In Vivo Low-Temperature Plasma Ionization Mass Spectrometry (LTP-MS) Reveals Regulation of 6-Pentyl-2H-Pyran-2-One (6-PP) as a Physiological Variable during Plant-Fungal Interaction
Abstract
:1. Introduction
- LTP allows chemically very diverse molecules to be ionized, since the plasma simultaneously contains positively or negatively charged particles.
- LTP systems are adaptable to any MS with an accessible atmospheric inlet, e.g., coupling with linear ion trap, quadrupole, or Fourier Transform-Ion Cyclotron Resonance (FTICR) spectrometers.
- The operating conditions of LTP-MS systems are more straightforward than conventional mass spectrometry ionization sources such as EI, ESI, or MALDI.
2. Methods
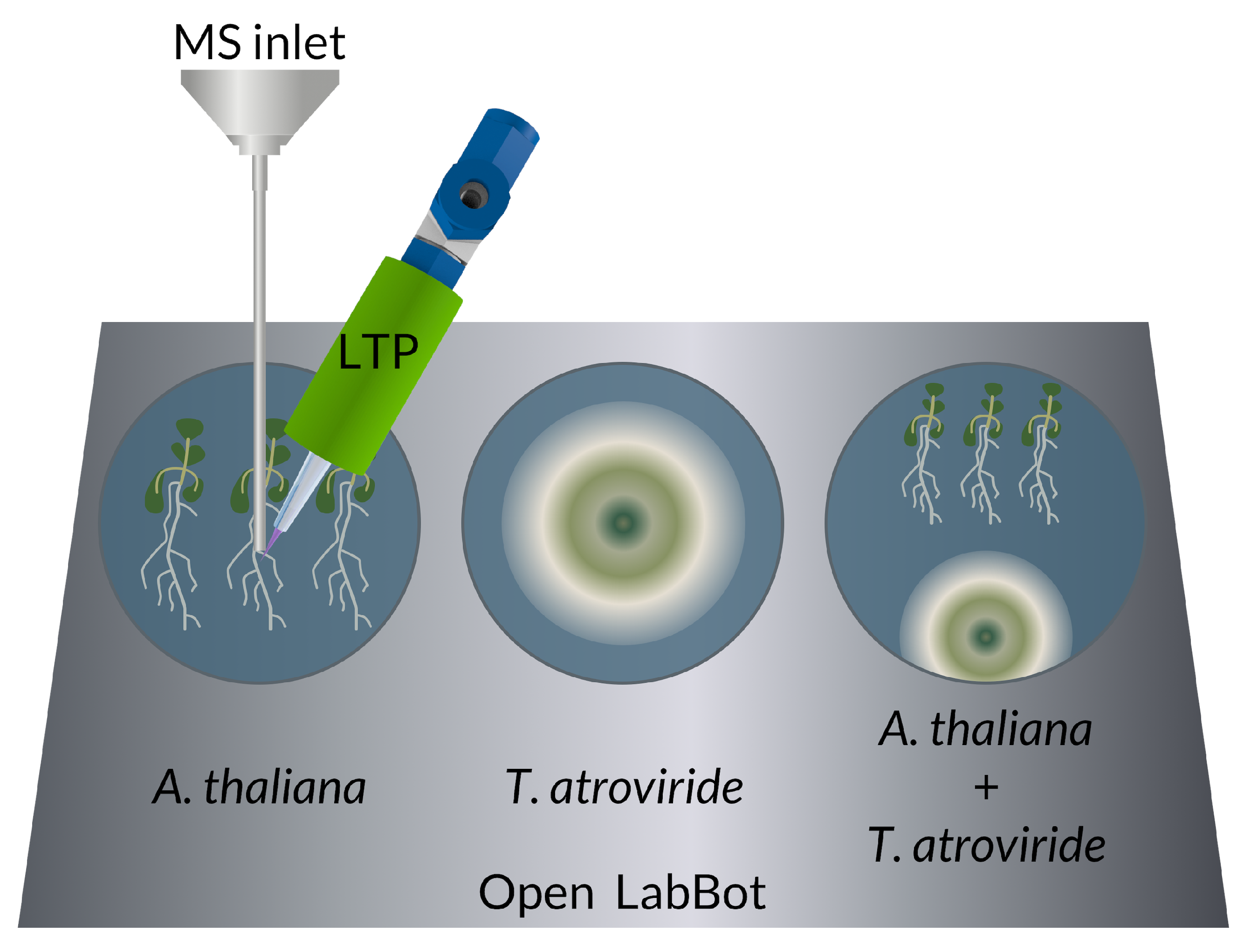
2.1. Biological Material
2.2. Plant Growth Conditions
2.3. Infection with T. atroviride
2.4. 6-PP Determination by Standardized SPME-GC-MS
2.5. 6-PP Determination by LTP-MS
2.6. 6-PP Kinetics and Other VOCs by LTP-MS during T. atroviride-A. thaliana Interaction
2.7. Data Analyses
3. Results
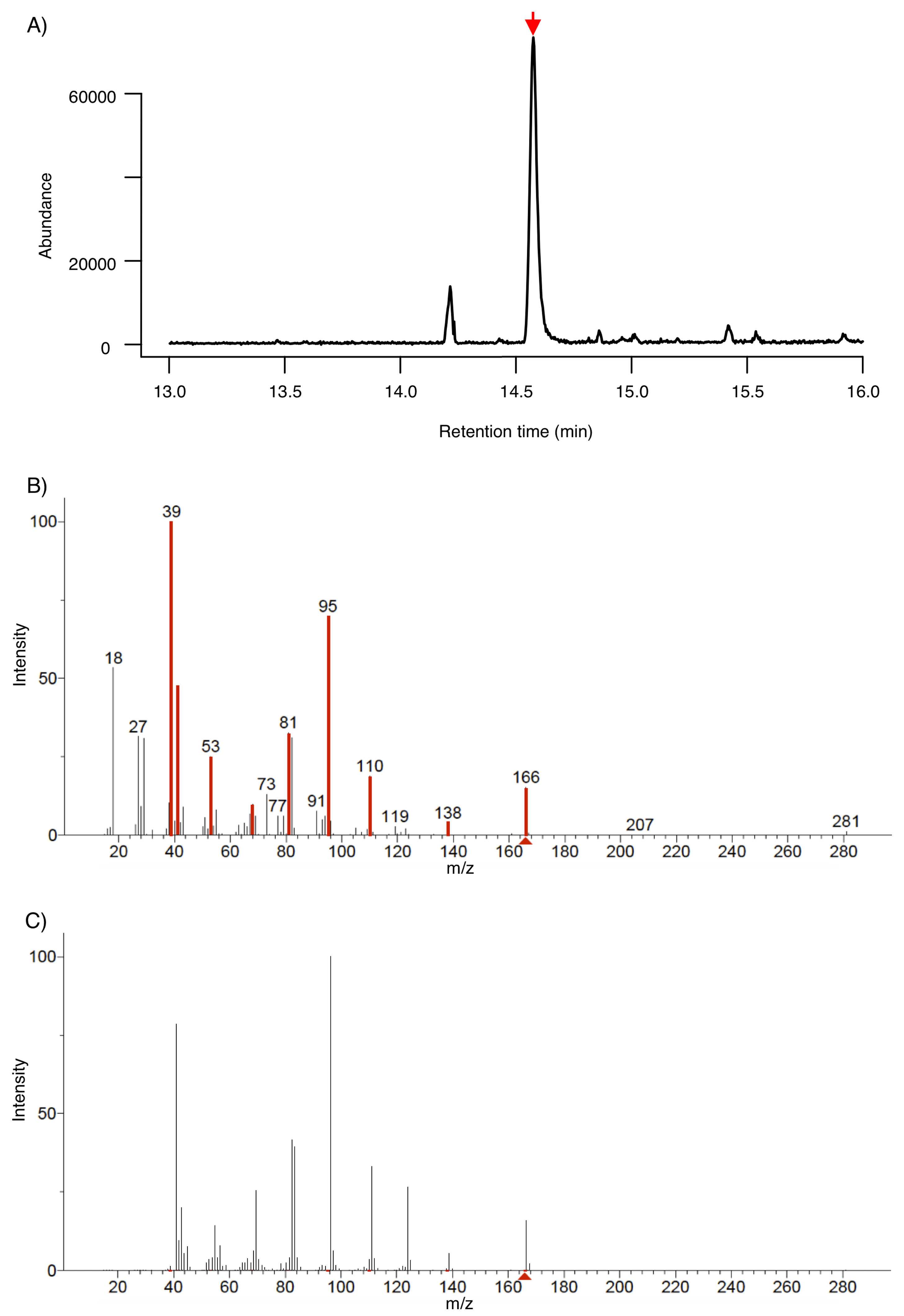
3.1. Identification of 6-PP by SPME-GC-MS
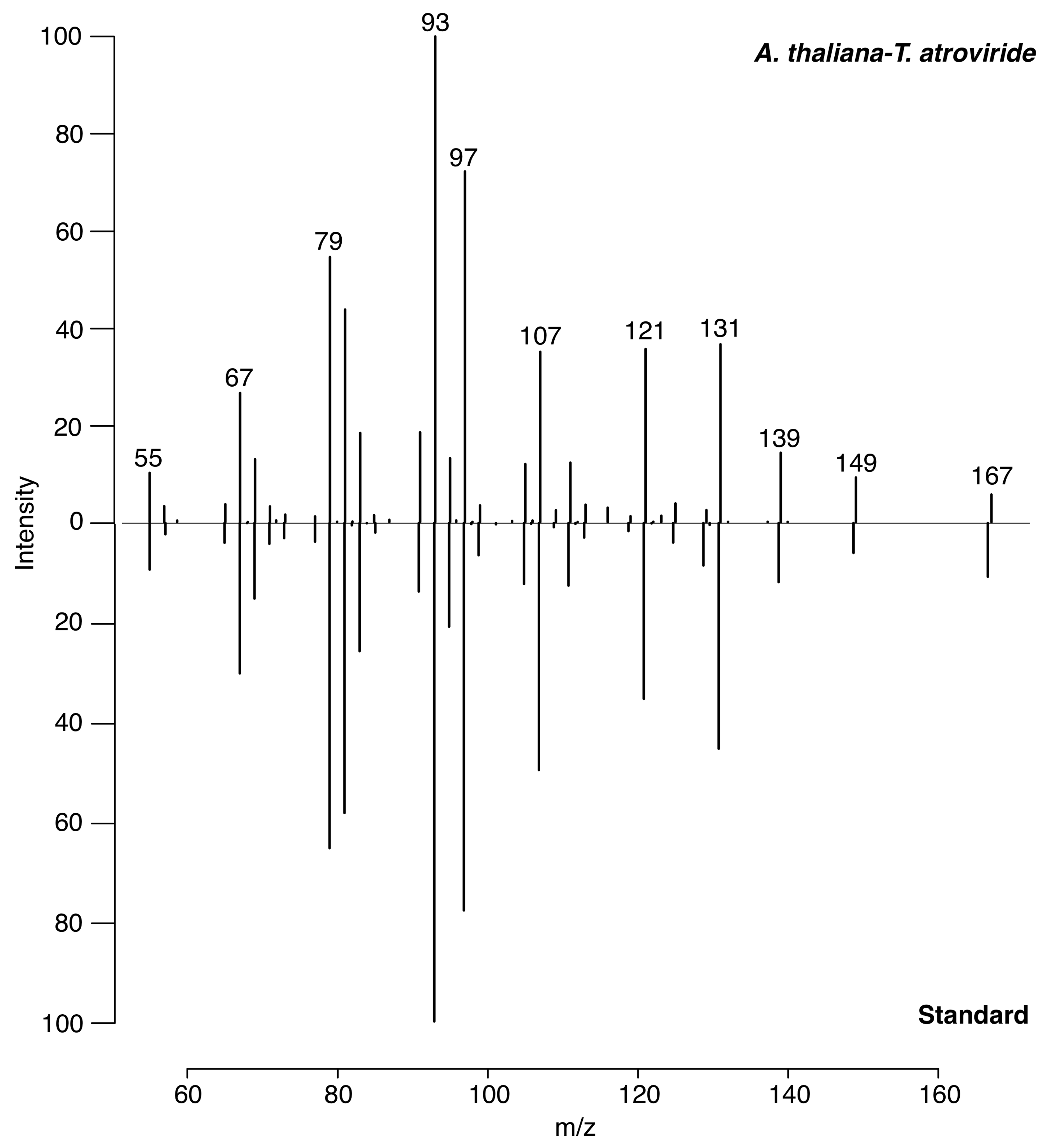
3.2. Identification of 6-PP by LTP-MS
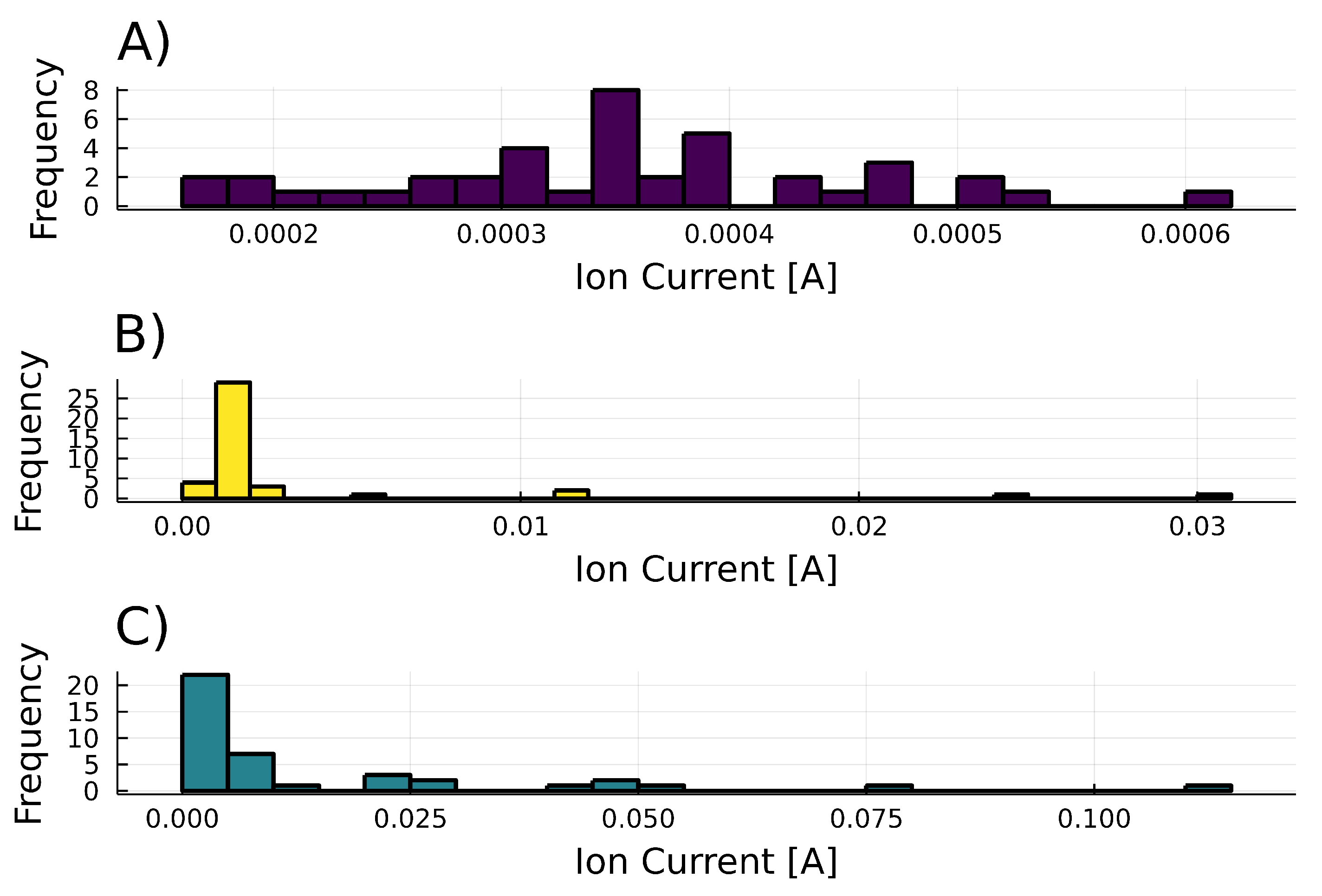
3.3. Time Series of 6-PP Kinetics Production
3.3.1. Time Evolution and Behavior
3.3.2. Frequencies and Statistical Moments
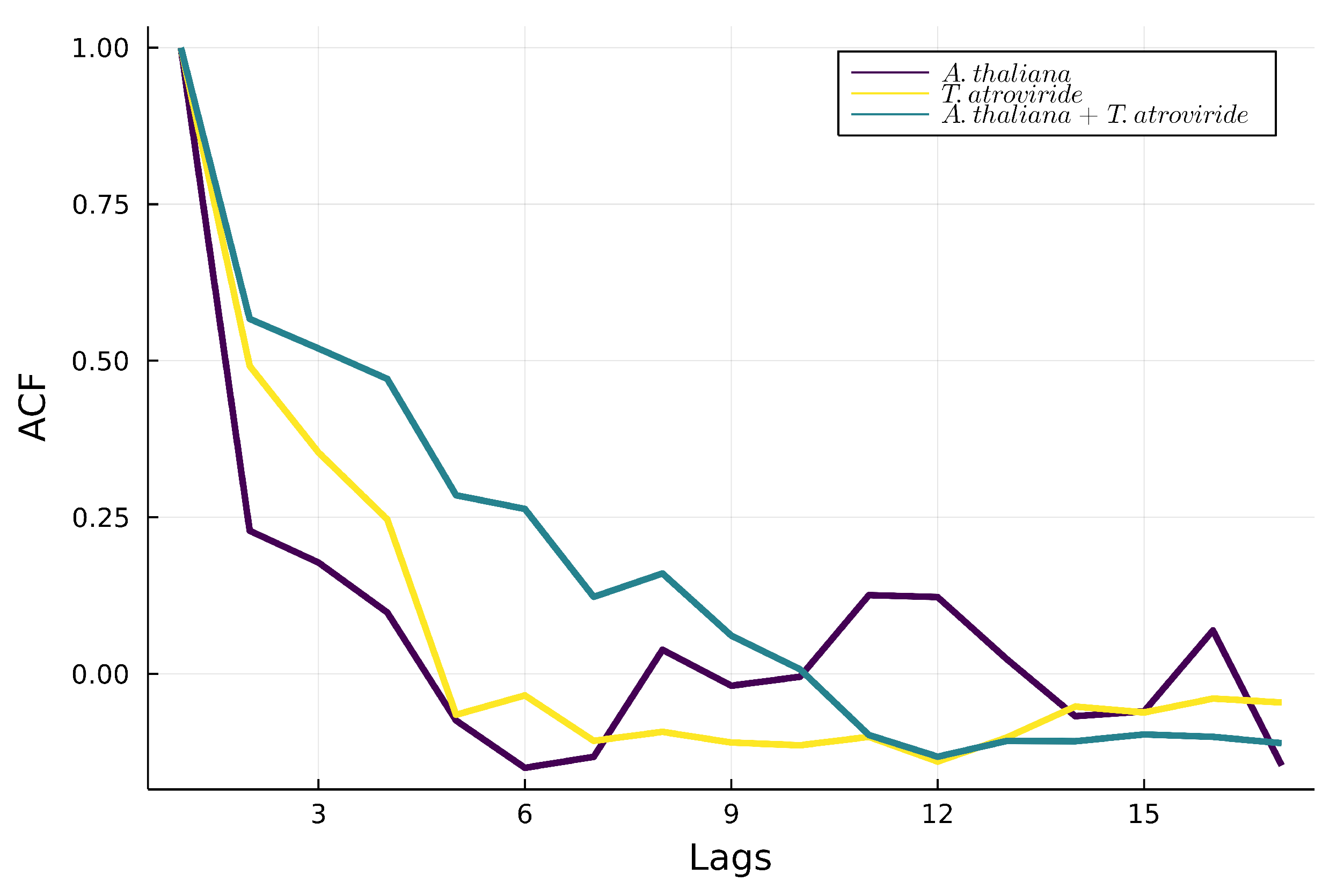
3.3.3. Autocorrelations
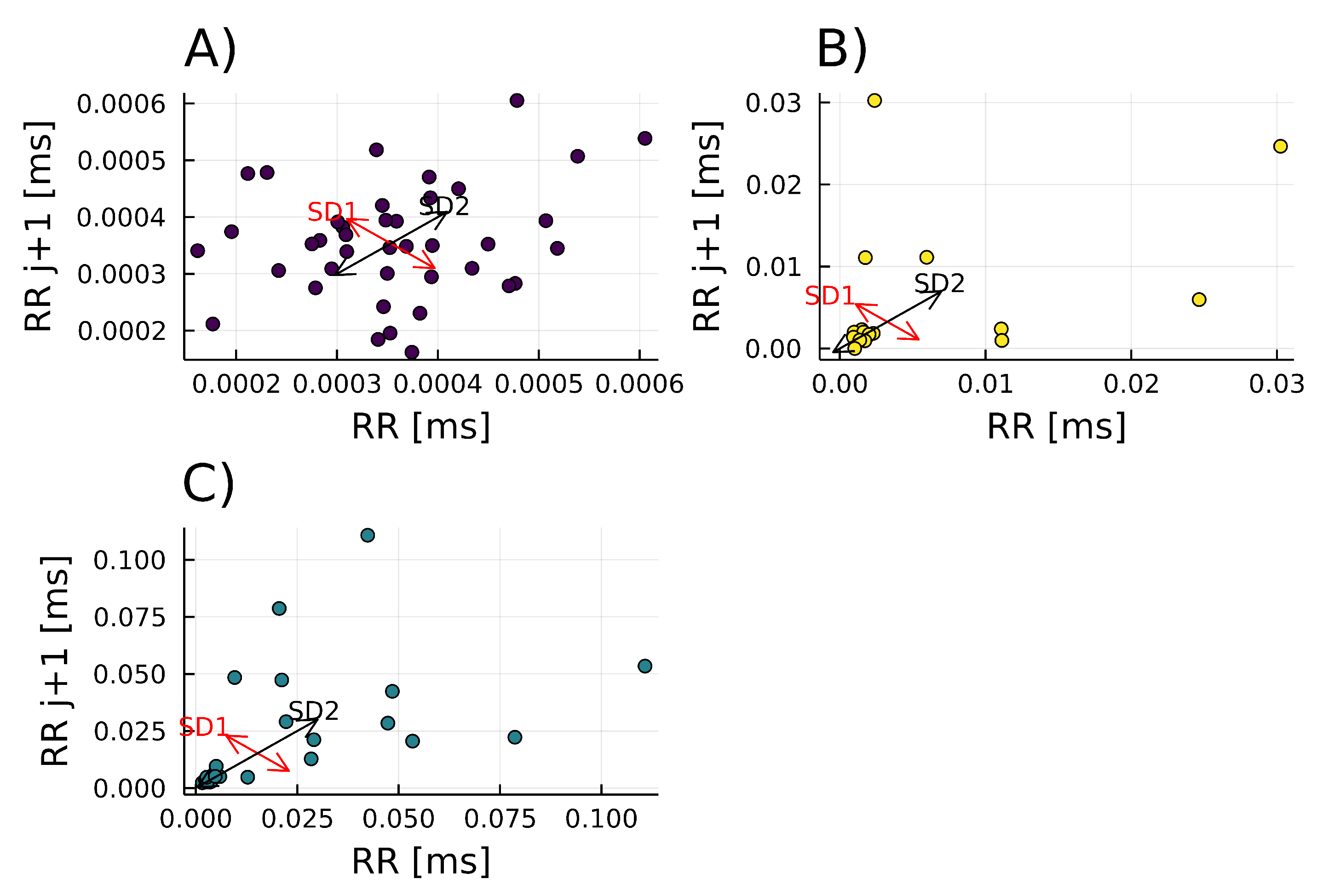
3.3.4. Poincaré Plot
4. Discussion
4.1. Dynamics of 6-PP Production in Plant-Fungal Interaction
4.2. Time-SERIES Analysis of Biological MS Data with Julia
4.3. LTP-MS for Monitoring VOCs in Biological Systems
- High fiber saturation with water results in poor VOC capture.
- Molecules are lost because of fiber selectivity.
- Extensive time of capture and separation for molecule identification, 1.3 h at least between both phases.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 6-PP | 6-Pentyl-2H-pyran-2-one |
| AIMS | ambient ionisation mass spectrometry |
| DART | direct analysis in real-time |
| DESI | desorption electrospray ionisation |
| EI | electron impact |
| ESI | electrospray |
| FID | flame ionization detection |
| fVOC | fungal volatile organic compound |
| GC | gas chromatography |
| HCl | hydrochloric acid |
| HPLC | high-performance liquid chromatography |
| HRV | heart rate variability |
| LTP | low-temperature plasma |
| LB | Luria-Bertani |
| MALDI | matrix-assisted laser desorption/ionisation |
| MA | methamphetamine |
| MS | mass spectrometry |
| MS medium | Murashige & Skoog medium |
| NIST | National Institute of Standards and Technology |
| PTR | proton-transfer reaction |
| ROS | reactive oxygen species |
| SA | salicylic acid |
| SoH | state of health |
| SPME | solid-phase microextraction |
| TIC | total ion current |
| VOC | volatile organic compound |
References
- Afridi, M.S.; Javed, M.A.; Ali, S.; De Medeiros, F.H.V.; Ali, B.; Salam, A.; Sumaira; Marc, R.A.; Alkhalifah, D.H.M.; Selim, S.; et al. New opportunities in plant microbiome engineering for increasing agricultural sustainability under stressful conditions. Front. Plant Sci. 2022, 13, 899464. [Google Scholar] [CrossRef] [PubMed]
- Salwan, R.; Rialch, N.; Sharma, V. Bioactive Volatile Metabolites of Trichoderma: An overview. In Secondary Metabolites of Plant Growth Promoting Rhizomicroorganisms; Singh, H.B., Keswani, C., Reddy, M.S., Sansinenea, E., García-Estrada, C., Eds.; Springer: Singapore, 2019; pp. 87–111. [Google Scholar] [CrossRef]
- Jeleń, H.; Błaszczyk, L.; Chełkowski, J.; Rogowicz, K.; Strakowska, J. Formation of 6-n-pentyl-2H-pyran-2-one (6-PAP) and other volatiles by different Trichoderma species. Mycol. Prog. 2014, 13, 589–600. [Google Scholar] [CrossRef] [Green Version]
- Brosset, A.; Blande, J.D. Volatile-mediated plant–plant interactions: Volatile organic compounds as modulators of receiver plant defence, growth, and reproduction. J. Exp. Bot. 2022, 73, 511–528. [Google Scholar] [CrossRef]
- González-Pérez, E.; Ortega-Amaro, M.A.; Salazar-Badillo, F.B.; Bautista, E.; Douterlungne, D.; Jiménez-Bremont, J.F. The Arabidopsis-Trichoderma interaction reveals that the fungal growth medium is an important factor in plant growth induction. Sci. Rep. 2018, 8, 16427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cordovez, V.; Mommer, L.; Moisan, K.; Lucas-Barbosa, D.; Pierik, R.; Mumm, R.; Carrion, V.J.; Raaijmakers, J.M. Plant Phenotypic and Transcriptional Changes Induced by Volatiles from the Fungal Root Pathogen Rhizoctonia solani. Front. Plant Sci. 2017, 8, 1262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spinelli, F.; Cellini, A.; Marchetti, L.; Mudigere, K.; Piovene, C. Emission and Function of Volatile Organic Compounds in Response to Abiotic Stress. In Abiotic Stress in Plants–Mechanisms and Adaptations; Shanker, A., Ed.; InTech: Rang-Du-Fliers, France, 2011. [Google Scholar] [CrossRef] [Green Version]
- Camarena-Pozos, D.A.; Flores-Núñez, V.M.; López, M.G.; Partida-Martínez, L.P. Fungal volatiles emitted by members of the microbiome of desert plants are diverse and capable of promoting plant growth. Environ. Microbiol. 2021, 23, 2215–2229. [Google Scholar] [CrossRef]
- Carillo, P.; Woo, S.L.; Comite, E.; El-Nakhel, C.; Rouphael, Y.; Fusco, G.M.; Borzacchiello, A.; Lanzuise, S.; Vinale, F. Application of Trichoderma harzianum, 6-Pentyl-α-pyrone and Plant Biopolymer Formulations Modulate Plant Metabolism and Fruit Quality of Plum Tomatoes. Plants 2020, 9, 771. [Google Scholar] [CrossRef] [PubMed]
- Kottb, M.; Gigolashvili, T.; Großkinsky, D.K.; Piechulla, B. Trichoderma volatiles effecting Arabidopsis: From inhibition to protection against phytopathogenic fungi. Front. Microbiol. 2015, 6, 995. [Google Scholar] [CrossRef]
- Garnica-Vergara, A.; Barrera-Ortiz, S.; Muñoz-Parra, E.; Raya-González, J.; Méndez-Bravo, A.; Macías-Rodríguez, L.; Ruiz-Herrera, L.F.; López-Bucio, J. The volatile 6-pentyl-2H-pyran-2-one from Trichoderma atroviride regulates Arabidopsis thaliana root morphogenesis via auxin signaling and ETHYLENE INSENSITIVE 2 functioning. New Phytol. 2016, 209, 1496–1512. [Google Scholar] [CrossRef] [Green Version]
- Alcalde-Vázquez, R.; Moreno-Pedraza, A.; Rosas-Román, I.; Guillén-Alonso, H.; Riedel, J.; Partida-Martínez, L.P.; Winkler, R. MoBiMS: A modular miniature mass analyzer for the real-time monitoring of gases and volatile compounds in biological systems. Microchem. J. 2022, 175, 107090. [Google Scholar] [CrossRef]
- Capuano, R.; Khomenko, I.; Grasso, F.; Messina, V.; Olivieri, A.; Cappellin, L.; Paolesse, R.; Catini, A.; Ponzi, M.; Biasioli, F.; et al. Simultaneous Proton Transfer Reaction-Mass Spectrometry and electronic nose study of the volatile compounds released by Plasmodium falciparum infected red blood cells in vitro. Sci. Rep. 2019, 9, 12360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-Jarquín, S.; Winkler, R. Low-temperature plasma (LTP) jets for mass spectrometry (MS): Ion processes, instrumental set-ups, and application examples. TrAC Trends Anal. Chem. 2017, 89, 133–145. [Google Scholar] [CrossRef]
- Alberici, R.M.; Simas, R.C.; Sanvido, G.B.; Romão, W.; Lalli, P.M.; Benassi, M.; Cunha, I.B.S.; Eberlin, M.N. Ambient mass spectrometry: Bringing MS into the “real world”. Anal. Bioanal. Chem. 2010, 398, 265–294. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Jarquín, S.; Herrera-Ubaldo, H.; de Folter, S.; Winkler, R. In vivo monitoring of nicotine biosynthesis in tobacco leaves by low-temperature plasma mass spectrometry. Talanta 2018, 185, 324–327. [Google Scholar] [CrossRef] [PubMed]
- Takáts, Z.; Wiseman, J.M.; Gologan, B.; Cooks, R.G. Mass Spectrometry Sampling Under Ambient Conditions with Desorption Electrospray Ionization. Science 2004, 306, 471–473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cody, R.B.; Laramée, J.A.; Durst, H.D. Versatile new ion source for the analysis of materials in open air under ambient conditions. Anal. Chem. 2005, 77, 2297–2302. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Gamez, G.; Zenobi, R. What can we learn from ambient ionization techniques? J. Am. Soc. Mass Spectrom. 2009, 20, 1947–1963. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Zhou, W.; Jin, W.; Zhou, J.; Handberg, E.; Zhu, Z.; Chen, H.; Jin, Q. Direct desorption/ionization of analytes by microwave plasma torch for ambient mass spectrometric analysis. J. Mass Spectrom. 2013, 48, 669–676. [Google Scholar] [CrossRef]
- Chingin, K.; Chen, H.; Gamez, G.; Zhu, L.; Zenobi, R. Detection of Diethyl Phthalate in Perfumes by Extractive Electrospray Ionization Mass Spectrometry. Anal. Chem. 2009, 81, 123–129. [Google Scholar] [CrossRef]
- Gamez, G.; Zhu, L.; Disko, A.; Chen, H.; Azov, V.; Chingin, K.; Krämer, G.; Zenobi, R. Real-time, in vivo monitoring and pharmacokinetics of valproic acidvia a novel biomarker in exhaled breath. Chem. Commun. 2011, 47, 4884–4886. [Google Scholar] [CrossRef]
- Feider, C.L.; Krieger, A.; DeHoog, R.J.; Eberlin, L.S. Ambient Ionization Mass Spectrometry: Recent Developments and Applications. Anal. Chem. 2019, 91, 4266–4290. [Google Scholar] [CrossRef] [PubMed]
- Harper, J.D.; Charipar, N.A.; Mulligan, C.C.; Zhang, X.; Cooks, R.G.; Ouyang, Z. Low-temperature plasma probe for ambient desorption ionization. Anal. Chem. 2008, 80, 9097–9104. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.K.; Horwitz, B.A.; Kenerley, C.M. Secondary metabolism in Trichoderma—A genomic perspective. Microbiology 2012, 158, 35–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trefz, P.; Schmidt, M.; Oertel, P.; Obermeier, J.; Brock, B.; Kamysek, S.; Dunkl, J.; Zimmermann, R.; Schubert, J.K.; Miekisch, W. Continuous Real Time Breath Gas Monitoring in the Clinical Environment by Proton-Transfer-Reaction-Time-of-Flight-Mass Spectrometry. Anal. Chem. 2013, 85, 10321–10329. [Google Scholar] [CrossRef]
- Fossion, R.; Rivera, A.L.; Estañol, B. A physicist’s view of homeostasis: How time series of continuous monitoring reflect the function of physiological variables in regulatory mechanisms. Physiol. Meas. 2018, 39, 084007. [Google Scholar] [CrossRef] [Green Version]
- Modell, H.; Cliff, W.; Michael, J.; McFarland, J.; Wenderoth, M.P.; Wright, A. A physiologist’s view of homeostasis. Adv. Physiol. Educ. 2015, 39, 259–266. [Google Scholar] [CrossRef] [Green Version]
- Peña, J.U.L.; Morales, F.S.; Carlos, J.C.; Fossion, R. Parallels between Homeostatic Regulation and Control Theory. AIP Conf. Proc. 2021, 2348, 040007. [Google Scholar] [CrossRef]
- Giordano, M. Homeostasis: An underestimated focal point of ecology and evolution. Plant Sci. 2013, 211, 92–101. [Google Scholar] [CrossRef]
- National Institute of Standards and Technology. NIST20: Updates to the NIST Tandem and Electron Ionization Spectral Libraries; NIST: Gaithersburg, MD, USA, 2020. [Google Scholar]
- Martínez-Jarquín, S.; Moreno-Pedraza, A.; Guillén-Alonso, H.; Winkler, R. Template for 3D Printing a Low-Temperature Plasma Probe. Anal. Chem. 2016, 88, 6976–6980. [Google Scholar] [CrossRef]
- Rosas-Román, I.; Ovando-Vázquez, C.; Moreno-Pedraza, A.; Guillén-Alonso, H.; Winkler, R. Open LabBot and RmsiGUI: Community development kit for sampling automation and ambient imaging. Microchem. J. 2020, 152, 104343. [Google Scholar] [CrossRef]
- Carpenter, R.H.S. Homeostasis: A plea for a unified approach. Adv. Physiol. Educ. 2004, 28, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Biswas, D.; Iglesias, P.A. Sensitivity minimization, biological homeostasis and information theory. Biol. Cybern. 2021, 115, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Steel, H.; Papachristodoulou, A. Design Constraints for Biological Systems That Achieve Adaptation and Disturbance Rejection. IEEE Trans. Control Netw. Syst. 2018, 5, 807–817. [Google Scholar] [CrossRef]
- Morgan Ernest, S.K.; Brown, J.H. Homeostasis and compensation: The role of species and resources in ecosystem stability. Ecology 2001, 82, 2118–2132. [Google Scholar] [CrossRef] [Green Version]
- Dyke, J.G.; Weaver, I.S. The Emergence of Environmental Homeostasis in Complex Ecosystems. PLoS Comput. Biol. 2013, 9, e1003050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harvey, I. Homeostasis and Rein Control: From Daisyworld to Active Perception; MIT Press: Cambridge, MA, USA, 2004; pp. 309–314. [Google Scholar]
- Moreno-Ruiz, D.; Fuchs, A.; Missbach, K.; Schuhmacher, R.; Zeilinger, S. Influence of Different Light Regimes on the Mycoparasitic Activity and 6-Pentyl-α-pyrone Biosynthesis in Two Strains of Trichoderma atroviride. Pathogens 2020, 9, 860. [Google Scholar] [CrossRef] [PubMed]
- Henríquez-Urrutia, M.; Spanner, R.; Olivares-Yánez, C.; Seguel-Avello, A.; Pérez-Lara, R.; Guillén-Alonso, H.; Winkler, R.; Herrera-Estrella, A.H.; Canessa, P.; Larrondo, L.F. Circadian oscillations in Trichoderma atroviride and the role of core clock components in secondary metabolism, development, and mycoparasitism against the phytopathogen Botrytis cinerea. eLife 2022, 11, e71358. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Cornejo, H.A.; Orozco-Granados, O.; Ramírez-Ordorica, A.; García-Juárez, P.; López-Bucio, J.; Macías-Rodríguez, L. Light and mycelial injury influences the volatile and non-volatile metabolites and the biocontrol properties of Trichoderma atroviride. Rhizosphere 2022, 22, 100511. [Google Scholar] [CrossRef]
- Fossion, R.; Rivera, A.L.; Alvarez-Milláan, L.; García-Iglesias, L.; Lecona, O.; Robles-Cabrera, A.; Esta˜nol, B. A Time-Series Approach to Assess Physiological and Biomechanical Regulatory Mechanisms. In 2019–20 MATRIX Annals; de Gier, J., Praeger, C.E., Tao, T., Eds.; Springer International Publishing: Cham, Switzerlands, 2021; Volume 4, pp. 265–277. [Google Scholar] [CrossRef]
- Barajas-Martínez, A.; Easton, J.F.; Rivera, A.L.; Martínez-Tapia, R.; de la Cruz, L.; Robles-Cabrera, A.; Stephens, C.R. Metabolic Physiological Networks: The Impact of Age. Front. Physiol. 2020, 11, 587994. [Google Scholar] [CrossRef]
- West, B.J. Homeostasis and Gauss statistics: Barriers to understanding natural variability: Homeostasis and Gauss statistics. J. Eval. Clin. Pract. 2010, 16, 403–408. [Google Scholar] [CrossRef]
- Guo, Y.; Jud, W.; Ghirardo, A.; Antritter, F.; Benz, J.P.; Schnitzler, J.; Rosenkranz, M. Sniffing fungi – phenotyping of volatile chemical diversity in Trichoderma species. New Phytol. 2020, 227, 244–259. [Google Scholar] [CrossRef] [PubMed]
- Winkler, R. (Ed.) Processing Metabolomics and Proteomics Data with Open Software: A Practical Guide, 1st ed.; Number 8 in New Developments in Mass Spectrometry; Royal Society of Chemistry: Cambridge, UK, 2020. [Google Scholar]
- Hansel, A.; Jordan, A.; Holzinger, R.; Prazeller, P.; Vogel, W.; Lindinger, W. Proton transfer reaction mass spectrometry: On-line trace gas analysis at the ppb level. Int. J. Mass Spectrom. Ion Process. 1995, 149–150, 609–619. [Google Scholar] [CrossRef]
- Li, L.; Chen, T.C.; Ren, Y.; Hendricks, P.I.; Cooks, R.G.; Ouyang, Z. Mini 12, miniature mass spectrometer for clinical and other applications–introduction and characterization. Anal. Chem. 2014, 86, 2909–2916. [Google Scholar] [CrossRef] [PubMed]
- Analytical Methods, C.A.N. A ‘Periodic Table’ of mass spectrometry instrumentation and acronyms. Anal. Methods 2017, 9, 5086–5090. [Google Scholar] [CrossRef]

| Condition | Skewness | Kurtosis |
|---|---|---|
| A. thaliana | 0.424891559 | −0.319935009 |
| T. atroviride | 3.51034121 | 11.65552257 |
| A. thaliana + T. atroviride | 2.442766689 | 6.019413496 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres-Ortega, R.; Guillén-Alonso, H.; Alcalde-Vázquez, R.; Ramírez-Chávez, E.; Molina-Torres, J.; Winkler, R. In Vivo Low-Temperature Plasma Ionization Mass Spectrometry (LTP-MS) Reveals Regulation of 6-Pentyl-2H-Pyran-2-One (6-PP) as a Physiological Variable during Plant-Fungal Interaction. Metabolites 2022, 12, 1231. https://doi.org/10.3390/metabo12121231
Torres-Ortega R, Guillén-Alonso H, Alcalde-Vázquez R, Ramírez-Chávez E, Molina-Torres J, Winkler R. In Vivo Low-Temperature Plasma Ionization Mass Spectrometry (LTP-MS) Reveals Regulation of 6-Pentyl-2H-Pyran-2-One (6-PP) as a Physiological Variable during Plant-Fungal Interaction. Metabolites. 2022; 12(12):1231. https://doi.org/10.3390/metabo12121231
Chicago/Turabian StyleTorres-Ortega, Rosina, Héctor Guillén-Alonso, Raúl Alcalde-Vázquez, Enrique Ramírez-Chávez, Jorge Molina-Torres, and Robert Winkler. 2022. "In Vivo Low-Temperature Plasma Ionization Mass Spectrometry (LTP-MS) Reveals Regulation of 6-Pentyl-2H-Pyran-2-One (6-PP) as a Physiological Variable during Plant-Fungal Interaction" Metabolites 12, no. 12: 1231. https://doi.org/10.3390/metabo12121231
APA StyleTorres-Ortega, R., Guillén-Alonso, H., Alcalde-Vázquez, R., Ramírez-Chávez, E., Molina-Torres, J., & Winkler, R. (2022). In Vivo Low-Temperature Plasma Ionization Mass Spectrometry (LTP-MS) Reveals Regulation of 6-Pentyl-2H-Pyran-2-One (6-PP) as a Physiological Variable during Plant-Fungal Interaction. Metabolites, 12(12), 1231. https://doi.org/10.3390/metabo12121231







