Development of Chelating Agent-Based Polymeric Gel System for Hydraulic Fracturing
Abstract
:1. Introduction
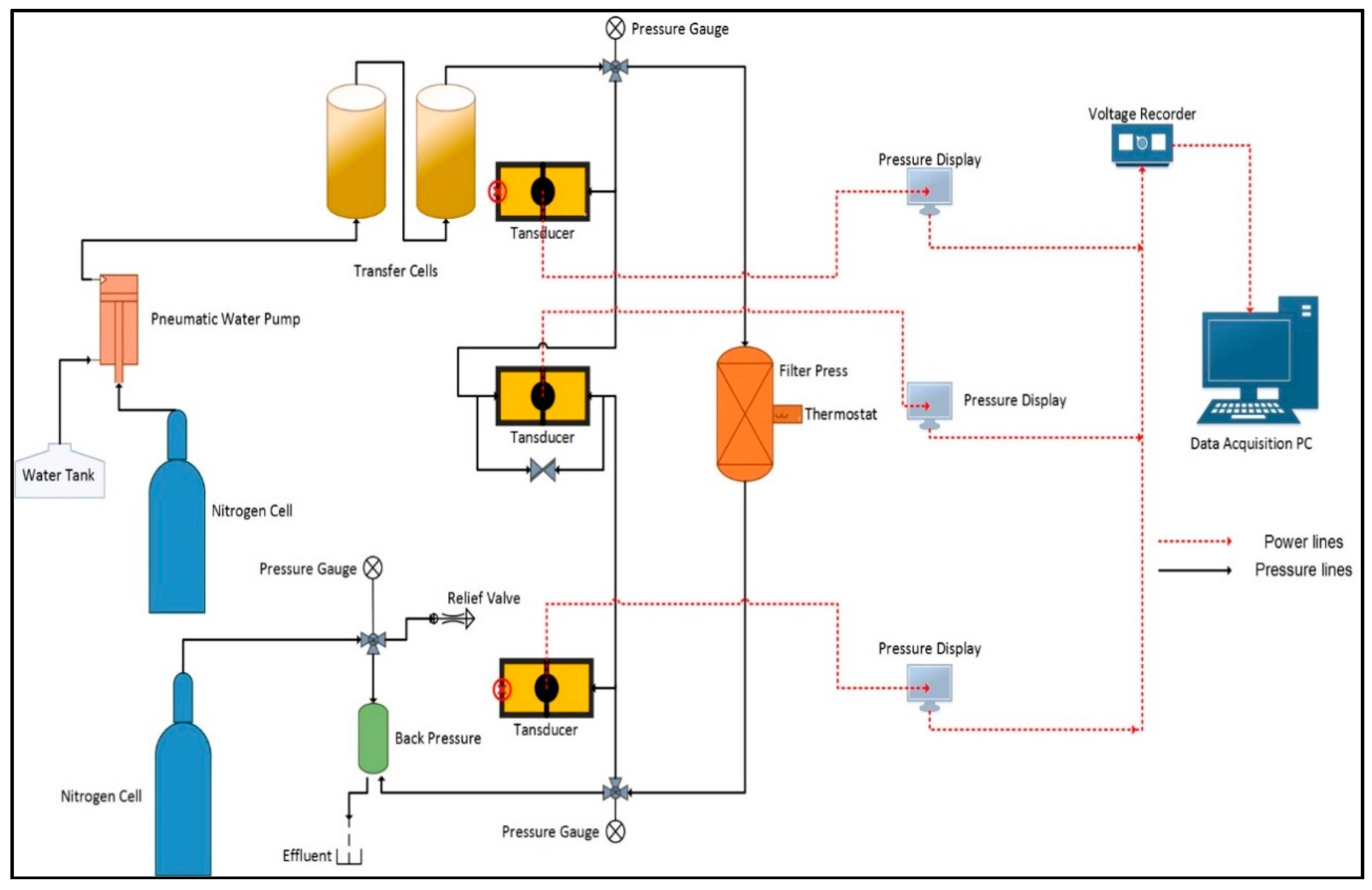
2. Experimental
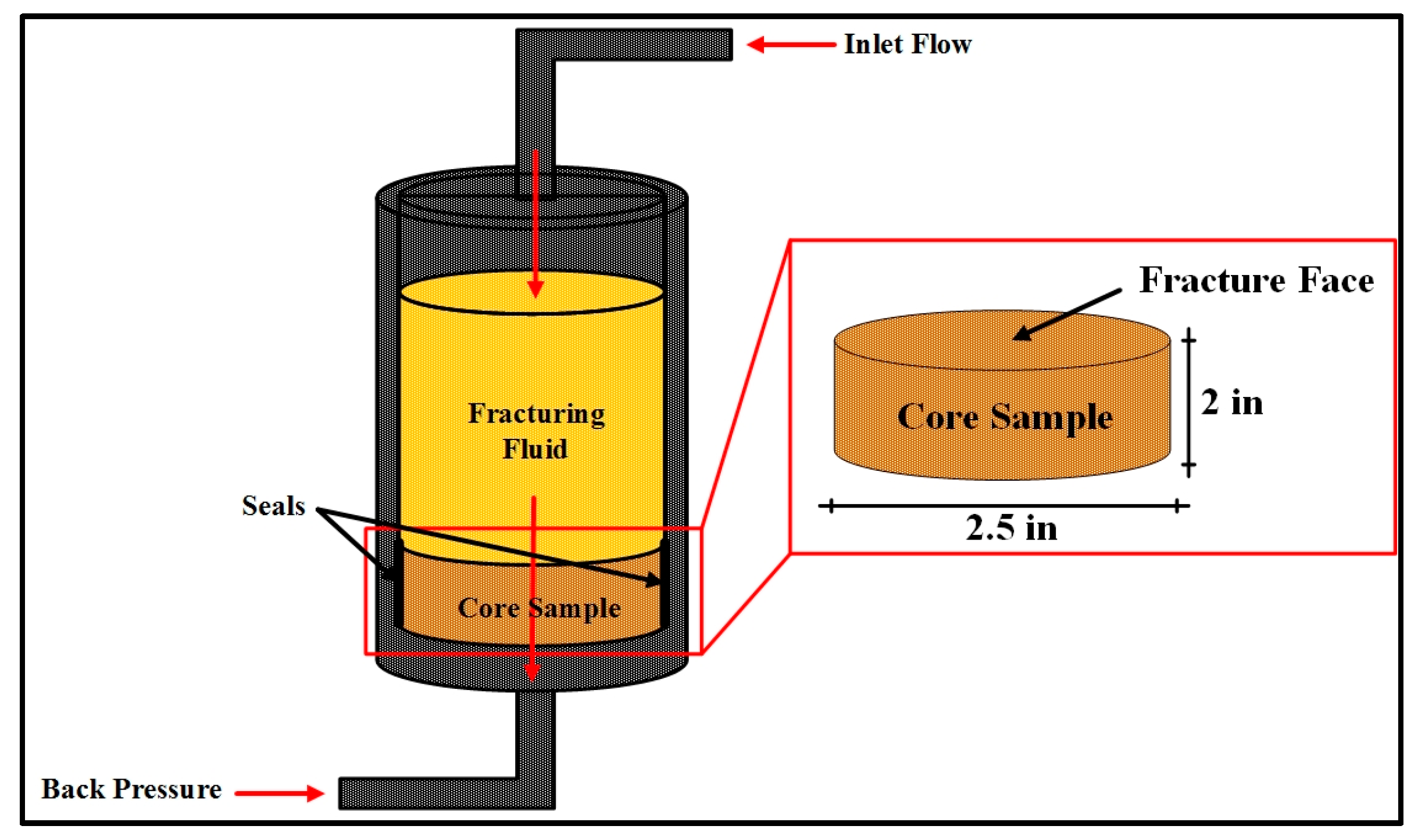
- Fill the cell from the top with the fracturing fluid, and tighten the cell top and connect the pressure lines coming from the transfer cells (Figure 2).
- Insert the core sample into the cell and tighten the cell bottom of the cell against the core sample to prevent leaking and attach the pressure lines leading to the back-pressure system.
- Set the temperature to the required value and allow enough time for the core sample to be heated (about 1 h).
- Apply the required pressure on the transfer cells, and open the valves leading to the core cell, and apply the required back pressure to the system, and open the valves leading to the core cell.
- Using the water pump, the injection rate was set to the required value and activated to start flooding the core sample, the pressure drop was monitored with time until the required pore volumes were injected. Effluents from some intervals were collected for analysis.
3. Results & Discussion
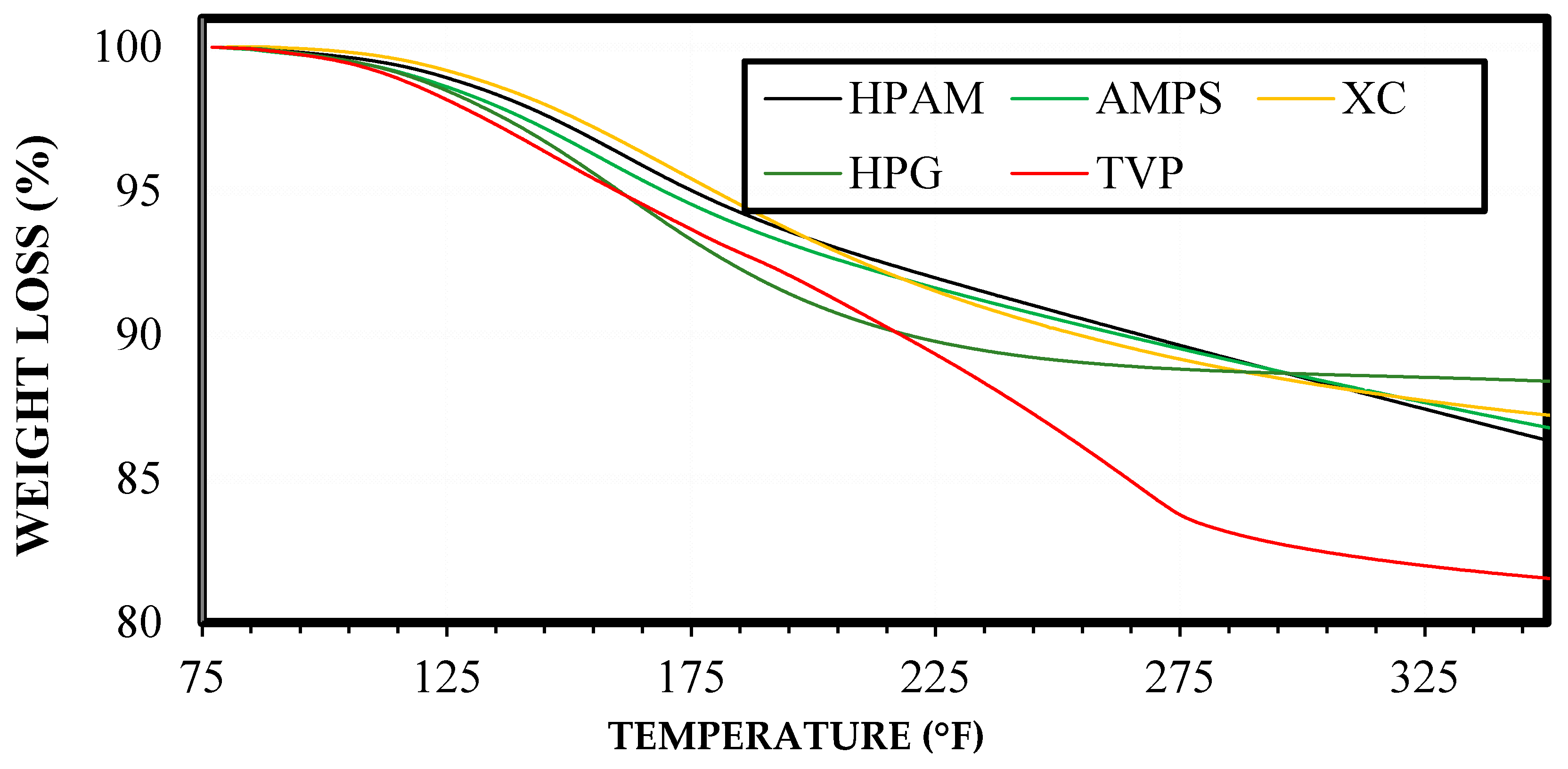
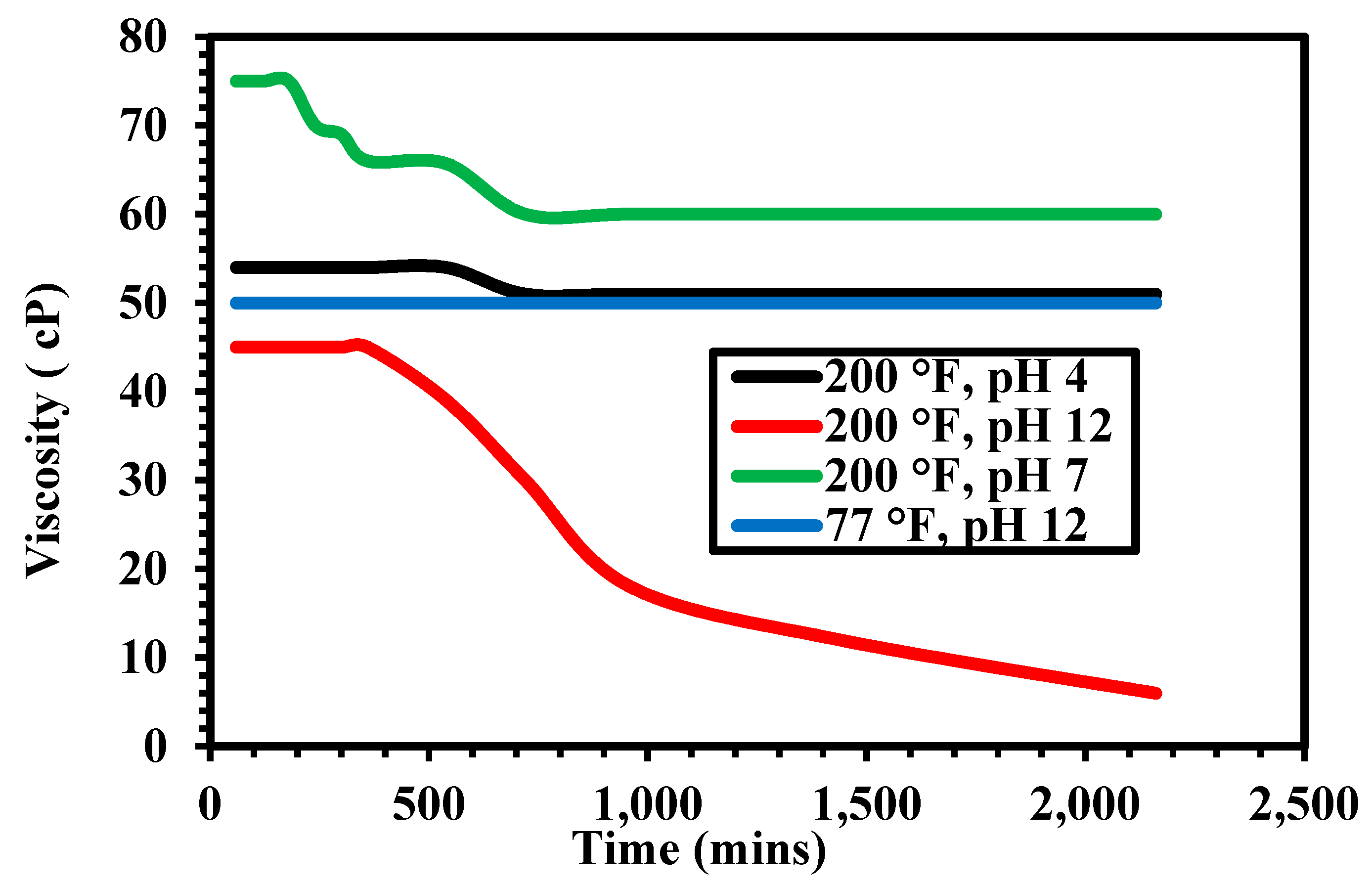
3.1. Thermal Stability
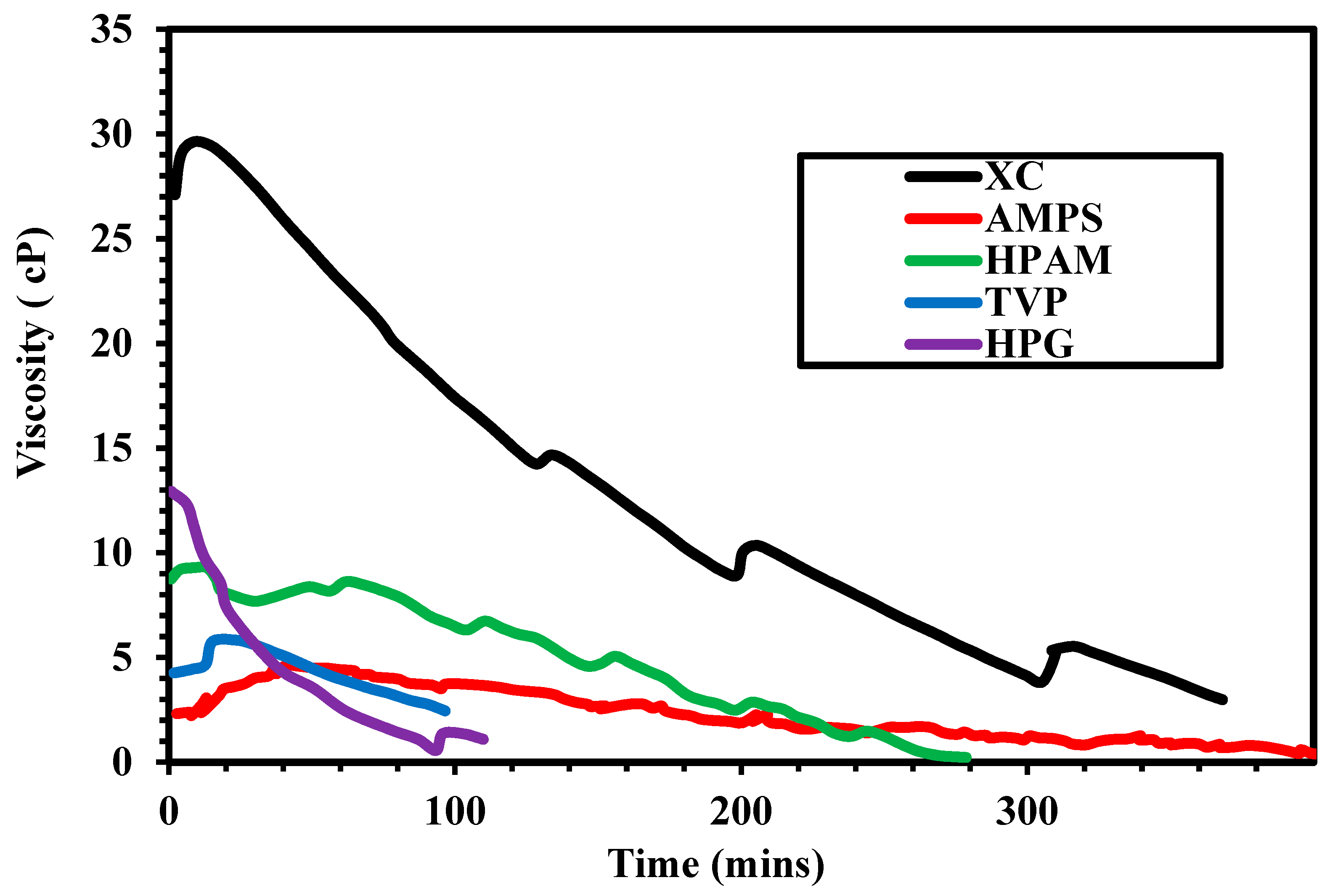
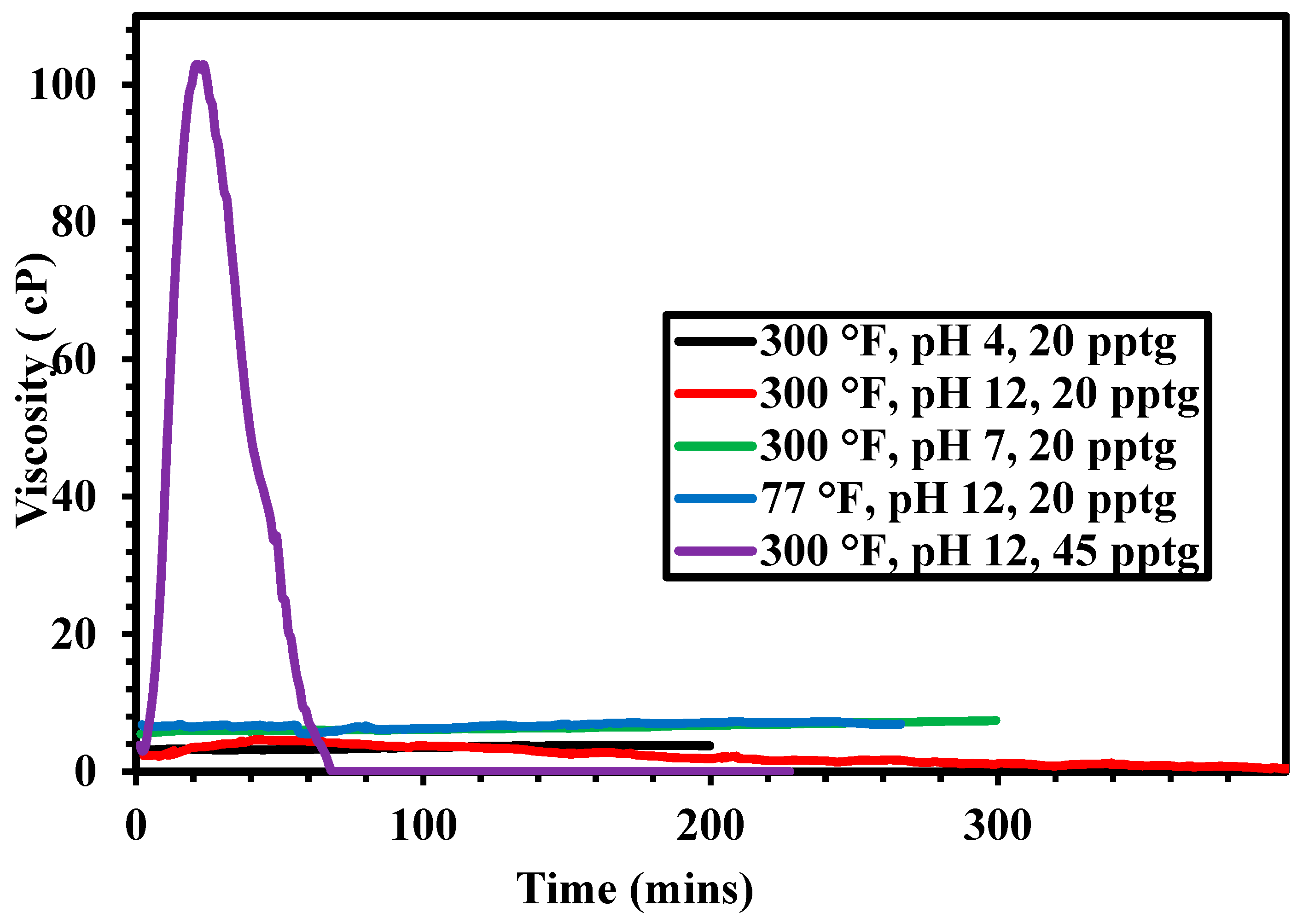
3.2. Rheological Properties
3.3. FTIR Analysis
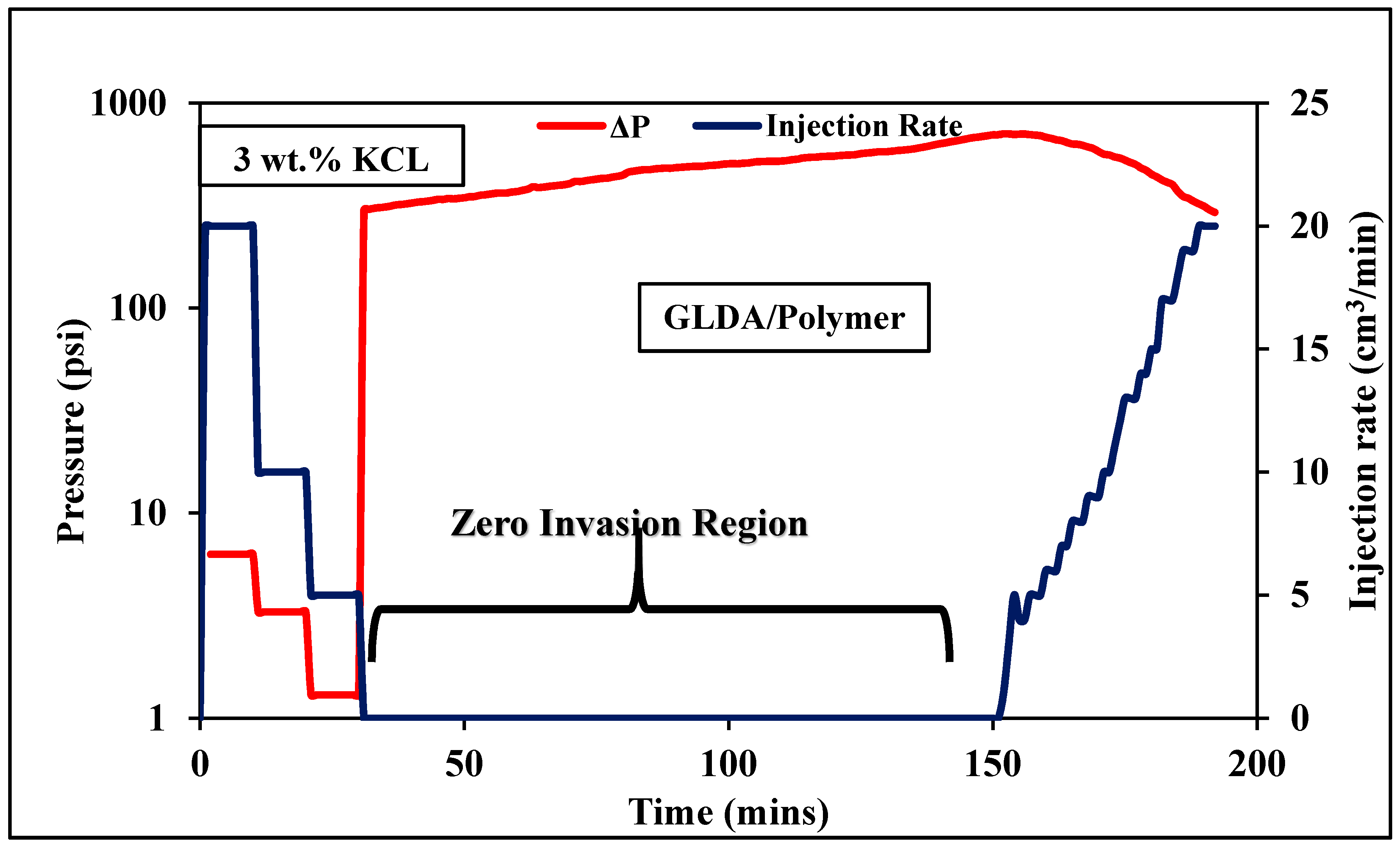
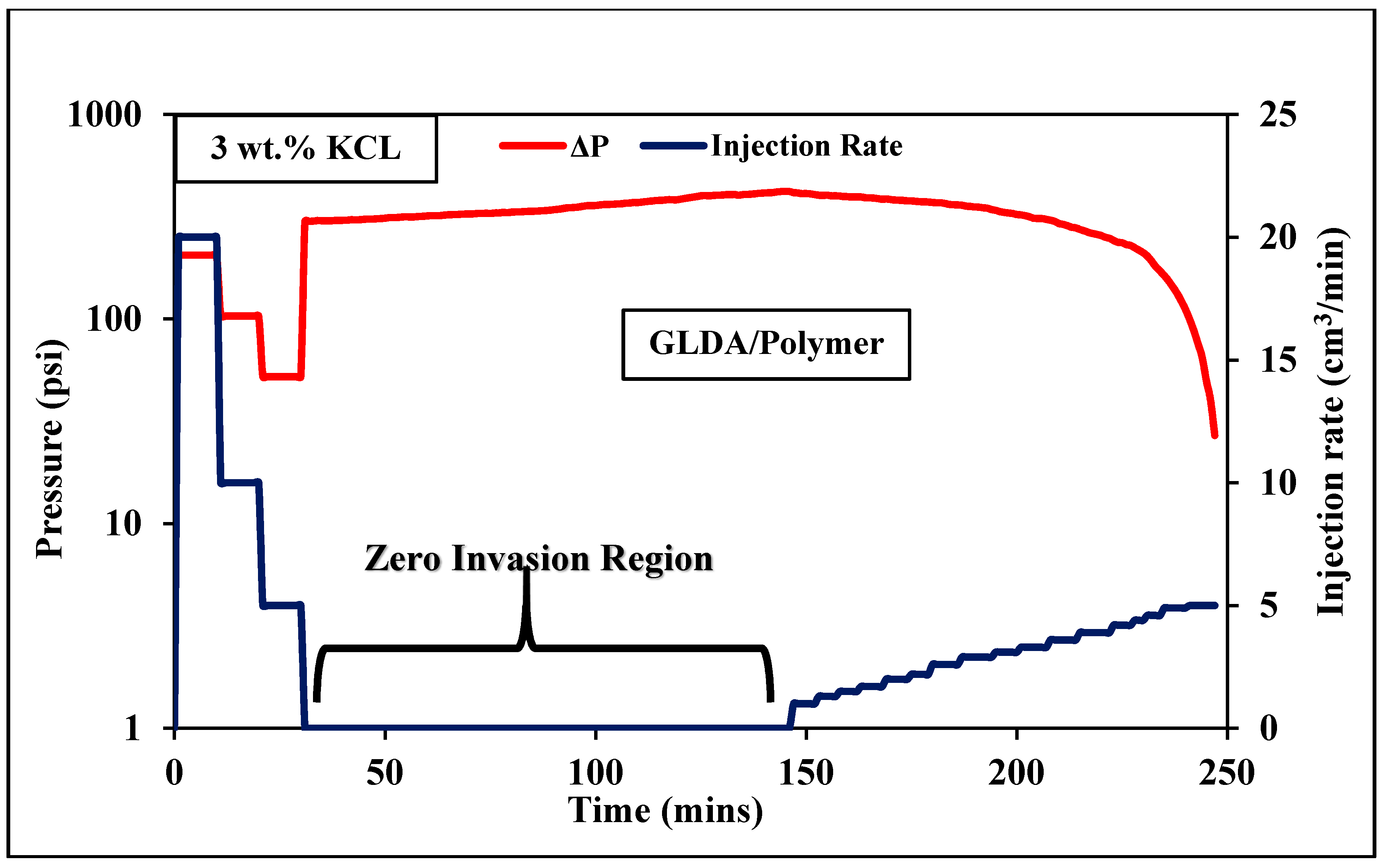
3.4. Coreflooding
3.4.1. High Permeability Coreflooding
3.4.2. Low Permeability Coreflooding
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Li, Q.; Xing, H.; Liu, J.; Liu, X. A review on hydraulic fracturing of unconventional reservoir. Petroleum 2015, 1, 8–15. [Google Scholar] [CrossRef]
- Das, A.; Chauhan, G.; Verma, A.; Kalita, P.; Ojha, K. Rheological and breaking studies of a novel single-phase surfactant-polymeric gel system for hydraulic fracturing application. J. Pet. Sci. Eng. 2018, 167, 559–567. [Google Scholar] [CrossRef]
- Zhao, J.; Fan, J.; Mao, J.; Yang, X.; Zhang, H.; Zhang, W. High performance clean fracturing fluid using a new tri-cationic surfactant. Polymers 2018, 10, 535. [Google Scholar] [CrossRef]
- Fogang, L.T.; Sultan, A.S.; Kamal, M.S. Understanding viscosity reduction of a long-tail sulfobetaine viscoelastic surfactant by organic compounds. RSC Adv. 2018, 8, 4455–4463. [Google Scholar] [CrossRef] [Green Version]
- Fogang, L.T.; Sultan, A.S.; Kamal, M.S. Comparing the Effects of Breakers on a Long-tail Sulfobetaine Viscoelastic Surfactant Solution for Well Stimulation. In Proceedings of the Abu Dhabi International Petroleum Exhibition & Conference, Abu Dhabi, United Arab Emirates, 7–10 November 2016. SPE-182915-MS. [Google Scholar]
- Ahmed, S.; Elraies, K.A.; Hashmet, M.R.; Alnarabiji, M.S. Empirical modeling of the viscosity of supercritical carbon dioxide foam fracturing fluid under different downhole conditions. Energies 2018, 11, 782. [Google Scholar] [CrossRef]
- Fan, M.; Li, J.; Liu, G. New method to analyse the cement sheath integrity during the volume fracturing of shale gas. Energies 2018, 11, 750. [Google Scholar] [CrossRef]
- Zhang, C.; Ranjith, P.G. Experimental study of matrix permeability of gas shale: An application to CO2-based shale fracturing. Energies 2018, 11, 702. [Google Scholar] [CrossRef]
- Liu, R.; Jian, Y.; Haung, N.; Sugimoto, S. Hydraulic properties of 3d crossed rock fractures by considering anisotropic aperture distributions. Adv. Geo Energy Res. 2018, 2, 113–121. [Google Scholar] [CrossRef]
- Parvizi, H.; Rezaei Gomari, S.; Nabhani, F.; Dehghan Monfared, A. Modeling the risk of commercial failure for hydraulic fracturing projects due to reservoir heterogeneity. Energies 2018, 11, 218. [Google Scholar] [CrossRef]
- Holditch, S.A. Tight gas sands. J. Pet. Technol. 2006, 58, 86–93. [Google Scholar] [CrossRef]
- Assiri, W.; Miskimins, J.L. The Water Blockage Effect on Desiccated Tight Gas Reservoir. In Proceedings of the SPE International Symposium and Exhibition on Formation Damage Control, Lafayette, LA, USA, 26–28 February 2014. SPE-168160-MS. [Google Scholar]
- Bennion, D.; Thomas, F.; Schulmeister, B.; Sumani, M. Determination of true effective in situ gas permeability in subnormally water-saturated tight gas reservoirs. J. Can. Pet. Technol. 2004, 43. [Google Scholar] [CrossRef]
- Wang, F.; Li, B.; Zhang, Y.; Zhang, S. Coupled thermo-hydro-mechanical-chemical modeling of water leak-off process during hydraulic fracturing in shale gas reservoirs. Energies 2017, 10, 1960. [Google Scholar] [CrossRef]
- Civan, F. Analyses of Processes, Mechanisms, and Preventive Measures of Shale-gas Reservoir Fluid, Completion, and Formation Damage. In Proceedings of the SPE International Symposium and Exhibition on Formation Damage Control, Lafayette, LA, USA, 26–28 February 2014. SPE-168164-MS. [Google Scholar]
- Middleton, R.S.; Carey, J.W.; Currier, R.P.; Hyman, J.D.; Kang, Q.; Karra, S.; Jiménez-Martínez, J.; Porter, M.L.; Viswanathan, H.S. Shale gas and non-aqueous fracturing fluids: Opportunities and challenges for supercritical CO2. Appl. Energy 2015, 147, 500–509. [Google Scholar] [CrossRef]
- Bertoncello, A.; Wallace, J.; Blyton, C.; Honarpour, M.; Kabir, C. Imbibition and Water Blockage in Unconventional Reservoirs: Well management implications during flowback and early production. In Proceedings of the SPE/EAGE European Unconventional Resources Conference and Exhibition, Vienna, Austria, 25–27 February 2014. SPE-167698-MS. [Google Scholar]
- Kesavan, S.; Prud’Homme, R.K. Rheology of guar and (hydroxypropyl) guar crosslinked by borate. Macromolecules 1992, 25, 2026–2032. [Google Scholar] [CrossRef]
- Jennings, A.R., Jr. Fracturing fluids-then and now. J. Pet. Technol. 1996, 48, 604–610. [Google Scholar] [CrossRef]
- Shah, S.; Lord, D.; Rao, B. Borate-crosslinked Fluid Rheology under Various ph, Temperature, and Shear History Conditions. In Proceedings of the SPE Production Operations Symposium, Oklahoma City, Oklahoma, 9–11 March 1997. SPE-37487-MS. [Google Scholar]
- Gaillard, N.; Thomas, A.; Favero, C. Novel Associative Acrylamide-based Polymers for Proppant Transport in Hydraulic Fracturing Fluids. In Proceedings of the SPE International Symposium on Oilfield Chemistry, The Woodlands, TX, USA, 8–10 April 2013. SPE-164072-MS. [Google Scholar]
- Holtsclaw, J.; Funkhouser, G.P. A crosslinkable synthetic-polymer system for high-temperature hydraulic-fracturing applications. SPE Drill. Complet. 2010, 25, 555–563. [Google Scholar] [CrossRef]
- Omeiza, A.; Samsuri, A. Viscoelastic surfactants application in hydraulic fracturing, it’s set back and mitigation—an overview. ARPN J. Eng. Appl. Sci. 2006, 9, 25–29. [Google Scholar]
- Samuel, M.; Polson, D.; Graham, D.; Kordziel, W.; Waite, T.; Waters, G.; Vinod, P.; Fu, D.; Downey, R. Viscoelastic Surfactant Fracturing Fluids: Applications in Low Permeability Reservoirs. In Proceedings of the SPE Rocky Mountain Regional/Low-Permeability Reservoirs Symposium and Exhibition, Denver, CO, USA, 12–15 March 2000. SPE-60322-MS. [Google Scholar]
- Harris, P. Application of Foam Fluids to Minimize Damage during Fracturing. In Proceedings of the International Meeting on Petroleum Engineering, Beijing, China, 24–27 March 1992. SPE-22394-MS. [Google Scholar]
- Ahmed, S.; Elraies, K.A.; Hanamertani, A.S.; Hashmet, M.R. Viscosity models for polymer free CO2 foam fracturing fluid with the effect of surfactant concentration, salinity and shear rate. Energies 2017, 10, 1970. [Google Scholar] [CrossRef]
- He, J.; Afolagboye, L.O.; Lin, C.; Wan, X. An experimental investigation of hydraulic fracturing in shale considering anisotropy and using freshwater and supercritical CO2. Energies 2018, 11, 557. [Google Scholar] [CrossRef]
- Mahmoud, M.A.; Nasr-El-Din, H.A.; De Wolf, C.A. High-temperature laboratory testing of illitic sandstone outcrop cores with hcl-alternative fluids. SPE Prod. Oper. 2015, 30, 43–51. [Google Scholar] [CrossRef]
- Mahmoud, M.A.; Nasr-El-Din, H.A.; De Wolf, C.; Alex, A. Sandstone Acidizing Using a New Class of Chelating Agents. In Proceedings of the SPE International Symposium on Oilfield Chemistry, The Woodlands, TX, USA, 11–13 April 2011. SPE-139815-MS. [Google Scholar]
- De Wolf, C.A.; Nasr-El-Din, H.; LePage, J.N.; Mahmoud, M.A.N.-E.-D.; Bemelaar, J.H. Environmentally Friendly Stimulation Fluids, Processes to Create Wormholes in Carbonate Reservoirs, and Processes to Remove Wellbore Damage in Carbonate Reservoirs. U.S. Patent 9150780B2, 6 October 2015. [Google Scholar]
- Mahmoud, M.A.N.E.-D.; Gadallah, M.A. Method of Maintaining Oil Reservoir Pressure. U.S. Patent 20140345868A1, 27 November 2014. [Google Scholar]
- Kamal, M.S.; Shakil Hussain, S.M.; Fogang, L.T. A zwitterionic surfactant bearing unsaturated tail for enhanced oil recovery in high-temperature high-salinity reservoirs. J. Surfactants Deterg. 2018, 21, 165–174. [Google Scholar] [CrossRef]
- Ahmad, H.M.; Kamal, M.S.; Al-Harthi, M.A. High molecular weight copolymers as rheology modifier and fluid loss additive for water-based drilling fluids. J. Mol. Liq. 2018, 252, 133–143. [Google Scholar] [CrossRef]
- Shahzad Kamal, M.; Sultan, A. Thermosensitive Water Soluble Polymers: A solution to High Temperature and High Salinity Reservoirs. In Proceedings of the SPE Kingdom of Saudi Arabia Annual Technical Symposium and Exhibition, Dammam, Saudi Arabia, 24–27 April 2017. SPE-188006-MS. [Google Scholar]
- Malik, I.A.; Al-Mubaiyedh, U.A.; Sultan, A.S.; Kamal, M.S.; Hussein, I.A. Rheological and thermal properties of novel surfactant-polymer systems for eor applications. Can. J. Chem. Eng. 2016, 94, 1693–1699. [Google Scholar] [CrossRef]
- Ahmad, H.M.; Kamal, M.S.; Al-Harthi, M.A. Rheological and filtration properties of clay-polymer systems: Impact of polymer structure. Appl. Clay Sci. 2018, 160, 226–237. [Google Scholar] [CrossRef]
- Mahmoud, M.; Abdelgawad, K.Z.; Elkatatny, S.M.; Akram, A.; Stanitzek, T. Stimulation of seawater injectors by glda (glutamic-di acetic acid). SPE Drill. Complet. 2016, 31, 178–187. [Google Scholar] [CrossRef]
- Ameur, Z.O.; Kudrashou, V.; Nasr-El-Din, H.; Forsyth, J.; Mahoney, J.; Daigle, B. Stimulation of High Temperature Sagd Producer Wells Using a Novel Chelating Agent (glda) and Subsequent Geochemical Modeling Using Phreeqc. In Proceedings of the SPE International Symposium on Oilfield Chemistry, The Woodlands, TX, USA, 13–15 April 2015. SPE-173774-MS. [Google Scholar]
- Braun, W.; De Wolf, C.A.; Nasr-El-Din, H.A. Improved Health, Safety and Environmental Profile of a New Field Proven Stimulation Fluid. In Proceedings of the SPE Russian Oil and Gas Exploration and Production Technical Conference and Exhibition, Moscow, Russia, 16–18 October 2012. SPE-157467-MS. [Google Scholar]




| Polymer | Abbreviation | Structure |
|---|---|---|
| Partially hydrolyzed polyacrylamide | HPAM | 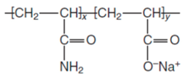 |
| Xanthan Gum | XC | 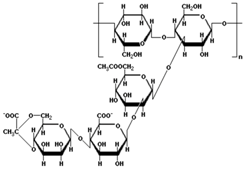 |
| Guar Gum | HPG |  |
| Thermoviscofying polymer | TVP | 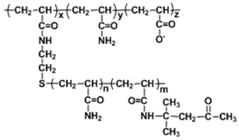 |
| Copolymer of 2-acrylamido-2-methylpropane sulfonic acid and acrylamide | AMPS |  |
| Chelating Agent | Abbreviation | Structure |
|---|---|---|
| glutamic acid diacetic acid | GLDA | 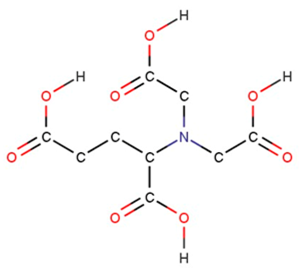 |
| Ethylenediaminetetraacetic acid | EDTA |  |
| Diethylenetriaminepentaacetic acid | DTPA |  |
| Sample | 1 | 2 |
|---|---|---|
| Type | Sandstone | Sandstone |
| Origin | Berea | Scioto |
| Diameter | 6.35 cm | 6.35 cm |
| Length | 5.08 cm | 5.08 cm |
| Pore Volume | 35.4 cm3 | 19.3 cm3 |
| Bulk Volume | 160.8 cm3 | 160.8 cm3 |
| Porosity | 22% | 12% |
| Pemreability | 151.2 mD | 3.837 mD |
| Minerals | Berea | Scioto |
|---|---|---|
| Quartz | 86 | 70 |
| Dolomite | 1 | - |
| Calcite | 2 | - |
| Feldspar | 3 | 2 |
| Kaolinite | 5 | Trace |
| Illite | 1 | 18 |
| Chlorite | 2 | 4 |
| Plagioclase | - | 5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamal, M.S.; Mohammed, M.; Mahmoud, M.; Elkatatny, S. Development of Chelating Agent-Based Polymeric Gel System for Hydraulic Fracturing. Energies 2018, 11, 1663. https://doi.org/10.3390/en11071663
Kamal MS, Mohammed M, Mahmoud M, Elkatatny S. Development of Chelating Agent-Based Polymeric Gel System for Hydraulic Fracturing. Energies. 2018; 11(7):1663. https://doi.org/10.3390/en11071663
Chicago/Turabian StyleKamal, Muhammad Shahzad, Marwan Mohammed, Mohamed Mahmoud, and Salaheldin Elkatatny. 2018. "Development of Chelating Agent-Based Polymeric Gel System for Hydraulic Fracturing" Energies 11, no. 7: 1663. https://doi.org/10.3390/en11071663
APA StyleKamal, M. S., Mohammed, M., Mahmoud, M., & Elkatatny, S. (2018). Development of Chelating Agent-Based Polymeric Gel System for Hydraulic Fracturing. Energies, 11(7), 1663. https://doi.org/10.3390/en11071663








