Continuous Hydrothermal Liquefaction of Biomass: A Critical Review
Abstract
:1. Introduction
1.1. Hydrothermal Liquefaction Basics
1.2. Why Continuous?
- Thermal transience. During batch operations, process conditions are not constant, because the system has to go from ambient conditions to the desired temperature and hence pressure and back. This transience makes it difficult to separate effects of temperature and time, which is sometimes overcome by using the severity index of Overend and Chornet [33], lumping these two into a single parameter. It is evident that, the faster this heating is, the more the effect of thermal transience can be neglected. This can actually be achieved when the experimental device has a reduced size [34].
- Difficulty in decoupling temperature and pressure. In most batch experiments, pressure is obtained by the heating up of the reactants. As a result, the experimental conditions are often those corresponding to saturation conditions of water, i.e., the points lying on the saturation line in the phase diagram. Pre-pressurizing the system with an inert gas can partially overcome this problem. However, high pressure increases the solubility of the inert gas in liquid water, thus making pre-pressurization less and less useful. In a continuous system, pressure and temperature can be controlled in a completely independent fashion.
- Different contact pattern. In a batch reactor, the reactants are usually completely mixed by means of an impeller or by shaking the reactor itself. This contact pattern could be substantially different from that achieved in a continuous reactor. For example, in a continuous stirred flow reactor (CSTR), although there is continuous mixing, new fresh reactant is continuously supplied and products are continuously removed. In a continuous tubular reactor, flow pattern (laminar or turbulent) can significantly change the outcomes of the process.
- Significant distance towards actual industrial implementation. The industrial utilization of batch type reactors is normally justified only for the production of high added-value products, often produced in limited amounts. This is definitely not the case for fuel production, which often accounts for production volumes in the order of thousands of barrels per day. Additionally, HTL requires a thorough optimization in order to reduce the energy consumption of the process, which can be effectively realized only in a continuous configuration.
1.3. Scope of This Review
2. Continuous HTL Systems at a Glance
2.1. Historic Processes
2.2. Research Plants and Setups
2.2.1. Pilot Plants
2.2.2. Bench-Scale Plants
2.3. (Near-) Commercial Processes
3. Interpreting Data from Continuous HTL Processing
3.1. The Influence of Dry Matter Concentration
3.2. The Influence of the Feedstock
4. HTL Products Processing
4.1. Bio-Crude Upgrading
4.2. Aqueous Phase Processing
5. Techno-Economic Considerations
- Optimization of the solid content of the HTL feed slurries.
- Optimization of biocrude yield.
- Optimization of HTL reactor liquid hourly space velocity (LHSV).
- Optimization of heat recovery and phase separation systems.
- Optimization of fuel yield from hydrotreating and minimization of hydrogen consumption.
- Combined large and small HTL scale and integration benefits (e.g., with existing refineries).
6. Final Remarks and Recommendations
6.1. Reactors and Plants
6.2. Developing a Common Paradigm for Product Processing and Results Reporting
6.3. Focusing on the Whole Chain: HTL + Hydrotreating
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- McKendry, P. Energy production from biomass (part 1): Overview of biomass. Bioresour. Technol. 2002, 83, 37–46. [Google Scholar] [CrossRef]
- Ragauskas, A.J.; Williams, C.K.; Davison, B.H.; Britovsek, G.; Cairney, J.; Eckert, C.A.; Frederick, W.J.; Hallett, J.P.; Leak, D.J.; Liotta, C.L.; et al. The Path Forward for Biofuels and Biomaterials. Science 2006, 311, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Wei, N.; Quarterman, J.; Jin, Y.S. Marine macroalgae: An untapped resource for producing fuels and chemicals. Trends Biotechnol. 2013, 31, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Biller, P. Hydrothermal liquefaction of aquatic Feedstocks. In Direct Thermochemical Liquefaction for Energy Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 101–125. [Google Scholar]
- Bridgwater, A.V. Renewable fuels and chemicals by thermal processing of biomass. Chem. Eng. J. 2003, 91, 87–102. [Google Scholar] [CrossRef]
- Naik, S.N.; Goud, V.V.; Rout, P.K.; Dalai, A.K. Production of first and second generation biofuels: A comprehensive review. Renew. Sustain. Energy Rev. 2010, 14, 578–597. [Google Scholar] [CrossRef]
- Oudenhoven, S.R.G.; Kersten, S.R.A. Thermochemical Conversion: An Introduction to Fast Pyrolysis. In Biomass as a Sustainable Energy Source for the Future: Fundamentals of Conversion Processes; De Jong, W., Van Ommen, J.R., Eds.; Wiley Blackwell: Hoboken, NJ, USA, 2014; pp. 359–387. ISBN 978-111830491-4. [Google Scholar]
- Mortensen, P.M.; Grunwaldt, J.D.; Jensen, P.A.; Knudsen, K.G.; Jensen, A.D. A review of catalytic upgrading of bio-oil to engine fuels. Appl. Catal. A Gen. 2011, 407, 1–19. [Google Scholar] [CrossRef]
- Toor, S.S.; Rosendahl, L.; Rudolf, A. Hydrothermal liquefaction of biomass: A review of subcritical water technologies. Energy 2011, 36, 2328–2342. [Google Scholar] [CrossRef]
- Gollakota, A.R.K.; Kishore, N.; Gu, S. A review on hydrothermal liquefaction of biomass. Renew. Sustain. Energy Rev. 2018, 81, 1378–1392. [Google Scholar] [CrossRef]
- Savage, P.E.; Levine, R.B.; Huelsman, C.M.; Crocker, M.; Davis, B.H.; Schüth, F. Hydrothermal Processing of Biomass. In Thermochemical Conversion of Biomass to Liquid Fuels and Chemicals; Royal Society of Chemistry: London, UK, 2010; Chapter 8; pp. 192–221. ISBN 1-84973-035-0. [Google Scholar]
- Pedersen, T.H.; Rosendahl, L.A. Production of fuel range oxygenates by supercritical hydrothermal liquefaction of lignocellulosic model systems. Biomass Bioenergy 2015, 83. [Google Scholar] [CrossRef]
- Jensen, C.U.; Rodriguez Guerrero, J.K.; Karatzos, S.; Olofsson, G.; Iversen, S.B. Fundamentals of HydrofactionTM: Renewable crude oil from woody biomass. Biomass Convers. Biorefin. 2017, 7, 495–509. [Google Scholar] [CrossRef]
- Savage, P.E. Organic Chemical Reactions in Supercritical Water. Chem. Rev. 1999, 99, 603–622. [Google Scholar] [CrossRef] [PubMed]
- Savage, P.E.; Gopalan, S.; Mizan, T.I.; Martino, C.J.; Brock, E.E. Reactions at supercritical conditions: Applications and fundamentals. AIChE J. 1995, 41, 1723–1778. [Google Scholar] [CrossRef]
- Dunn, K.G.; Hobson, P.A. Hydrothermal liquefaction of lignin. In Sugarcane-Based Biofuels and Bioproducts; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; ISBN 9781118719862. [Google Scholar]
- Xu, Y.; Hu, X.; Yu, H.; Wang, K.; Cui, Z. Hydrothermal catalytic liquefaction mechanisms of agal biomass to bio-oil. Energy Sources Part A Recover. Util. Environ. Eff. 2016, 30, 1478–1484. [Google Scholar] [CrossRef]
- Jasiūnas, L.; Pedersen, T.H.; Toor, S.S.; Rosendahl, L.A. Biocrude production via supercritical hydrothermal co-liquefaction of spent mushroom compost and aspen wood sawdust. Renew. Energy 2017, 111, 392–398. [Google Scholar] [CrossRef]
- Zhu, Z.; Toor, S.S.; Rosendahl, L.; Yu, D.; Chen, G. Influence of alkali catalyst on product yield and properties via hydrothermal liquefaction of barley straw. Energy 2015, 80, 284–292. [Google Scholar] [CrossRef]
- Savage, P.E. A perspective on catalysis in sub- and supercritical water. J. Supercrit. Fluids 2009, 47, 407–414. [Google Scholar] [CrossRef]
- Promdej, C.; Matsumura, Y. Temperature effect on hydrothermal decomposition of glucose in sub-and supercritical water. Ind. Eng. Chem. Res. 2011, 50, 8492–8497. [Google Scholar] [CrossRef]
- Zhu, Z.; Rosendahl, L.; Toor, S.S.; Yu, D.; Chen, G. Hydrothermal liquefaction of barley straw to bio-crude oil: Effects of reaction temperature and aqueous phase recirculation. Appl. Energy 2015, 137, 183–192. [Google Scholar] [CrossRef]
- Akhtar, J.; Amin, N.A.S. A review on process conditions for optimum bio-oil yield in hydrothermal liquefaction of biomass. Renew. Sustain. Energy Rev. 2011, 15, 1615–1624. [Google Scholar] [CrossRef]
- Peterson, A.A.; Vogel, F.; Lachance, R.P.; Fröling, M.; Antal, M.J., Jr.; Tester, J.W. Thermochemical biofuel production in hydrothermal media: A review of sub- and supercritical water technologies. Energy Environ. Sci. 2008, 1, 32. [Google Scholar] [CrossRef] [Green Version]
- Jensen, C.U.; Guerrero, J.K.R.; Karatzos, S.; Olofsson, G.; Iversen, S.B. HydrofactionTM of forestry residues to drop-in renewable transportation fuels. In Direct Thermochemical Liquefaction for Energy Applications; Rosendahl, L., Ed.; Woodhead Publishing—Elsevier: Sawston, UK, 2017; pp. 319–345. ISBN 978-0-08-101029-7. [Google Scholar]
- Osada, M.; Sato, T.; Watanabe, M.; Shirai, M.; Arai, K. Catalytic gasification of wood biomass in subcritical and supercritical water. Combust. Sci. Technol. 2006, 178, 537–552. [Google Scholar] [CrossRef]
- Pedersen, T.H.; Grigoras, I.F.; Hoffmann, J.; Toor, S.S.; Daraban, I.M.; Jensen, C.U.; Iversen, S.B.; Madsen, R.B.; Glasius, M.; Arturi, K.R.; et al. Continuous hydrothermal co-liquefaction of aspen wood and glycerol with water phase recirculation. Appl. Energy 2016, 162, 1034–1041. [Google Scholar] [CrossRef]
- Sintamarean, I.M.; Grigoras, I.F.; Jensen, C.U.; Toor, S.S.; Pedersen, T.H.; Rosendahl, L.A. Two-stage alkaline hydrothermal liquefaction of wood to biocrude in a continuous bench-scale system. Biomass Convers. Biorefin. 2017, 7. [Google Scholar] [CrossRef]
- Wagner, W.; Pruß, A. The IAPWS formulation 1995 for the thermodynamic properties of ordinary water substance for general and scientific use. J. Phys. Chem. Ref. Data 2002, 31, 387–535. [Google Scholar] [CrossRef]
- Uematsu, M.; Franck, E.U. Static dielectric constant of water and steam. J. Phys. Chem. Ref. Data 1980, 9, 1291–1306. [Google Scholar] [CrossRef]
- Marshall, W.L.; Franck, E.U. Ion product of water substance, 0–1000 °C, 1–10,000 bars. New international formulation and its background. J. Phys. Chem. Ref. Data 1981, 10, 295–304. [Google Scholar] [CrossRef]
- Xu, D.; Savage, P.E. Characterization of biocrudes recovered with and without solvent after hydrothermal liquefaction of algae. Algal Res. 2014, 6, 1–7. [Google Scholar] [CrossRef]
- Overend, R.P.; Chornet, E. Fractionation of lignocellulosics by steam aqueous pretreatments. Phil. Trans. R. Soc. Lond. A 1987, 321, 523–536. [Google Scholar] [CrossRef]
- Pedersen, T.H. Hydro Thermal Liquefaction of Biomass and Model Compounds; Aalborg University: Aalborg, Denmark, 2016; ISBN 9788771124972. [Google Scholar]
- Elliott, D.C.; Biller, P.; Ross, A.B.; Schmidt, A.J.; Jones, S.B. Hydrothermal liquefaction of biomass: Developments from batch to continuous process. Bioresour. Technol. 2015, 178, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Thigpen, P.L.; Berry, W.L., Jr. Liquid fuels from wood by continuous operation of the Albany, Oregon biomass liquefaction facility. In Proceedings of the Energy from Biomass and Wastes VI: Symposium, Lake Buena Vista, FL, USA, 25–29 January 1982; pp. 1057–1095. [Google Scholar]
- Schaleger, L.L.; Figueroa, C.; Davis, H.G. Direct liquefaction of biomass: Results from operation of continuous bench scale unit in liquefaction of water slurries of Douglas fir wood. In Proceedings of the 4th Symposium on Biotechnology in Energy Production and Conservation, Gatlinburg, TN, USA, 11–14 May 1982. [Google Scholar]
- Molton, P.M.; Fassbender, A.G.; Brown, M.D. STORS: The Sludge-to-Oil Reactor System; Report No. EPA/600/S2-86/034; U.S. Enviromental Protection Agency (EPA): Cincinnati, OH, USA, 1986. [Google Scholar]
- Itoh, S.; Suzuki, A.; Nakamura, T.; Yokoyama, S.Y. Production of heavy oil from sewage sludge by direct thermochemical liquefaction. Desalination 1994, 98, 127–133. [Google Scholar] [CrossRef]
- Goudriaan, F.; Peferoen, D.G.R. Liquid fuels from biomass via a hydrothermal process. Chem. Eng. Sci. 1990, 45, 2729–2734. [Google Scholar] [CrossRef]
- Goudriaan, F.; Naber, J.E. Biomass to liquid fuels via HTU®. In Biomass Power for the World: Transformations to Effective Use; Van Swaaij, W., Kersten, S., Palz, W., Eds.; Pan Stanford Publishing Pte. Ltd.: New York, NY, USA, 2015; pp. 631–664. ISBN 978-981-4669-24-5. [Google Scholar]
- Elliott, D.C.; Hart, T.R.; Schmidt, A.J.; Neuenschwander, G.G.; Rotness, L.J.; Olarte, M.V.; Zacher, A.H.; Albrecht, K.O.; Hallen, R.T.; Holladay, J.E. Process development for hydrothermal liquefaction of algae feedstocks in a continuous-flow reactor. Algal Res. 2013, 2, 445–454. [Google Scholar] [CrossRef] [Green Version]
- Albrecht, K.O.; Zhu, Y.; Schmidt, A.J.; Billing, J.M.; Hart, T.R.; Jones, S.B.; Maupin, G.; Hallen, R.; Ahrens, T.; Anderson, D. Impact of heterotrophically stressed algae for biofuel production via hydrothermal liquefaction and catalytic hydrotreating in continuous-flow reactors. Algal Res. 2016, 14, 17–27. [Google Scholar] [CrossRef] [Green Version]
- Elliott, D.C.; Hart, T.R.; Neuenschwander, G.G.; Rotness, L.J.; Roesijadi, G.; Zacher, A.H.; Magnuson, J.K. Hydrothermal processing of macroalgal feedstocks in continuous-flow reactors. ACS Sustain. Chem. Eng. 2014, 2, 207–215. [Google Scholar] [CrossRef]
- Elliott, D.C.; Schmidt, A.J.; Hart, T.R.; Billing, J.M. Conversion of a wet waste feedstock to biocrude by hydrothermal processing in a continuous-flow reactor: Grape pomace. Biomass Convers. Biorefin. 2017, 7, 455–465. [Google Scholar] [CrossRef]
- Marrone, P.A.; Elliott, D.C.; Billing, J.M.; Hallen, R.T.; Hart, T.R.; Kadota, P.; Moeller, J.C.; Randel, M.A.; Schmidt, A.J. Bench-scale evaluation of hydrothermal processing technology for conversion of wastewater solids to fuels. Water Environ. Res. 2018, 90, 329–342. [Google Scholar] [CrossRef] [PubMed]
- Jazrawi, C.; Biller, P.; Ross, A.B.; Montoya, A.; Maschmeyer, T.; Haynes, B.S. Pilot plant testing of continuous hydrothermal liquefaction of microalgae. Algal Res. 2013, 2, 268–277. [Google Scholar] [CrossRef] [Green Version]
- He, Y.; Liang, X.; Jazrawi, C.; Montoya, A.; Yuen, A.; Cole, A.J.; Neveux, N.; Paul, N.A.; de Nys, R.; Maschmeyer, T.; et al. Continuous hydrothermal liquefaction of macroalgae in the presence of organic co-solvents. Algal Res. 2016, 17, 185–195. [Google Scholar] [CrossRef]
- Ocfemia, K.S.; Zhang, Y.; Funk, T. Hydrothermal processing of swine manure into oil using a continuous reactor system: Development and testing. Trans. ASABE 2006, 49, 533–541. [Google Scholar] [CrossRef]
- Ocfemia, K.S.; Zhang, Y.; Funk, T. Hydrothermal processing of swine manure into oil using a continuous reactor system: Effects of operating parameters on oil yield and quality. Trans. ASABE 2006, 49, 1897–1904. [Google Scholar] [CrossRef]
- Suesse, A.R.; Norton, G.A.; Van Leeuwen, J. Pilot-Scale Continuous-Flow Hydrothermal Liquefaction of Filamentous Fungi. Energy Fuels 2016, 30, 7379–7386. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.D.H.; Maschietti, M.; Belkheiri, T.; Åmand, L.E.; Theliander, H.; Vamling, L.; Olausson, L.; Andersson, S.I. Catalytic depolymerisation and conversion of Kraft lignin into liquid products using near-critical water. J. Supercrit. Fluids 2014, 86, 67–75. [Google Scholar] [CrossRef]
- Nguyen, T.D.H.; Maschietti, M.; Åmand, L.E.; Vamling, L.; Olausson, L.; Andersson, S.I.; Theliander, H. The effect of temperature on the catalytic conversion of Kraft lignin using near-critical water. Bioresour. Technol. 2014, 170, 196–203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belkheiri, T.; Mattsson, C.; Andersson, S.I.; Olausson, L.; Åmand, L.E.; Theliander, H.; Vamling, L. Effect of pH on Kraft Lignin Depolymerisation in Subcritical Water. Energy Fuels 2016, 30, 4916–4924. [Google Scholar] [CrossRef]
- Belkheiri, T.; Andersson, S.I.; Mattsson, C.; Olausson, L.; Theliander, H.; Vamling, L. Hydrothermal liquefaction of kraft lignin in sub-critical water: The influence of the sodium and potassium fraction. Biomass Convers. Biorefin. 2018, 8, 585–595. [Google Scholar] [CrossRef]
- Belkheiri, T.; Andersson, S.I.; Mattsson, C.; Olausson, L.; Theliander, H.; Vamling, L. Hydrothermal Liquefaction of Kraft Lignin in Subcritical Water: Influence of Phenol as Capping Agent. Energy Fuels 2018, 32, 5923–5932. [Google Scholar] [CrossRef]
- Hammerschmidt, A.; Boukis, N.; Hauer, E.; Galla, U.; Dinjus, E.; Hitzmann, B.; Larsen, T.; Nygaard, S.D. Catalytic conversion of waste biomass by hydrothermal treatment. Fuel 2011, 90, 555–562. [Google Scholar] [CrossRef]
- Hammerschmidt, A.; Boukis, N.; Galla, U.; Dinjus, E.; Hitzmann, B. Conversion of yeast by hydrothermal treatment under reducing conditions. Fuel 2011, 90, 3424–3432. [Google Scholar] [CrossRef]
- Hammerschmidt, A.; Boukis, N.; Galla, U.; Zevaco, T.; Dinjus, E.; Hitzmann, B. Influence of the heating rate and the potassium concentration of the feed solution on the hydrothermal liquefaction of used yeast and apple pomace under reducing conditions. Biomass Convers. Biorefin. 2015, 5, 125–139. [Google Scholar] [CrossRef]
- Barreiro, D.L.; Gómez, B.R.; Hornung, U.; Kruse, A.; Prins, W. Hydrothermal Liquefaction of Microalgae in a Continuous Stirred-Tank Reactor. Energy Fuels 2015, 29, 6422–6432. [Google Scholar] [CrossRef]
- Biller, P.; Sharma, B.K.; Kunwar, B.; Ross, A.B. Hydroprocessing of bio-crude from continuous hydrothermal liquefaction of microalgae. Fuel 2015, 159, 197–205. [Google Scholar] [CrossRef]
- Mørup, A.J.; Becker, J.; Christensen, P.S.; Houlberg, K.; Lappa, E.; Klemmer, M.; Madsen, R.B.; Glasius, M.; Iversen, B.B. Construction and Commissioning of a Continuous Reactor for Hydrothermal Liquefaction. Ind. Eng. Chem. Res. 2015, 54, 5935–5947. [Google Scholar] [CrossRef]
- Biller, P.; Madsen, R.B.; Klemmer, M.; Becker, J.; Iversen, B.B.; Glasius, M. Effect of hydrothermal liquefaction aqueous phase recycling on bio-crude yields and composition. Bioresour. Technol. 2016, 220, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Anastasakis, K.; Biller, P.; Madsen, R.; Glasius, M.; Johannsen, I.; Anastasakis, K.; Biller, P.; Madsen, R.B.; Glasius, M.; Johannsen, I. Continuous Hydrothermal Liquefaction of Biomass in a Novel Pilot Plant with Heat Recovery and Hydraulic Oscillation. Energies 2018, 11, 2695. [Google Scholar] [CrossRef]
- Patel, B.; Hellgardt, K. Hydrothermal upgrading of algae paste in a continuous flow reactor. Bioresour. Technol. 2015, 191, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Wagner, J.L.; Le, C.D.; Ting, V.P.; Chuck, C.J. Design and operation of an inexpensive, laboratory-scale, continuous hydrothermal liquefaction reactor for the conversion of microalgae produced during wastewater treatment. Fuel Process. Technol. 2017, 165, 102–111. [Google Scholar] [CrossRef] [Green Version]
- Wądrzyk, M.; Janus, R.; Vos, M.P.; Brilman, D.W.F. Effect of process conditions on bio-oil obtained through continuous hydrothermal liquefaction of Scenedesmus sp. microalgae. J. Anal. Appl. Pyrolysis 2018, 134, 415–426. [Google Scholar] [CrossRef]
- Licella Pty Ltd. Corporate Website. Available online: http://www.licella.com.au (accessed on 25 April 2018).
- Muradel Pty Ltd. Corporate Website. Available online: http://www.muradel.com.au (accessed on 26 April 2018).
- Genifuel Corp. Corporate Website. Available online: http://www.genifuel.com (accessed on 23 April 2018).
- Marrone, P.A. Genifuel Hydrothermal Processing Bench-Scale Technology Evaluation Project; Water Environment and Reuse Foundation: Alexandria, VA, USA, 2016; ISBN 9781780408408. [Google Scholar]
- ENI S.p.A. From Waste to Biofuel. Available online: https://www.eni.com/en_IT/innovation/technological-platforms/bio-refinery/waste-to-fuel.page (accessed on 29 October 2018).
- Caretta, A.; Riccò, M.; Bosetti, A.; Burattini, M.; Carnelli, L.; Miglio, R.; Volpato, C.B. Waste to fuel—Conversion of waste into energy. In Proceedings of the 9th European Congress of Chemical Engineering, The Hague, The Netherlands, 21–24 April 2013. [Google Scholar]
- Toor, S.S.; Rosendahl, L.; Nielsen, M.P.; Glasius, M.; Rudolf, A.; Iversen, S.B. Continuous production of bio-oil by catalytic liquefaction from wet distiller’s grain with solubles (WDGS) from bio-ethanol production. Biomass Bioenergy 2012, 36, 327–332. [Google Scholar] [CrossRef]
- Unsal, M.; Livatyali, H.; Aksoy, P.; Gul, S.; Onoglu, A. CatLiq-Catalytic hydrothermal liquefaction process from pilot scale to demo scale. J. Fundam. Renew. Energy Appl. 2015, 5, 69. [Google Scholar] [CrossRef]
- Altaca Enerji Corporate Website. Available online: http://www.altacaenerji.com/ (accessed on 29 August 2018).
- Changing World Technologies Inc. Corporate Website. Available online: www.changingworldtech.com (accessed on 1 June 2018).
- Roberts, M.; Williams, J.; Halberstadt, P.; Sanders, D.; Adams, T. Animal waste to marketable products. In Proceedings of the Natural Gas Technologies Conference, Phoenix, AZ, USA, 8–11 February 2004. [Google Scholar]
- Sintamarean, I.-M. Feedstock Preparation and Physico-Chemical Characterization. Optimization of Feedstocks for Continuous HTL and Optimum Yield; Aalborg University: Aalborg, Denmark, 2017; ISBN 9788771129434. [Google Scholar]
- Appell, H.R.; Fu, Y.C.; Illig, E.G.; Steffgen, F.W.; Miller, R.D. Conversion of Cellulosic Wastes to Oil; Report No. 8013; U.S. Bureau of Mines: Washington, DC, USA, 1975.
- Appell, H.R.; Fu, Y.C.; Friedman, S.; Yavorsky, P.M.; Wender, I. Converting Organic Wastes to Oil: A Replenishable Energy Source; Report No. 7560; U.S. Bureau of Mines: Washington, DC, USA, 1971.
- Bergius, F. Die Anwendung Hoher Drücke bei Chemischen Vorgängen und Eine Nachbildung des Entstehungsprozesses der Steinkohle; Knapp: Halle/Saale, Germany, 1913. [Google Scholar]
- Lindemuth, T.E. Carboxylolysis of Biomass. In Biomass Conversion Processes for Energy and Fuels; Sofer, S.S., Zaborsky, O.R., Eds.; Springer: Boston, MA, USA, 1981; pp. 187–200. ISBN 978-1-4757-0303-0. [Google Scholar]
- Elliott, D.C. Hydrothermal Processing. In Thermochemical Processing of Biomass; Brown, R.C., Ed.; John Wiley & Sons: Chichester, UK, 2011; pp. 200–231. ISBN 9780470721117. [Google Scholar]
- Stevens, D.J. Review and Analysis of the 1980–1989 Biomass Thermochemical Conversion Program; Report No. NREL/TP-421-7501; National Renewable Energy Laboratory (NREL): Golden, CO, USA, 1994. [Google Scholar]
- Willner, T.; Brunner, G. Umwandlung von Holz unter dem Einfluß von Wasserstoff und Wasser unter höheren Drücken. Chem. Ing. Tech. 1994, 66, 72–74. [Google Scholar] [CrossRef]
- Behrendt, F.; Neubauer, Y.; Oevermann, M.; Wilmes, B.; Zobel, N. Direct liquefaction of biomass. Chem. Eng. Technol. 2008, 31, 667–677. [Google Scholar] [CrossRef]
- Pedersen, T.H.; Jensen, C.U.; Sandström, L.; Rosendahl, L.A. Full characterization of compounds obtained from fractional distillation and upgrading of a HTL biocrude. Appl. Energy 2017, 202, 408–419. [Google Scholar] [CrossRef]
- Maschmeyer, T.; Humphreys, L.J. Methods for Biofuel Production. Patent No. WO 2011/123897 A1, 13 October 2011. [Google Scholar]
- Maschmeyer, T. Processing of Organic Matter. Patent No. WO 2012/092644 A1, 12 July 2012. [Google Scholar]
- Sustainable Development Technology Canada. Pulp Mill Biocrude Demonstration Project. Available online: https://www.sdtc.ca/en/portfolio/projects/pulp-mill-biocrude-demonstration-project (accessed on 2 August 2018).
- Lane, J. The Wonder from Down Under, and Canada’ll Fund ‘er: Canfor Picks Up $13M for Licella Biofuels Project. Available online: http://www.biofuelsdigest.com/bdigest/2017/03/14/the-wonder-from-down-under-and-canadall-fund-er-canfor-picks-up-13m-for-licella-biofuels-project/ (accessed on 2 August 2018).
- Lane, J. The Silver in Silva: The Story of Steeper Energy and SGF’s’s $59M Advanced Biofuels Project in Norway. Available online: http://www.biofuelsdigest.com/bdigest/2018/01/16/the-silver-in-silva-the-story-of-steeper-energys-59m-advanced-biofuels-project-in-norway (accessed on 17 April 2018).
- Chinnasamy, S.; Bhaskar, S.; Nallasivam, J.; Kumar Ratha, S.; Lewis, D.M.; Meenakshisundaram, A.; Lavanya, M.; Selvavathi, C. Method for Processing Algae, Carbonaceous Feedstocks, and Their Mixtures to Biocrude and Its Conversion into Biofuel Products. Patent No. US 2017/0198223 A1, 13 July 2017. [Google Scholar]
- Bosetti, A.; Bianchi, D.; Franzosi, G.; Ricci, M. Process for the Production of Bio-Oil from Solid Urban Waste. Patent No. WO 2011/030196 A1, 17 March 2011. [Google Scholar]
- Voegele, E. NextFuels Targets Palm Oil Waste for Advanced Biofuel Production. Available online: http://biomassmagazine.com/articles/9327/nextfuels-targets-palm-oil-waste-for-advanced-biofuel-production (accessed on 29 August 2018).
- Iversen, S.B.; Felsvang, K.S.; Larsen, T.; Lüthje, V. Method and Apparatus for Converting Organic Material. Patent No. US 7,678,163 B2, 16 March 2010. [Google Scholar]
- Nielsen, R.P.; Olofsson, G.; Søgaard, E.G. CatLiq—High pressure and temperature catalytic conversion of biomass: The CatLiq technology in relation to other thermochemical conversion technologies. Biomass Bioenergy 2012, 39, 399–402. [Google Scholar] [CrossRef]
- Baskis, P.T. Thermal Depolymerizing Reforming Process and Apparatus. Patent No. US 5,269,947, 14 December 1993. [Google Scholar]
- Biller, P.; Roth, A. Hydrothermal Liquefaction: A Promising Pathway Towards Renewable Jet Fuel. In Biokerosene; Springer: Berlin/Heidelberg, Germany, 2018; pp. 607–635. ISBN 978-3-662-53063-4. [Google Scholar]
- Sustainability Matters Pilot Project to Turn Biosolids into Crude Oil. Available online: https://www.sustainabilitymatters.net.au/content/energy/news/pilot-project-to-turn-biosolids-into-crude-oil-763693234 (accessed on 3 October 2018).
- Channiwala, S.A.; Parikh, P.P. A unified correlation for estimating HHV of solid, liquid and gaseous fuels. Fuel 2002, 81, 1051–1063. [Google Scholar] [CrossRef]
- Dãrãban, I.M.; Rosendahl, L.A.; Pedersen, T.H.; Iversen, S.B. Pretreatment methods to obtain pumpable high solid loading wood-water slurries for continuous hydrothermal liquefaction systems. Biomass Bioenergy 2015, 81, 437–443. [Google Scholar] [CrossRef]
- Sintamarean, I.M.; Pedersen, T.H.; Zhao, X.; Kruse, A.; Rosendahl, L.A. Application of Algae as Cosubstrate to Enhance the Processability of Willow Wood for Continuous Hydrothermal Liquefaction. Ind. Eng. Chem. Res. 2017, 56, 4562–4571. [Google Scholar] [CrossRef]
- Berglin, E.J.; Enderlin, C.W.; Schmidt, A.J. Review and Assessment of Commercial Vendors/Options for Feeding and Pumping Biomass Slurries for Hydrothermal Liquefaction; Report No. PNNL-21981; Pacific Northwest National Laboratory (PNNL): Richland, WA, USA, 2012. [Google Scholar]
- Elliott, D.C. Historical developments in hydroprocessing bio-oils. Energy Fuels 2007, 21, 1792–1815. [Google Scholar] [CrossRef]
- Baker, E.G.; Elliott, D.C. Method of Upgrading Oils Containing Hydroxyaromatic Hydrocarbon Compounds to Highly Aromatic Gasoline. Patent No. US 5,180,868, 19 January 1993. [Google Scholar]
- Gevert, B.S.; Otterstedt, J.E. Upgrading of directly liquefied biomass to transportation fuels—Hydroprocessing. Biomass 1987, 13, 105–115. [Google Scholar] [CrossRef]
- Gevert, S.B.; Andersson, P.B.W.; Sandqvist, S.P.; Järås, S.G.; Tokarz, M.T. Hydroprocessing of Directly Liquefied Biomass with Large-Pore Catalysts. Energy Fuels 1990, 4, 78–81. [Google Scholar] [CrossRef]
- Kunwar, B.; Deilami, S.D.; Macaskie, L.E.; Wood, J.; Biller, P.; Sharma, B.K. Nanoparticles of Pd supported on bacterial biomass for hydroprocessing crude bio-oil. Fuel 2017, 209, 449–456. [Google Scholar] [CrossRef]
- Patel, B.; Arcelus-Arrillaga, P.; Izadpanah, A.; Hellgardt, K. Catalytic Hydrotreatment of algal biocrude from fast Hydrothermal Liquefaction. Renew. Energy 2017, 101, 1094–1101. [Google Scholar] [CrossRef]
- López Barreiro, D.; Gómez, B.R.; Ronsse, F.; Hornung, U.; Kruse, A.; Prins, W. Heterogeneous catalytic upgrading of biocrude oil produced by hydrothermal liquefaction of microalgae: State of the art and own experiments. Fuel Process. Technol. 2016, 148, 117–127. [Google Scholar] [CrossRef]
- Jensen, C.U.; Hoffmann, J.; Rosendahl, L.A. Co-processing potential of HTL bio-crude at petroleum refineries. Part 2: A parametric hydrotreating study. Fuel 2015, 165, 536–543. [Google Scholar] [CrossRef]
- Jensen, C.U.; Rosendahl, L.A.; Olofsson, G. Impact of nitrogenous alkaline agent on continuous HTL of lignocellulosic biomass and biocrude upgrading. Fuel Process. Technol. 2017, 159, 376–385. [Google Scholar] [CrossRef]
- Elliott, D.C.; Wang, H.; French, R.; Deutch, S.; Iisa, K. Hydrocarbon liquid production from biomass via hot-vapor-filtered fast pyrolysis and catalytic hydroprocessing of the bio-oil. Energy Fuels 2014, 28, 5909–5917. [Google Scholar] [CrossRef]
- Elliott, D.C. Catalytic hydrothermal gasification of biomass. Biofuels Bioprod. Biorefin. 2008, 2, 254–265. [Google Scholar] [CrossRef]
- Mawhood, R.; Gazis, E.; de Jong, S.; Hoefnagels, R.; Slade, R. Production pathways for renewable jet fuel: A review of commercialization status and future prospects. Biofuels Bioprod. Biorefin. 2016, 10, 462–484. [Google Scholar] [CrossRef]
- Lane, J. Eight under $70: Which Biofuels Ventures Can Beat out Cheap Oil? Available online: http://www.biofuelsdigest.com/bdigest/2015/01/12/eight-under-70-which-biofuels-ventures-can-beat-out-cheap-oil/ (accessed on 17 April 2018).
- De Jong, S.; Hoefnagels, R.; Faaij, A.; Slade, R.; Mawhood, R.; Junginger, M. The feasibility of short-term production strategies for renewable jet fuels—A comprehensive techno-economic comparison. Biofuels Bioprod. Biorefin. 2015, 9, 778–800. [Google Scholar] [CrossRef]
- Zhu, Y.; Biddy, M.J.; Jones, S.B.; Elliott, D.C.; Schmidt, A.J. Techno-economic analysis of liquid fuel production from woody biomass via hydrothermal liquefaction (HTL) and upgrading. Appl. Energy 2014, 129, 384–394. [Google Scholar] [CrossRef]
- Pedersen, T.H.; Hansen, N.H.; Pérez, O.M.; Cabezas, D.E.V.; Rosendahl, L.A. Renewable hydrocarbon fuels from hydrothermal liquefaction: A techno-economic analysis. Biofuels Bioprod. Biorefin. 2018, 12, 213–223. [Google Scholar] [CrossRef]
- Ou, L.; Thilakaratne, R.; Brown, R.C.; Wright, M.M. Techno-economic analysis of transportation fuels from defatted microalgae via hydrothermal liquefaction and hydroprocessing. Biomass Bioenergy 2015, 72, 45–54. [Google Scholar] [CrossRef]
- Knorr, D.; Lukas, J.; Schoen, P. Production of Advanced Biofuels via Liquefaction. Hydrothermal Liquefaction Reactor Design; Report No. 30352.00/01; National Renewable Energy Laboratory (NREL): Golden, CO, USA, 2013. [Google Scholar]
- Anastasakis, K.; Johannsen, I.; Madsen, R.B.; Biller, P. Assessing hydrothermal liquefaction of lignocellulosic biomass, microalgae and sewage sludge at pilot scale. In Proceedings of the 26th European Biomass Conference and Exhibition, Copenhagen, Denmark, 14–17 May 2018. [Google Scholar]
- Castello, D.; Rosendahl, L. Coprocessing of pyrolysis oil in refineries. In Direct Thermochemical Liquefaction for Energy Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 293–317. ISBN 9780081010297. [Google Scholar]



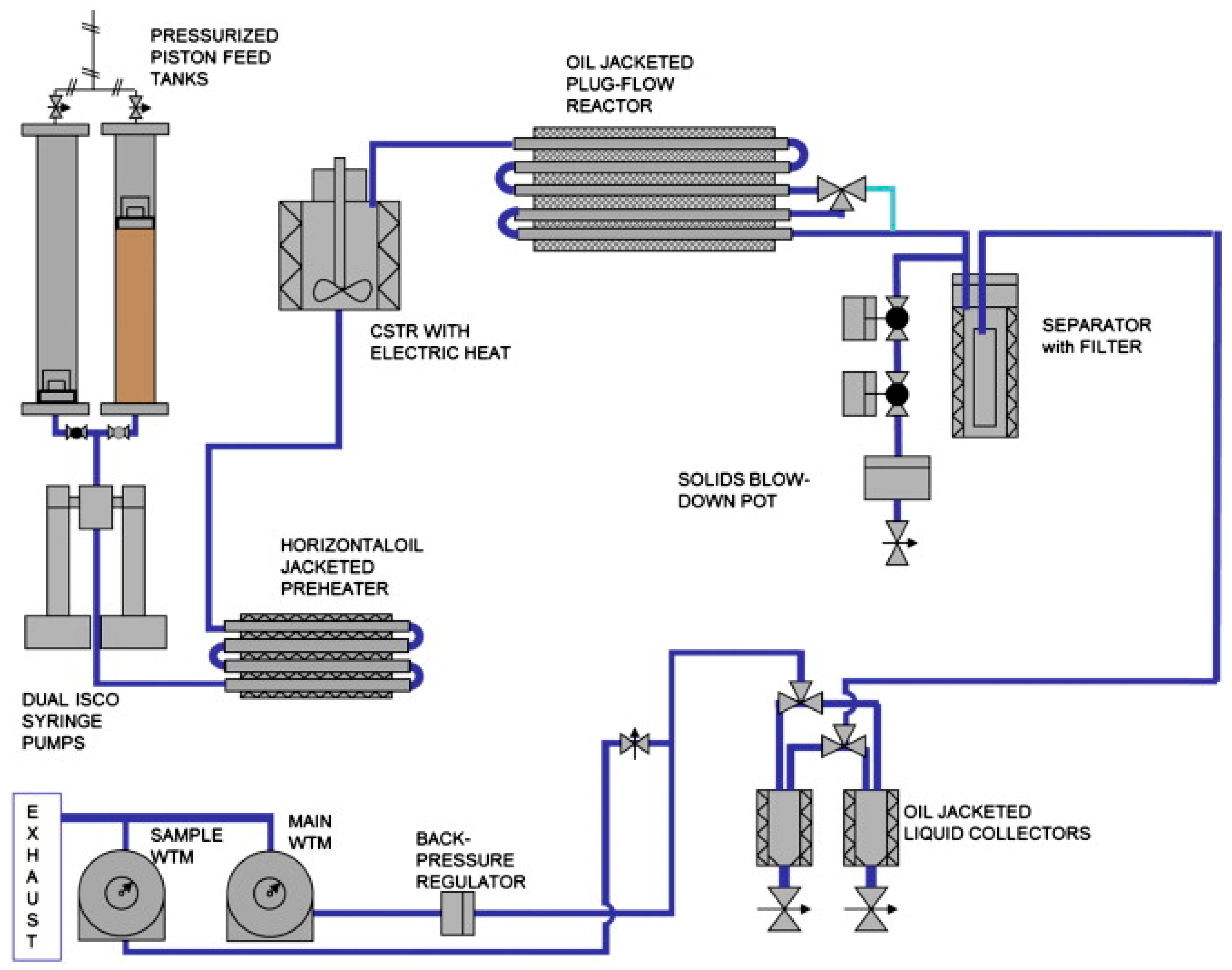






| Process/Plant | Reactor Concept | Biomass | Throughput (kg/h) | Pressure (bar) | Temp. (°C) | Residence Time (min) | Catalyst | Ref. |
|---|---|---|---|---|---|---|---|---|
| PDU–PERC Albany, USA | Tubular/stirred | Wood | 230–270 | 207 | 330–340 | 19–100 | Na2CO3 | [36] |
| PDU–LBL Albany, USA | Tubular/stirred | Wood | 43–360 | 207 | 340–345 | 11.3–465 | Na2CO3 | [36] |
| LBL Berkeley, USA | Stirred | Wood | ~1 | 200–230 | 330–350 | ~20 | - | [37] |
| STORS–EPA, USA | Column | Sewage sludge | 30 | 86–148 | 275–305 | 90 | Na2CO3 | [38] |
| STORS–Organo Corp., Japan | Column | Sewage sludge | 240 | 88–98 | 290–300 | N/A | - | [39] |
| HTU® process Shell, The Netherlands | Tubular | Wood | 10 | 180 | 350 | 6 | - | [40] |
| HTU® process Biofuels B.V., The Netherlands | Tubular | Sugar beet pulp, onion pulp | 100 | 180 | 350 | 15 | - | [41] |
| Pacific Northwest National Laboratories (PNNL), USA | Stirred + tubular | Algae | 1.5 | 200 | 350 | 27–50 | - | [42,43] |
| Macroalgae | - | [44] | ||||||
| Grape pomace | Na2CO3 | [45] | ||||||
| Wastewater solids | - | [46] | ||||||
| University of Sydney, Australia | Coils in sandbath | Algae | 24–40 | 200–250 | 350 | 15–20 | - | [47,48] |
| University of Illinois, USA | Stirred | Swine manure | 0.9–2.0 | 103 | 305 | 40–80 | - | [49,50] |
| Iowa State University, USA | Tubular | Fungi | 3.0–7.5 | 270 | 300–400 | 11–31 | - | [51] |
| Chalmers University of Technology, Sweden | Fixed bed, with recycle loop | Kraft lignin | 1–2 | 250 | 350 | 6–11 | ZrO2, K2CO3 | [52,53,54,55,56] |
| Aalborg University, Denmark | Tubular | Wood/glycerol | 20 | 300–350 | 390–420 | 15 | K2CO3 | [27] |
| Wood | [28] | |||||||
| Karlsruhe Institute of Technology (KIT), Germany | Tubular, with recirculation | Waste biomass | 0.29–0.63 | 250 | 330–350 | 5–10 | K2CO3, ZrO2 | [57] |
| Tubular, with MeOH gasifier | Yeast, pomace | 0.06–0.61 | 200–250 | 330–450 | 1–30 | K2CO3, ZrO2 | [58,59] | |
| Stirred | Algae | 0.76 | 200 | 350 | 15 | - | [60] | |
| University of Leeds, UK | Coils in sandbath | Chlorella | 0.6–2.4 | 185 | 350 | 1.4–5.8 | - | [61] |
| Aarhus University, Denmark | Tubular (bench) | Dried digested grains with solubles | 0.36–1.44 | 250 | 250–350 | ~20 | K2CO3 | [62,63] |
| Tubular (pilot), with oscillator | Wood, sewage sludge, Spirulina | 60 | 220 | 350 | 10 | KOH, no | [64] | |
| Imperial College London, UK | Tubular, hexane as co-solvent | Algae | 0.03–0.24 | 180 | 300–380 | 0.5–4 | - | [65] |
| Bath University, UK | Concentric tubular | Wastewater algae | 0.18–0.42 | 160 | 302–344 | 17.7–41.8 | - | [66] |
| University of Twente, The Netherlands | Coils in sandbath | Scenedesmus sp. | 0.06–0.33 | 150–300 | 250–350 | 7–30 | - | [67] |
| Cat-HTR™ process Licella, Australia | N/A | Pulp/paper, plastics | 10,000 t/y 2 | N/A | N/A | N/A | Yes | [68] |
| Hydrofaction™ process Steeper Energy 3, Denmark-Canada | Tubular | Wood | 20 | 300–350 | 390–420 | 15 | K2CO3 | [13] |
| Green2black™ process Muradel, Australia | Tubular | Tires, algae | 168 | 200 | 360 | 10 | N/A | [69] |
| HTP process Genifuel, USA | Stirred + tubular 1 | Sewage sludge | N/A | 200 | 350 | 45 | N/A | [70,71] |
| W2F process ENI S.p.A., Italy | N/A | Organic fraction of municipal solid waste | 1–5 | 100 | 250–310 | 60–120 | N/A | [72,73] |
| CatLiq® process SCF Technologies, Denmark | Stirred | Wet digested grains with solubles | 30 | 250 | 350 | 1–15 | ZrO2 | [74] |
| CatLiq® process Altaca Enerji, Turkey | N/A | Different wastes and residues | 15,000 2 | 250 | 350 | N/A | Yes | [75,76] |
| TDP process Changing World Technologies, USA | N/A | Turkey waste | 8500 | N/A | 200–300 4 | N/A | - | [77,78] |
| Location | Biomass | HTL Bio-Crude | Catalyst | Upgraded Bio-Crude | Overall Yield (wt. %) | C Yield (wt. %) | Ref. | ||
|---|---|---|---|---|---|---|---|---|---|
| HHV (MJ/kg) | H/C (-) | HHV (MJ/kg) | H/C (-) | ||||||
| Chalmers Institute of Technology, Sweden 1 | Wood | 36.3 | 1.38 | CoMo/Al2O3 | 42.5 | 1.44 | N/A | N/A | [106,108] |
| University of Leeds, UK | Algae (Chlorella) | 36.1 | 1.55 | NiMo | 41.5 | 1.57 | 34.3 | 49.0 | [61] |
| University of Illinois, USA 2 | Algae (Chlorella) | 35.7 | 1.45 | Pd/C | 43.1 | 1.64 | 26.5 | 38.7 | [110] |
| Karlsruhe Institute of Technology, Germany | Algae (Scenedesmus) | 36.3 | 1.52 | Pt/Al2O3 | 41.9 | 1.60 | 31.5 | 54.9 | [112] |
| HZSM-5 | 40.7 | 1.50 | 31.5 | 54.2 | |||||
| Algae (Nannochloropsis) | 37 | 1.63 | Pt/Al2O3 | 43.2 | 1.67 | 33.9 | 52.6 | ||
| HZSM-5 | 42.3 | 1.62 | 27.6 | 42.5 | |||||
| Imperial College London, UK | Algae (Nannochloropsis) | 36.5 | 1.58 | NiMo/Al2O3 | 38.3 | 1.89 | 35.7 | 60.0 | [111] |
| Ru/Al2O3 | 43.9 | 1.67 | 23.5 | 45.0 | |||||
| Pt/Al2O3 | 45.4 | 1.78 | 23.5 | 45.2 | |||||
| Pd/Al2O3 | 44.2 | 1.73 | 24.1 | 45.7 | |||||
| Pt/C | 44.9 | 1.78 | 24.1 | 46.0 | |||||
| Ru/C | 43.7 | 1.77 | 25.9 | 48.5 | |||||
| Pd/C | 42.2 | 1.76 | 26.6 | 48.8 | |||||
| Aalborg University, Denmark | Aspen wood | 38.6 | 1.40 | NiMo/Al2O3 | 41.1 | 1.57 | N/A | N/A | [114] |
| Hardwood | 40.4 | 1.48 | NiMo/Al2O3 | 43.9 | 1.67 | N/A | N/A | [113] | |
| Steeper Energy, Denmark-Canada | Pine/spruce | 37.2 | 1.34 | NiMo/Al2O3 | 42.2 | 1.57 | 36.0 | 63.3 | [25] |
| 38.0 | 1.34 | NiMo/Al2O3 | 43.2 | 1.71 | 38.1 | 66.1 | |||
| 38.6 | 1.26 | NiW/SiO2/Al2O3 Pd/Al2O3 | 43.5 | 1.58 | N/A | N/A | |||
| Pacific Northwest National Laboratory (PNNL), USA | Wood 1 | 36.3 | 1.29 | CoMo/Al2O3 | 45.2 | 1.75 | N/A | 68.9 | [106] |
| Algae (Solix) | 39.5 | 1.52 | CoMo/Al2O3 | 46.3 | 2.00 | 42.3 | 72.3 | [116] | |
| Algae (NB238) | 39.8 | 1.59 | CoMo/Al2O3 | 45.4 | 1.87 | 32.1 | 57.5 | ||
| Algae (Cellana low lipid) | 39.5 | 1.62 | CoMo/Al2O3 | 45.2 | 1.92 | 51.4 | 83.5 | ||
| Algae (Cellana high lipid) | 39.9 | 1.63 | CoMo/Al2O3 | 45.7 | 2.00 | 51.3 | 83.9 | ||
| Algae (Chlorella standard lipid) | 39.4 | 1.47 | CoMo/Al2O3 | 45.6 | 1.98 | 30.1 | 53.1 | [43] | |
| Algae (Chlorella high lipid) | 39.4 | 1.72 | CoMo/Al2O3 | 47.1 | 2.04 | 63.9 | 86.8 | ||
| Primary sludge | 37.9 | 1.57 | CoMo/Al2O3 | 46.1 | 2.00 | 28.6 | 50.7 | [46] | |
| Digested solids | 38.1 | 1.40 | CoMo/Al2O3 | 45.7 | 1.92 | 32.0 | 70.5 | ||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castello, D.; Pedersen, T.H.; Rosendahl, L.A. Continuous Hydrothermal Liquefaction of Biomass: A Critical Review. Energies 2018, 11, 3165. https://doi.org/10.3390/en11113165
Castello D, Pedersen TH, Rosendahl LA. Continuous Hydrothermal Liquefaction of Biomass: A Critical Review. Energies. 2018; 11(11):3165. https://doi.org/10.3390/en11113165
Chicago/Turabian StyleCastello, Daniele, Thomas Helmer Pedersen, and Lasse Aistrup Rosendahl. 2018. "Continuous Hydrothermal Liquefaction of Biomass: A Critical Review" Energies 11, no. 11: 3165. https://doi.org/10.3390/en11113165






