1. Introduction
Nowadays, it becomes more important to ensure low carbon emissions from industry and power plants. This may be done with the help of bio-methane from biogas upgrading. To fulfill the quality requirements for supplying bio-methane to the natural gas grid, separation from CO2 is required. This task can be done via continuous temperature swing adsorption (TSA) within a bubbling fluidized bed system.
Temperature swing adsorption based on a moving bed is often used in the field of flue gas cleaning by CO
2-capture and shows an acceptable effectiveness [
1]. The recently proposed TSA-system [
2] by the authors of this work for biogas upgrading uses fluidized bed technology in order to achieve higher heat transfer rates compared to fixed or moving beds [
3,
4]. The separation process is operating at medium temperatures and atmospheric conditions [
2]. For excellent process control, it is essential to maintain a certain temperature within the fluidized bed due to the adsorption isotherms and therefore adsorption/desorption activity of the CO
2 on the amine adsorbents. During the exothermic adsorption step active cooling is required and vice-versa active heating during the endothermic desorption step. Hence, in-bed heat exchangers are applied to the fluidized bed TSA-process to avoid deviation from efficient adsorption/desorption temperatures. The resulting heat transfer coefficient (HTC) between the fluidized bed and immersed heat exchangers influences the thermodynamic driving force for separation and moreover, the overall performance and economics of a TSA-process [
2]. However, heat transfer between a fluidized bed and an immersed object depends on a number of process-factors [
5,
6,
7,
8,
9,
10,
11,
12], including particle (i.e., type of Geldart) and gas properties, in-bed object geometry (i.e., heat exchanger layout) and of course fluidization rate. The TSA-process considers particles of Geldart type B [
13] in terms of solids well-mixed behaviors [
13] for acceptable CO
2 capture performance [
14].
In general, the heat transfer coefficient
between an immersed tube-wall and a fluidized bed is shown in Equation (
1) and can be split into three terms of particle convection
, gas convection
and radiation
.
The particle convection describes the conducted heat transfer from the particle to the surface between a thin remaining gas layer. In most gas-solids fluidized beds, the particle convection is predominat [
15]. The gas convection describes the heat transport between gas and surface. It becomes important for denser and larger particles [
15]. The radiative term of the heat transfer coefficient becomes significant only for bed temperatures above 400 °C (870 K) [
16] and can be neglected for TSA-applications (i.e., max. process-temperature 120 °C (390 K)).
Literature offers many experimental and numerical investigations on the heat transfer coefficients from wall-to-bed in fluidized beds as well as mathematical correlations for HTC. For example, some heat transfer models are based on a cluster renewal approach model [
5,
17,
18,
19], on a fuzzy logic model [
17], or a semi-empirical packet-renewal model [
6] to predict mechanistic HTC. Zarghami et al. [
20] developed a model for departure rate of particle from the surface element to the fluidized bed. The models by Molerus et al. [
21,
22], Natusch et al. [
23] and Martin [
24] are able to describe the fluidized bed HTC as a function of the fluidization gas velocity. This property of the mathematical models is particularly important for the design of optimized in-bed heat exchangers of TSA-systems. The last named models have been developed for single tubes immersed in fluidized bed.
To consider tube bundle configurations for heat exchanger design, some models are designed to calculate tube bundle reduction factors [
11,
25], which can be multiplied to the single tube models. According to Hofer et al. [
26], the particle diameter, tube diameter and tube bundle spacing have a significant influence on the heat transfer rates and furthermore, the models by Natusch et al. [
23] and Molerus et al. [
21,
22] lead to qualitative and quantitative differences compared to the obtained results.
Therefore, this work will first experimentally investigate the HTC for Geldart type B amine layered bulk material, specially developed for CO2 capture applications. A test facility was set up and heat exchange measurements were carried out on single tubes and in particular tube bundles in different configurations within a fluidized bed similar to a TSA-reactor. The aim is to provide information about which HTC values can be expected from the experimental investigations of the adsorbent material and thus, which mathematical models can predict them best. Suitable mathematical models can be used for dimensioning in-bed heat exchangers for TSA reactors and full heat integration can be achieved.
The second part of the work will be a numerical analysis with the computer fluid dynamics (CFD) program ANSYS
® Fluent
® using Euler–Euler method of the proposed test facility in order to investigate flow characteristics and heat transfer of it. Furthermore, only a few numerical studies on both regime and heat transfer have been performed on bubbling fluidized beds with external circulation. This numerical study aims to show how the flow characteristics and heat transfer coefficient is influenced by the geometric shape of the immersed in-line heat exchanger surface. For this, the Euler–Euler method was combined with the kinetic theory of granular flows. The kinetic theory of granular flows was used in various heat transfer studies in a fluidized bed to predict the heat transfer coefficients of a fluidized bed with an immersed object [
27]. The influence of different thermal conductivities, drag models and specularity coefficients on the fluidization regime and heat transfer is also shown. The previously obtained experimental data from the test facility can be used for validation. Furthermore, the proposed modeling of a fluidized bed with external material inlet and outlet is supposed to create a simulation that allows faster and more flexible investigations of different heat exchanger geometries for multi-stage fluidized bed TSA-systems. This means, that the time-consuming design and construction of cold flow models with different heat exchanger geometries for performance studies can be avoided.
2. Experimental Investigation
For experimental investigations on heat transfer between bubbling fluidized bed and immersed bare tubes, a novel test facility at atmospheric test conditions with temperature swing adsorption process characteristics (i.e., external material circulation and amine layered bulk material) has been constructed and deployed.
2.1. Materials and Experimental Test Facility
2.1.1. Test Facility
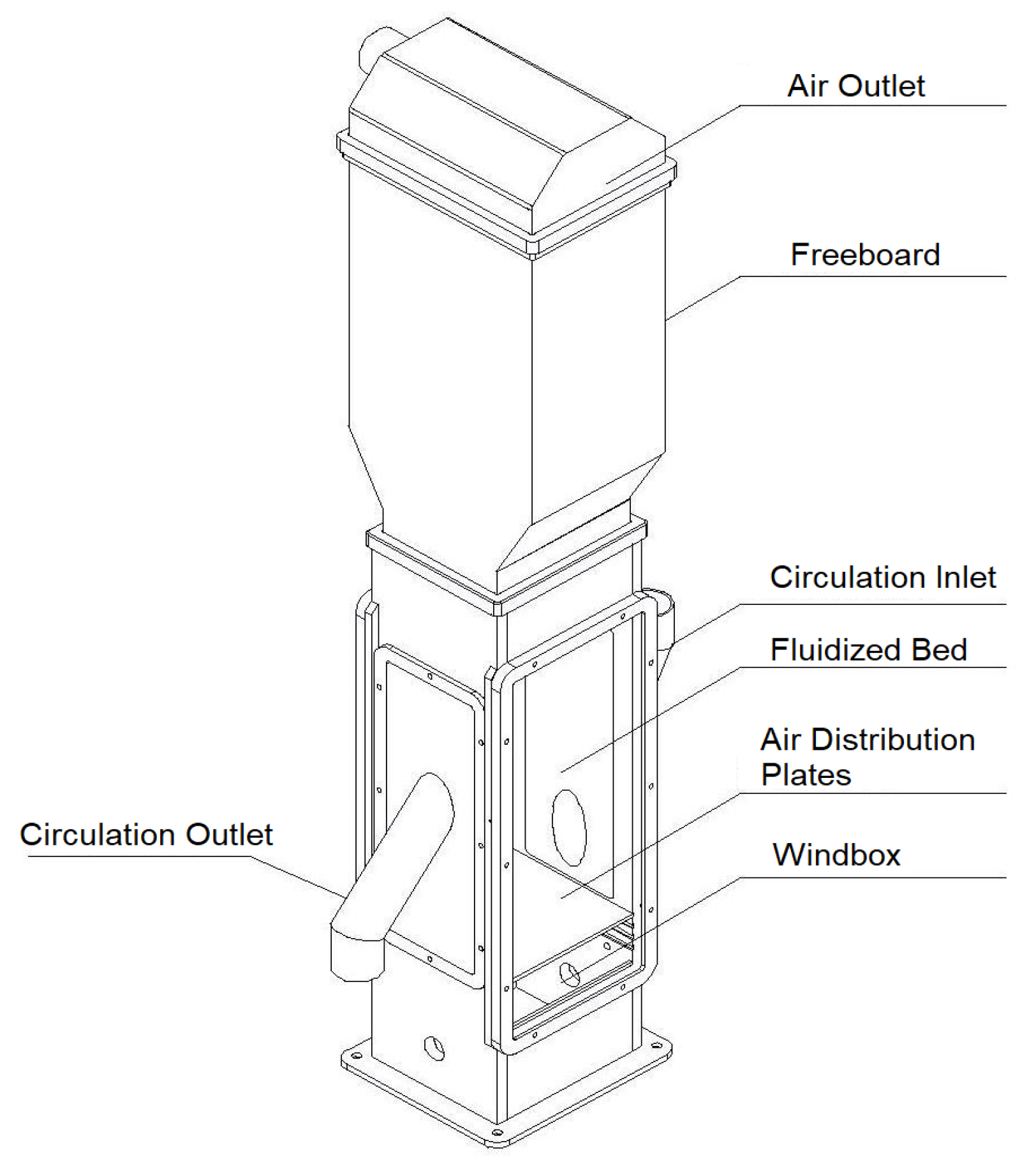
Figure 1 shows the main parts of the test facility. In the bottom, air was introduced into the windbox and the gas distribution plate enables a homogenous distribution of air for fluidization. To enable an external bulk material circulation, two sideward placed tubes facilitated material transport. Thus, the fluidized bed test facility transports the solids material through the bed in a cross-current flow with the immersed heat exchanger layout. The aim was to indicate a counter-cross heat exchanger, but nevertheless real fluidized bed regime behaviors within the test facility were similar to a cross-current application. Above the fluidized bed, the arising freeboard with the air outlet is located on the top of the facility.
The dimension of the bed height was up to 320 mm at superficial gas velocity of 0.13 m/s in a square cross section with a lateral length of 200 mm.
Figure 2 shows the test facility with and without bulk material and on the right the aluminum dummies in a staggered layout. To control the external circulating rate of the test facility, air was used to adapt the solids material throughput in the L-valve and therefore the transport within the riser into the air/solids material separator. The throughput of the solids material quantity was set via a pre-experimental evaluation. This was determined by the volume flow of the air and the cross-section of the L-valve. After separation the solids material flows back into the fluidized bed. Dry compressed air was used as the fluidization agent and can be provided with a volume flow in the range of 2.5–100 Nm
/h. Due to absolutely dry air behaviors, it had to be moisturized through a moistening system to avoid possible electrostatic potential of the bulk material. Hence, the relative humidity of the compressed air can be adjusted in the range between 0% and 40%.
The heating probe (
Figure 3) was divided into three sections: in the center an electrically heated copper part is placed, surrounded by two acrylic glass ends to ensure that the entire heat is transferred to the fluidized bed.
The electrical power consumption of the heating probe
was measured and two thermocouples (1/3DIN standard) determined the temperature of the heating probe (
and
). The surface temperature of the copper part was set to 65 °C (338 K), by measuring and controlling the voltage
U and current
I of the power supplier. Two separate thermocouples (1/3DIN standard) were placed on the acrylic glass plates at different positions (
Figure 4) to measure the bed temperature (
and
). Furthermore, as a result of the mixing due to the bubble movement, a fluidized bed had a high effective thermal conductivity in the vertical direction. As a consequence, temperature gradients were immediately reduced and almost isothermal conditions occur [
28]. Thus, the position of the thermocouples was chosen that the position
was immediately after the heating probe and the position
in the bed inner (staggered and single) or on the opposite of the heating probe and
, respectively, (in-line) for a good prediction of the HTC between heating probe and fluidized bed. The electric power consumption and all temperatures were recorded during the measurements. The following
Table 1 gives an overview of the different experimental test facility parameters.
The experimental solids circulation rate (SCR) was set to 100 kg/h during all operations. The test facility contained removable acrylic glass plates for different tube layouts. The acrylic glass plates had seven rows in transverse and four or three holes in longitudinal direction, depending on the arrangement (
Figure 4). To imitate immersed heat exchangers, these plates held aluminum dummies with a diameter of d
t 25 mm and the heating probe in the same dimension, already shown in
Figure 2 (middle and right).
Figure 4 shows even the different geometry properties where
is the horizontal spacing and
the diagonal spacing (
Table 1). The permeability
is the rate of mean particle diameter and minimum free distance between tube walls.
2.1.2. Bulk Material
The measurements were conducted at ambient conditions with Lewatit
® VP OC 1065 as bulk material. Lewatit
® VP OC 1065 consists of a polystyrene polymer and functionalized with primary amine groups [
29].
Table 2 illustrates the important material data. This adsorption material was rated as thermally and mechanically stable and shows excellent CO
2 capture properties in a continuous TSA process [
29].
The particle diameter of Lewatit
® VP OC 1065 is clearly in the Geldart type B range [
13], as indicated in
Figure 5.
The minimal fluidization velocity
can be estimated at 0.105 m/s for the inserted Lewatit
® VP OC 1065, according to Equation (
2) [
30].
The Archimedes number is the ratio of the buoyancy force of the particles to the viscous forces of the fluidization gas.
The collision factor for solid–solid impact was the ratio between the relative velocities and is usually determined experimentally. Since the bulk material was also a polystyrene variant, a collision factor of 0.92 is selected [
31].
2.2. Measurement Procedure
The measurements of the power consumption of the heating probe and the temperatures of heat probe and fluidized bed are needed to calculate the HTC at adjusted conditions. The measured bed temperature was calculated as arithmetic mean between and as well as the heating probe temperature as arithmetic mean between and . In order to calculate the HTC from the measured data, the measured temperature of the heating probe was defined as the surface temperature of the heating probe. With this assumption the apparent heat transfer coefficient can be calculated as follows
The bed temperature and the power were measured after reaching the thermodynamic equilibrium at approx. 10 min and further, the sampling frequency of the temperature signal was set to 5 s during measurement.
2.3. Measurement Uncertainty
An analysis of measurements required an examination on uncertainties of the measured results. The calculation of uncertainty was conducted according to DIN 1319 [
32] and enabled a qualitative analysis of the measurement results. The mathematical method subdivided the measured values into the true value, the random error and the systematic error. The assumption was made that rough errors were prevented. These errors were not avoidable but random errors can reduced by increasing the number of recorded measurands of the same measured values under repeatability conditions. Systematic errors are dividable into known and unknown systematic errors. The propagation of the uncertainty is given through the well known measured uncertainties for the applied system. According to Equation (
5),
F is the determined function of the
n measured values
. The uncertainty, which is defined as the absolute error, has the same unit as the measured value.
The apparent heat transfer coefficient is determined with Equation (
6) and the assumption of
.
The heat transfer coefficient is a function of heating probe surface
, temperature difference
between bubbling fluidized bed (FB) and heating probe (HP) and electrical power supply
. The uncertainty of the heat transfer coefficient is given by evaluating the partial derivations with respect to
,
or
in terms of applying the law of uncertainty propagation and follows Equation (
7).
Equation (
7) shows that the temperature difference between heating probe and fluidized bed has an important influence on the measurement uncertainty for the heat transfer coefficient.
The structured relative uncertainties of the heat transfer coefficient over the the number of measurements
are illustrated in
Figure 6. The results of the calculated measurement uncertainty indicated that approximately 80% of the measured values had a relative uncertainty beneath
.
2.4. Mathematical Models
Mathematical models from the literature were used to calculate heat transfer coefficients in fluidized beds to compare them with the experimentally determined HTCs. The models and factors are summarized in
Table 3.
The correlation published by Molerus et al. [
21,
22] and Natusch et al. [
23] are able to describe heat transfer coefficients of single tubes within a fluidized bed but were developed under different particle and gas properties than those used in this study. Molerus et al. [
21,
22] conducted experiments with vertical single tubes and established a detailed calculation for particle and gas convective heat transfer. The model by Molerus et al. [
21,
22] is valid in the range of Geldart types A–D. The model presented by Natusch et al. [
23] is only valid for Geldart type B particles and with a mean diameter of 650
m. The relevant coefficients C1 to C5 for Natusch et al. [
23] are displayed in
Table 3 for the used particle diameter.
Furthermore, a heat exchanger tube diameter factor model proposed by Petrie et al. [
11] can be multiplied to the model by Molerus et al. [
21,
22] to consider the impact of tube diameters. Lechner et al. [
25] developed a tube bundle reduction factor for comparison theoretical and experimental heat transfer coefficients. Furthermore, a factor model which considers Geldart type B is introduced by Lechner et al. [
25], that can be applied to the model by Molerus et al. [
21,
22] and Natusch et al. [
23].
2.5. Experimental Results and Discussion
The HTCs of single tube and tube bundle experiments by varying fluidization gas velocity in the range of 0.1 m/s to 0.5 m/s for 650
m amine particles were carried out.
Figure 7 shows the heat transfer coefficients measurements for a single tube and both tube bundles with in-line and staggered arrangement. With regard to the same tube diameter of the single tube and the tube bundles it can be observed that the HTCs were nearly the same. Further, a deviation of the measurement results can be observed by higher fluidization rates. That contradicts the assumption that HTCs from single tube immersed in fluidized beds are usually higher than for corresponding tube bundles due to higher bubble size and bubble velocity and thus, higher particle exchange rates [
33]. However, it must be mentioned that with increasing fluidization gas velocity (i.e., 0.4 m/s) the single tube had a significantly increased HTC. Furthermore, it has been noted in literature that staggered arrangement had marginally higher HTCs than in-line arrangement [
25]. This phenomena was only obtained after a fluidization velocity of 0.35 m/s, whereby with increasing fluidization velocity the staggered layout exhibits a higher HTC. The highest rates were achieved at 0.45 m/s fluidization gas velocity for the single tube and 0.3 m/s fluidization gas velocity for tube bundles. For fluidization gas velocity of 0.3 m/s a homogeneous bed temperature of ∼
was measured after 10 min.
Due to rising high pressure in the windbox of the test facility by increasing the superficial gas velocity, the experimental series stopped at velocities of 0.5 m/s. It should be pointed out that the maximal heat transfer coefficient was obtained within this velocity range.
First of all, in contrast to the observed HTC from the experiments, the calculated tube bundle models show significantly smaller HTC than single tubes (
Figure 8). The calculated heat transfer coefficient by Molerus et al. [
21,
22] for a single tube was compared to the experimental results for the single tube and is shown in
Figure 8. The calculated heat transfer coefficient by Natusch et al. [
23] for a single tube deviates more than 30% (Table 8) from the experimental results and thus, was not further represented in
Figure 8. The model by Molerus et al. [
21,
22] was applied with the tube diameter factor model by Petrie et al. [
11] and compared with the measurement results.
The tube bundle measurement results were compared with the introduced models applied with the different factors for inline and staggered layouts, respectively. The initial point was the model by Molerus et al. [
21,
22] multiplied with the tube bundle factor by Lechner et al. [
25] and in separate cases multiplied additionally with the tube diameter factor by Petrie et al. [
11]. All used mathematical models were compared, as mentioned before, with the measurement results in
Figure 8. The deviation of the heat transfer coefficients between measurement and models are shown in a
corridor.
The simple model by Molerus et al. [
21,
22] for gas velocities over 0.2 m/s deviated with rising gas velocity more from the obtained heat transfer coefficients. The model by Molerus et al. [
21,
22] with the additional tube diameter factor by Petrie et al. [
11] would be the closest approximation to experimental results for single tubes but after exceeding
m/s the modeled values decreased and the measured values remained approximately constant. The nearest approximation for tube bundle arrangement in-line was given through the model by Molerus et al. [
21,
22] applied with the factor by Lechner et al. [
25] and the tube diameter factor by Petrie et al. [
11].
In general, the mathematical models were underestimated for this bulk material and tube bundle geometry. In the following, possible causes for these quantitative discrepancies are listed.
Due to the very high porosity of the amine layered particles used for CO
2 adsorption compared to denser glass beads which are often used for such heat transfer measurements in the literature. Many experiments have different particle size distributions of the bulk material and therefore another mean particle [
26]. Molerus et al. [
21,
22] conducted heat transfer experiments with glass beads at a particle size between 110–670
m.
Hofer et al. [
26] points out that experiments for the established models were conducted with various test facilities like different designs of gas distributors and different cross-sectional fluidized bed shapes. Likewise, the various measuring instruments can play an important role in the determination of the HTC.
Although the gas flow was continuously humidified and kept at approximately 40% relative humidity, a slight static charge of the particles could be observed during the experiment, which can lead to agglomeration of the particles.
4. Conclusions
The experimental investigations on heat transfer within a bubbling fluidized bed test facility with Geldart type B amine bulk material showed that, the single bare tube with 25 mm outer diameter have approximately equal measured results as both tube bundle arrangements. Moreover, in-line and staggered configuration also have identical HTCs which contradicts the finding that staggered have overall slightly higher HTCs than in-line layouts. This could only be determined in the last third of the measurement. In the same way, single tubes should have significantly higher HTCs.
In order to predict heat transfer coefficients when it comes to dimensioning for TSA heat exchangers, the model proposed by Molerus et al. [
21,
22] applied with the tube diameter factor by Petrie et al. [
11] are in a good agreement with the obtained experimental results. Nevertheless, the comparison of the experimentally obtained data with tube bundle modeled data point out, that the literature models are not able to describe the experimental results. The usability of the mathematical models depends, as shown in the evaluation, on the particle properties. For this reason, it is unclear which model can be used to dimension TSA processes for optimal heat integration. Thus, future experimental series with the introduced fluidized bed test facility will be conducted with different heat exchanger layouts, other tube diameters and finned tubes for heat transfer measurements.
A numerical study has been done of the test facility in terms of fluidization regime and heat transfer coefficient investigations. This work used the Euler–Euler method to describe the multiphase system. For a flow mechanical and thermal modeling of granular multiphase flows by CFD it is necessary to model the particle properties as well as the interaction forces between gas and solids very precisely. In order to obtain a more accurate representation of the fluidized bed at the bed surface in the dilute regime, it may be necessary to consider the diameter distribution of the particles or to use a modified drag model which uses experimentally determined values. Anyway, this tool can be used for modeling regimes of a fluidized bed TSA-reactor with external circulation.
For a more accurate determination of the heat transfer coefficient, the influencing factors on it must be identified. Since the thermal conductivity of both phases has a large influence on it, the modeling should be checked according to Zehner und Schlünder and adapted if necessary.
Further investigations of the particle properties can help to improve the modeling of the multiphase system with Euler–Euler. The interaction between gas and solids can possibly be improved using modified drag models. The effects of a mesh refinement on the tube, which contradicts the Euler–Euler approach, should be considered with regard to the effects on the fluidization regime and heat transfer. A numerical analysis with an alternative software (i.e., OPENFOAM®) can also help to classify the results. Nevertheless, the first step for the development of a simulation was taken for TSA-applications.