: Differential Physiological Responses and Adaptation Capacities of Dominant Halophytes. Sustainability 2021, 13, 11282. [Google Scholar] [CrossRef]
- Ibraheem, F.; Al-Zahrani, A.; Mosa, A. Physiological Adaptation of Three Wild Halophytic Suaeda Species: Salt Tolerance Strategies and Metal Accumulation Capacity. Plants 2022, 11, 537. [Google Scholar] [CrossRef]
- Sahin, U.; Ekinci, M.; Ors, S.; Turan, M.; Yildiz, S.; Yildirim, E. Effects of individual and combined effects of salinity and drought on physiological, nutritional and biochemical properties of cabbage (Brassica oleracea var. capitata). Sci. Hortic. 2018, 240, 196–204. [Google Scholar] [CrossRef]
- Chatterjee, A.; Solankey, S.S. Functional physiology in drought tolerance of vegetable crops an approach to mitigate climate change impact. Clim. Dyn. Hortic. Sci. 2015, 1, 149–171. [Google Scholar]
- Raza, A.; Mehmood, S.S.; Tabassum, J.; Batool, R. Targeting Plant Hormones to Develop Abiotic Stress Resistance in Wheat. In Wheat Production in Changing Environments; Springer: Singapore, 2019; pp. 557–577. [Google Scholar]
- Sun, C.; Li, X.; Hu, Y.; Zhao, P.; Xu, T.; Sun, J.; Gao, X. Proline, sugars, and antioxidant enzymes respond to drought stress in the leaves of strawberry plants. Kor. J. Hort. Sci. Technol. 2015, 33, 625–632. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.K. Plant salt tolerance. Trends Plant Sci. 2001, 6, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Parihar, P.; Singh, S.; Singh, R.; Vijay Pratap Singh, V.P.; Prasad, S.M. Effect of salinity stress on plants and its tolerance strategies: A review. Environ. Sci. Pollut. Res. 2015, 22, 4056–4075. [Google Scholar] [CrossRef] [PubMed]
- Aghaei, K.; Ehsanpour, A.A.; Komatsu, S. Proteome analysis of potato under salt stress. J. Proteome Res. 2008, 7, 4858–4868. [Google Scholar] [CrossRef]
- Zörb, C.; Schmitt, S.; Mühling, K.H. Proteomic changes in maize roots after short-term adjustment to saline growth conditions. Proteomics 2010, 10, 4444–4449. [Google Scholar] [CrossRef]
- Koyro, H.W.; Ahmad, P.; Geissler, N. Abiotic stress responses in plants: An overview. In Environmental Adaptations and Stress Tolerance of Plants in the Era of Climate Change; Ahmad, P., Prasad, M.N.V., Eds.; Springer: New York, NY, USA, 2012; pp. 1–28. [Google Scholar]
- Nemhauser, J.L.; Hong, F.; Chory, J. Different plant hormones regulate similar processes through largely nonoverlapping transcriptional responses. Cell 2006, 126, 467–475. [Google Scholar] [CrossRef] [Green Version]
- Cochard, H. Cavitation in trees. Comptes Rendus Phys. 2006, 7, 1018–1026. [Google Scholar] [CrossRef]
- Brodribb, T.J.; Holbrook, N.M.; Edwards, E.J.; Gutierrez, M.V. Relations between stomatal closure, leaf turgor and xylem vulnerability in eight tropical dry forest trees. Plant Cell Environ. 2003, 26, 443–450. [Google Scholar] [CrossRef]
- Seki, M.; Umezawa, T.; Urano, K.; Shinozaki, K. Regulatory metabolic networks in drought stress responses. Curr. Opin. Plant Biol. 2007, 10, 296–302. [Google Scholar] [CrossRef]
- Couee, I.; Sulmon, C.; Gouesbet, G.; Amrani, A.E. Involvement of soluble sugars in reactive oxygen species balance and responses to oxidative stress in plants. J. Exp. Bot. 2006, 57, 449–459. [Google Scholar] [CrossRef] [Green Version]
- Abebe, T.; Guenzi, A.C.; Martin, B.; Cushman, J.C. Tolerance of mannitol-accumulating transgenic wheat to water stress and salinity. Plant Physiol. 2003, 131, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avonce, N.; Leyman, B.; Mascorro-Gallardo, J.O.; Van Dijck, P.; Thevelein, J.M.; Iturriaga, G. The Arabidopsis trehalose-6-P synthase AtTPS1 gene is a regulator of glucose, abscisic acid, and stress signaling. Plant Physiol. 2004, 136, 3649–3659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miranda, J.A.; Avonce, N.; Suarez, R.; Thevelein, J.M.; Van Dijck, P.; Iturriaga, G. A bifunctional TPS-TPP enzyme from yeast confers tolerance to multiple and extreme abiotic-stress conditions in transgenic Arabidopsis. Planta 2007, 226, 1411–1421. [Google Scholar] [CrossRef] [Green Version]
- Garg, A.K.; Kim, J.K.; Owens, T.G.; Ranwala, A.P.; Choi, Y.D.; Kochian, L.V.; Wu, R.J. Trehalose accumulation in rice plants confers high tolerance levels to different abiotic stresses. Proc. Natl. Acad. Sci. USA 2002, 99, 15898–15903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, Q.; Ashraf, M.; Athar, H.U.R. Exogenously applied proline at different growth stages enhances growth of two maize cultivars grown under water defi cit conditions. Pak. J. Bot. 2007, 39, 1133–1144. [Google Scholar]
- Yan, H.; Gang, L.Z.; Zhao, C.Y.; Guo, W.Y. Effects of exogenous proline on the physiology of soybean plantlets regenerated from embryos in vitro and on the ultrastructure of their mitochondria under NaCl stress. Soybean Sci. 2000, 9, 314–319. [Google Scholar]
- Djilianov, D.; Georgieva, T.; Moyankova, D.; Atanassov, A.; Shinozaki, K.; Smeeken, S.C.M. Improved abiotic stress tolerance in plants by accumulation of osmoprotectants—gene transfer approach. Biotechnol. Biotechnol. Equip. 2005, 19, 63–71. [Google Scholar] [CrossRef] [Green Version]
- Salinas, R.; Sánchez, E.; Ruíz, J.M.; Lao, M.T.; Romero, L. Proline, Betaine, and Choline Responses to Different Phosphorus Levels in Green Bean. Commun. Soil Sci. Plant Anal. 2013, 44, 465–472. [Google Scholar] [CrossRef]
- Jaarsma, R.; de Vries, R.S.M.; de Boer, A.H. Effect of Salt Stress on Growth, Na+ Accumulation and Proline Metabolism in Potato (Solanum Tuberosum) Cultivars. PLoS ONE 2013, 8, e60183. [Google Scholar] [CrossRef]
- De la Torre-González, A.; Montesinos-Pereira, D.; Blasco, B.; Ruiz, J.M. Influence of the Proline Metabolism and Glycine Betaine on Tolerance to Salt Stress in Tomato (Solanum Lycopersicum, L.) Commercial Genotypes. J. Plant Physiol. 2018, 231, 329–336. [Google Scholar] [CrossRef]
- Hannachi, S.; Van Labeke, M.-C. Salt Stress Affects Germination, Seedling Growth and Physiological Responses Differentially in Eggplant Cultivars (Solanum melongena L.). Sci. Hortic. 2018, 228, 56–65. [Google Scholar] [CrossRef]
- Anower, M.R.; Peel, M.D.; Mott, I.W.; Wu, Y. Physiological Processes Associated with Salinity Tolerance in an Alfalfa Half-Sib Family. J. Agron. Crop Sci. 2017, 203, 506–518. [Google Scholar] [CrossRef]
- Sarabi, B.; Bolandnazar, S.; Ghaderi, N.; Ghashghaie, J. Genotypic Differences in Physiological and Biochemical Responses to Salinity Stress in Melon (Cucumis Melo L.) Plants: Prospects for Selection of Salt Tolerant Landraces. Plant Physiol. Biochem. 2017, 119, 294–311. [Google Scholar] [CrossRef] [PubMed]
- Abdelaal, K.A.A.; Attia, K.A.; Niedbała, G.; Wojciechowski, T.; Hafez, Y.; Alamery, S.; Alateeq, T.K.; Arafa, S.A. Mitigation of Drought Damages by Exogenous Chitosan and Yeast Extract with Modulating the Photosynthetic Pigments, Antioxidant Defense System and Improving the Productivity of Garlic Plants. Horticulturae 2021, 7, 510. [Google Scholar] [CrossRef]
- Ben Hassine, A.; Ghanem, M.E.; Bouzid, S.; Lutts, S. An inland and a coastal population of the Mediterranean xero-halophyte species Atriplex halimus L. differ in their ability to accumulate proline and glycinebetaine in response to salinity and water stress. J. Exp. Bot. 2008, 59, 1315–1326. [Google Scholar] [CrossRef] [Green Version]
- Park, E.J.; Jeknic, Z.; Pino, M.T.; Murata, N.; Chen, T.H. Glycine betaine accumulation is more effective in chloroplasts than in the cytosol for protecting transgenic tomato plants against abiotic stress. Plant Cell Environ. 2007, 30, 994–1005. [Google Scholar] [CrossRef]
- Hoque, M.A.; Banu, M.N.; Okuma, E.; Amako, K.; Nakamura, Y.; Shimoishi, Y.; Murata, Y. Exogenous proline and glycine betaine increase NaCl-induced ascorbate-glutathione cycle enzyme activities, and proline improves salt tolerance more than glycine betaine in tobacco bright yellow-2 suspension-cultured cells. J. Plant Physiol. 2007, 164, 1457–1468. [Google Scholar] [CrossRef]
- Kurepin, L.V.; Ivanov, A.G.; Zaman, M.; Pharis, R.P.; Allakhverdiev, S.I.; Hurry, V.; Hüner, N.P. Stress-related hormones and glycine betaine interplay in protection of photosynthesis under abiotic stress conditions. Photosynth. Res. 2015, 126, 221–235. [Google Scholar] [CrossRef]
- Ashraf, M.; Nawaz, K.; Raza, S. Growth enhancement in two potential cereal crops, maize and wheat by exogenous application of glycine betaine. In Biosaline Agriculture and High Salinity Tolerance; Abdelly, C., Öztürk, M., Ashraf, M., Grignon, C., Eds.; Birkhäuser: Basel, Switzerland, 2008; pp. 21–35. [Google Scholar]
- Faisal, M.; Imran, K.; Muhammad, B.C.; Rizwan, M.; Athar, M.; Rashid, W.K.; Sameer, H.Q. Exogenously applied Glycine betain alleviates chromium toxicity in pea by reducing CR uptake and improving antioxidant defense system. Appl. Ecol. Environ. Res. 2022, 20, 4295–4307. [Google Scholar] [CrossRef]
- Ding, P.; Ding, Y. Stories of salicylic acid: A plant defense hormone. Trends Plant Sci. 2020, 25, 549–565. [Google Scholar] [CrossRef]
- Nolan, T.M.; Vukasinovic, N.; Liu, D.; Russinova, E.; Yin, Y. Brassi-nosteroids: Multidimensional regulators of plant growth, development and stress responses. Plant Cell 2020, 32, 298–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raza, A.; Salehi, H.; Rahman, M.A.; Zahid, Z.; Madadkar Haghjou, M.; Najafi-Kakavand, S.; Zhuang, W. Plant hormones and neurotransmitter interactions mediate antioxidant defenses under induced oxidative stress in plants. Front. Plant Sci. 2022, 13, 961872. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R.; Blumwald, E. The roles of ROS and ABA in systemic acquired acclimation. Plant Cell 2015, 27, 64–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, L.; Zhang, H.; Sun, L.; Jiao, Y.; Zhang, G.; Miao, C.; Hao, F. NADPH oxidase Atrboh D and Atrboh F function in ROS-dependent regulation of Na+/K+ homeostasis in Arabidopsis under salt stress. J. Exp. Bot. 2012, 63, 305–317. [Google Scholar] [CrossRef] [PubMed]
- Rejeb, K.B.; Lefebvre-De Vos, D.; Disquet, I.L.; Leprince, A.-S.; Bordenave, M.; Maldiney, R.; Jdey, A.; Abdelly, C.; Savoure, A. Hydrogen peroxide produced by NADPH oxidases increases proline accumulation during salt or mannitol stress in Arabidopsis thaliana. New Phytol. 2015, 208, 1138–1148. [Google Scholar] [CrossRef] [Green Version]
- Choudhury, F.K.; Rivero, R.M.; Blumwald, E.; Mittler, R. Reactive oxygen species, abiotic stress and stress combination. Plant J. 2017, 90, 856–867. [Google Scholar] [CrossRef]
- Saini, K.; AbdElgawad, H.; Markakis, M.N.; Schoenaers, S.; Asard, H.; Prinsen, E.; Beemster, G.T.S.; Vissenberg, K. Perturbation of auxin homeostasis and signaling by PINOID overexpression induces stress responses in Arabidopsis. Front. Plant Sci. 2017, 8, 1308. [Google Scholar] [CrossRef] [Green Version]
- Colebrook, E.H.; Thomas, S.G.; Phillips, A.L.; Hedden, P. The role of gibberellin signaling in plant responses to abiotic stress. J. Exp. Biol. 2014, 217, 67–75. [Google Scholar] [CrossRef] [Green Version]
- Sirhindi, G.; Mushtaq, R.; Gill, S.S.; Sharma, P.; Abd_Allah, E.F.; Ahmad, P. Jasmonic acid and methyl jasmonate modulate growth, photosynthetic activity and expression of photosystem II subunit genes in Brassica oleracea L. Sci. Rep. 2020, 10, 9322. [Google Scholar] [CrossRef]
- Mir, M.A.; Sirhindi, G.; Alyemeni, M.N.; Alam, P.; Ahmad, P. Jasmonic acid improves growth performance of soybean under nickel toxicity by regulating nickel uptake, redox balance, and oxidative stress metabolism. J. Plant Growth Regul. 2018, 37, 1195–1209. [Google Scholar] [CrossRef]
- Avalbaev, A.; Yuldashev, R.; Fedorova, K.; Somov, K.; Vysotskaya, L.; Allagulova, C.; Shakirova, F. Exogenous methyl jasmonate regulates cytokinin content by modulating cytokinin oxidase activity in wheat seedlings under salinity. J. Plant Physiol. 2016, 191, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Bali, S.; Kaur, P.; Jamwal, V.L.; Gandhi, S.G.; Sharma, A.; Ohri, P.; Bhardwaj, R.; Ali, M.A.; Ahmad, P. Seed priming with jasmonic acid counteracts root knot nematode infection in tomato by modulating the activity and expression of antioxidative enzymes. Biomolecules 2020, 10, 98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmadi, F.I.; Karimi, K.; Struik, P.C. Effect of exogenous application of methyl jasmonate on physiological and biochemical characteristics of Brassica napus L. cv. Talaye under salinity stress. S. Afr. J. Bot. 2018, 115, 5–11. [Google Scholar] [CrossRef]
- Tavallali, V.; Karimi, S. Methyl jasmonate enhances salt tolerance of almond rootstocks by regulating endogenous phytohormones, antioxidant activity and gas exchange. J. Plant Physiol. 2019, 234–235, 98–105. [Google Scholar] [CrossRef]
- El Nahhas, N.; AlKahtani, M.; Abdelaal, K.A.A.; Al Husnain, L.; AlGwaiz, H.; Hafez, Y.M.; Attia, K.; El-Esawi, M.; Ibrahim, M.; Elkelish, A. Biochar and jasmonic acid application attenuates antioxidative systems and improves growth, physiology, nutrient uptake and productivity of faba bean (Vicia faba L.) irrigated with saline water. Plant Physiol. Biochem. 2021, 166, 807–817. [Google Scholar] [CrossRef]
- Howladar, S.M. Exogenous applications of biochar and A-tocopherol improve the performance of salt-stressed tomato plants. Umm Al-Qura Univ. J. Appl. Sci. 2016, 3, 16. [Google Scholar]
- Gowtham, H.G.; Singh, S.B.; Shilpa, N.; Aiyaz, M.; Nataraj, K.; Udayashankar, A.C.; Sayyed, R.Z. Insight into Recent Progress and Perspectives in Improvement of Antioxidant Machinery upon PGPR Augmentation in Plants under Drought Stress: A Review. Antioxidants 2022, 11, 1763. [Google Scholar] [CrossRef]
- El-Zohri, M.; Al-Wadaani, N.A.; Bafeel, S.O. Foliar sprayed green zinc oxide nanoparticles mitigate drought-induced oxidative stress in tomato. Plants 2021, 10, 2400. [Google Scholar] [CrossRef]
- Jovović, Z.; Popović, T.; Velimirović, A.; Milić, V.; Dolijanović, Ž.; Šilj, M.; Poštić, D. Efficacy of Chemical Weed Control in Potato (Solanum tuberosum L.). Agron. Knowl. J. 2013, 14, 487–495. [Google Scholar] [CrossRef] [Green Version]
- Fayez, K.; Kristen, U. The influence of herbicides on the growth and proline content of primary roots and on the ultrastructure of root caps. Environ. Exp. Bot. 1996, 36, 71–81. [Google Scholar] [CrossRef]
- Baker, H.G. The Genetics of Colonizing Species. In Characteristics and Modes of Origin of Weeds; Baker, H.G., Stebbins, G.L., Eds.; Academic Press: Cambridge, MA, USA, 1965; pp. 147–172. [Google Scholar]
- Reddy, T.Y.; Reddy, G.H.S. Principles of Agronomy; Kalyani Publishers: New Delhi, India, 2007; pp. 384–420. [Google Scholar]
- Ferrari, R.C. Exploring C4–CAM plasticity within the Portulaca oleracea complex. Sci. Rep. 2010, 10, 14237. [Google Scholar] [CrossRef] [PubMed]
- Sureshkumar, R.; Durairaj, N.; Marimuthu, S.; Muthukumar, M. Weed characters and indices of transplanted rice as influenced by different weed management practices. Int. J. Agric. Sci. 2016, 8, 2221–2223. [Google Scholar]
- Patil, S.; Bainade, S.P. A review integrated weed management practices in cotton. Pharma Innov. J. 2022, 11, 565–656. [Google Scholar]
- Eslami, S.V. Weed management in conservation agriculture systems. In Recent Advances in Weed Management; Chauhan, B.S., Mahajan, G., Eds.; Springer: New York, NY, USA, 2014; pp. 87–124. [Google Scholar]
- Zimdahl, R.L. Fundamentals of Weed Science, 5th ed.; Academic Press: San Diego, CA, USA, 2018. [Google Scholar]
- Ali, A.H.; Ahmad, S.A.; Sarwar, S.; Arooj, N.; Ahmood, M.M.; Shahzad, A. Impact of integrated weed management on flat-sown cotton (Gossypium hirsutum L.). J. Anim. Plant Sci. 2013, 23, 1185–1192. [Google Scholar]
- Mortensen, D.A.; Egan, J.F.; Maxwell, B.D.; Ryan, M.R.; Smith, R.G. Navigating a critical juncture for sustainable weed management. BioScience 2012, 62, 75–84. [Google Scholar] [CrossRef] [Green Version]
- Bartel, C.; Archontoulis, S.V.; Lenssen, A.W.; Moore, K.J.; Huber, I.L.; Laird, D.; Dixon, P. Modeling perennial groundcover effects on annual maize grain crop growth with the Agricultural Production Systems sIMulator. Agron, J. 2020, 112, 1895–1910. [Google Scholar] [CrossRef]
- Moore, K.J.; Anex, R.P.; Elobeid, A.E.; Fei, S.; Flora, C.B.; Goggi, A.S.; Jacobs, K.L.; Jha, P.; Kaleita, A.L.; Karlen, D.L.; et al. Regenerating agricultural landscapes with perennial groundcover for intensive crop production. Agronomy 2019, 9, 458. [Google Scholar] [CrossRef] [Green Version]
- Teasdale, J.R.; Brandsæter, L.O.; Calegari, A.; Skora, F. Cover crops and weed management. In Non-Chemical Weed Management: Principles, Concepts and Technology; Upadhyaya, M.K., Blackshaw, R.E., Eds.; CABI: Wallingford, UK, 2007; pp. 49–64. [Google Scholar]
- Médiène, S.; Valantin-Morison, M.; Sarthou, J.-P.; De Tourdonnet, S.; Gosme, M.; Bertrand, M.; Roger-Estrade, J.; Aubertot, J.-N.; Rusch, A.; Motisi, N.; et al. Agroecosystem management and biotic interactions: A review. Agron. Sustain. Dev. 2012, 31, 491–514. [Google Scholar] [CrossRef] [Green Version]
- Petit, S.; Cordeau, S.; Chauvel, B.; Bohan, D.; Guillemin, J.-P.; Steinberg, C. Biodiversity-based options for arable weed management. A review. Agron. Sustain. Dev. 2018, 38, 48. [Google Scholar] [CrossRef] [Green Version]
- Eberlein, C.; Sheaffer, C.; Oliveira, V. Corn growth and yield in an alfalfa living mulch system. J. Prod. Agric. 1992, 5, 332–339. [Google Scholar] [CrossRef]
- Verret, V.; Gardarin, A.; Pelzer, E.; Médiène, S.; Makowski, D.; Valantin-Morison, M. Can legume companion plants control weeds without decreasing crop yield? A meta-analysis. Field Crops Res. 2017, 204, 158–168. [Google Scholar] [CrossRef]
- Leoni, F.; Lazzaro, M.; Carlesi, S.; Moonen, A.-C. Legume ecotypes and commercial cultivars differ in performance and potential suitability for use as permanent living mulch in Mediterranean vegetable systems. Agronomy 2020, 10, 1836. [Google Scholar] [CrossRef]
- Ellis, D.; Guillard, K.; Adams, R. Purslane as a living mulch in broccoli production. Am. J. Altern. Agric. 2000, 15, 50–59. [Google Scholar] [CrossRef]
- Sanders, Z.; Andrews, J.; Saha, U.; Vencill, W.; Lee, R.; Hill, N. Optimizing agronomic practices for clover persistence and corn yield in a white clover–corn living mulch system. Agron. J. 2017, 109, 2025–2032. [Google Scholar] [CrossRef]
- Bhaskar, V.; Bellinder, R.; Reiners, S.; Westbrook, A.S.; DiTommaso, A. Significance of herbicide order in sequential applications to target weeds in a sunn hemp living mulch. Weed Technol. 2021, 35, 565–573. [Google Scholar] [CrossRef]
- Boselli, R.; Anders, N.; Fiorini, A.; Ganimede, C.; Faccini, N.; Marocco, A.; Schulz, M.; Tabaglio, V. Improving weed control in sustainable agro-ecosystems: Role of cultivar and termination timing of rye cover crop. Ital. J. Agron. 2021, 16, 1807. [Google Scholar] [CrossRef]
- Abou Chehade, L.; Puig, C.G.; Souto, C.; Antichi, D.; Mazzoncini, M.; Pedrol, N. Rye (Secale cereale L.) and squarrose clover (Trifolium squarrosum L.) cover crops can increase their allelopathic potential for weed control when used mixed as dead mulch. Ital. J. Agron. 2021, 16, 1869. [Google Scholar] [CrossRef]
- Scavo, A.; Restuccia, A.; Mauromicale, G. Allelopathy: General principles and basic aspects for agroecosystem control. In Sustainable Agriculture Reviews; Gaba, S., Smith, B., Lichtfouse, E., Eds.; Springer: Cham, Switzerland, 2018; Volume 28, pp. 47–101. [Google Scholar]
- Tesio, F.; Ferrero, A. Allelopathy, a chance for sustainable weed management. Int. J. Sust. Dev. World 2010, 17, 377–389. [Google Scholar] [CrossRef]
- Cordeau, S.; Triolet, M.; Wayman, S.; Steinberg, C.; Guillemin, J.P. Bioherbicides: Dead in the water? A review of the existing products for integrated weed management. Crop Prot. 2016, 87, 44–49. [Google Scholar] [CrossRef]
- Schwarzländer, M.; Hinz, H.L.; Winston, R.L.; Day, M.D. Biological control of weeds: An analysis of introductions, rates of establishment and estimates of success, worldwide. BioControl 2018, 63, 319–331. [Google Scholar] [CrossRef] [Green Version]
- Müller-Schärer, H.; Collins, A.R. Integrated Weed Management. In Encyclopedia of Environmental Management; Jorgensen, S.E., Ed.; Taylor and Francis: New York, NY, USA, 2012. [Google Scholar]
- Charudattan, R. Biological control of weeds by means of plant pathogens: Significance for integrated weed management in modern agro-ecology. BioControl 2001, 46, 229–260. [Google Scholar] [CrossRef]
- Berti, A.; Onofri, A.; Zanin, G.; Sattin, M. Sistema integrato di gestione della lotta alle malerbe (IWM). In Malerbologia; Catizone, P., Zanin, G., Eds.; Pàtron Editore: Bologna, Italy, 2001; pp. 659–710. [Google Scholar]
- Scavo, A.; Mauromicale, G. Integrated Weed Management in Herbaceous Field Crops. Agronomy 2020, 10, 466. [Google Scholar] [CrossRef] [Green Version]
- Mauromicale, G.; Occhipinti, A.; Mauro, R.P. Selection of shade-adapted subterranean clover species for cover cropping in orchards. Agron. Sustain. Dev. 2010, 30, 473–480. [Google Scholar] [CrossRef] [Green Version]
- Mauro, R.P.; Occhipinti, A.; Longo, A.M.G.; Mauromicale, G. Effects of shading on chlorophyll content, chlorophyll fluorescence and photosynthesis of subterranean clover. J. Agron. Crop. Sci. 2011, 197, 57–66. [Google Scholar] [CrossRef]
- McKenzie-Gopsill, A.G.; Amirsadeghi, S.; Earl, H.J.; Jones, A.M.P.; Lukens, L.; Lee, E.; Swanton, C.J. Early physiological and biochemical responses of soybean to neighboring weeds under resource-independent competition. Weed Res. 2019, 59, 288–299. [Google Scholar] [CrossRef]
- Yamazaki, J.Y. Is light quality involved in the regulation of the photosynthetic apparatus in attached rice leaves? Photosynth. Res. 2010, 105, 63–71. [Google Scholar] [CrossRef]
- Scebba, F.; Canaccini, F.; Castagna, A.; Bender, J. Physiological and biochemical stress responses in grassland species are influenced by both early-season ozone exposure and interspecific competition. Environ. Pollut. 2006, 142, 540–548. [Google Scholar] [CrossRef]
- Tanaka, T.; Abbas, H.K.; Duke, S.O. Structure-dependent phytotoxicity of fumonisins and related compounds in a duckweed bioassay. Phytochemistry 1993, 33, 779–785. [Google Scholar] [CrossRef]


| ROS | Radical Type | Production Site | Mode of Action | Scavenging System |
|---|---|---|---|---|
| Superoxide (O2•−) | Free radical | Chloroplast, mitochondria, peroxisomes, apoplast, electron transfer chains | Reacts with Fe–S proteins Dismutates to H2O2 | SOD, flavonoids, ascorbate |
| Hydroxyl radical (OH•) | Free radical | Iron and H2O2 (Fenton reaction) | Reacts with all biomolecules including DNA, RNA, lipids and proteins | Flavonoids, proline, sugars, ascorbate |
| Hydrogen peroxide (H2O2) | Non-radical | Chloroplast, mitochondria, Peroxisomes, cytosol, apoplast | Reacts with proteins by attacking cysteine and methionine residues. Reacts with heme proteins. Reacts with DNA. | APX, CAT, GPX, PER, PRX, ascorbate, glutathione |
| Singlet oxygen (1O2) | Non-radical | Chloroplasts, nuclei, membranes | Oxidizes lipids, proteins (Trp, His, Tyr, Met, and Cys residues) and G residues of DNA | Carotenoids and α-tocopherol |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelaal, K.; Alsubeie, M.S.; Hafez, Y.; Emeran, A.; Moghanm, F.; Okasha, S.; Omara, R.; Basahi, M.A.; Darwish, D.B.E.; Ibrahim, M.F.M.; et al. Physiological and Biochemical Changes in Vegetable and Field Crops under Drought, Salinity and Weeds Stresses: Control Strategies and Management. Agriculture 2022, 12, 2084. https://doi.org/10.3390/agriculture12122084
Abdelaal K, Alsubeie MS, Hafez Y, Emeran A, Moghanm F, Okasha S, Omara R, Basahi MA, Darwish DBE, Ibrahim MFM, et al. Physiological and Biochemical Changes in Vegetable and Field Crops under Drought, Salinity and Weeds Stresses: Control Strategies and Management. Agriculture. 2022; 12(12):2084. https://doi.org/10.3390/agriculture12122084
Chicago/Turabian StyleAbdelaal, Khaled, Moodi Saham Alsubeie, Yaser Hafez, Amero Emeran, Farahat Moghanm, Salah Okasha, Reda Omara, Mohammed A. Basahi, Doaa Bahaa Eldin Darwish, Mohamed F. M. Ibrahim, and et al. 2022. "Physiological and Biochemical Changes in Vegetable and Field Crops under Drought, Salinity and Weeds Stresses: Control Strategies and Management" Agriculture 12, no. 12: 2084. https://doi.org/10.3390/agriculture12122084
APA StyleAbdelaal, K., Alsubeie, M. S., Hafez, Y., Emeran, A., Moghanm, F., Okasha, S., Omara, R., Basahi, M. A., Darwish, D. B. E., Ibrahim, M. F. M., El-Yazied, A. A., Rashwan, E. A., Elkelish, A., Mady, M. A., & Ibraheem, F. (2022). Physiological and Biochemical Changes in Vegetable and Field Crops under Drought, Salinity and Weeds Stresses: Control Strategies and Management. Agriculture, 12(12), 2084. https://doi.org/10.3390/agriculture12122084









