Phase Change Materials (PCM) for Solar Energy Usages and Storage: An Overview
Abstract
:1. Introduction
2. Phase Change Materials (PCMs)
3. PCMs Solar Power Generation
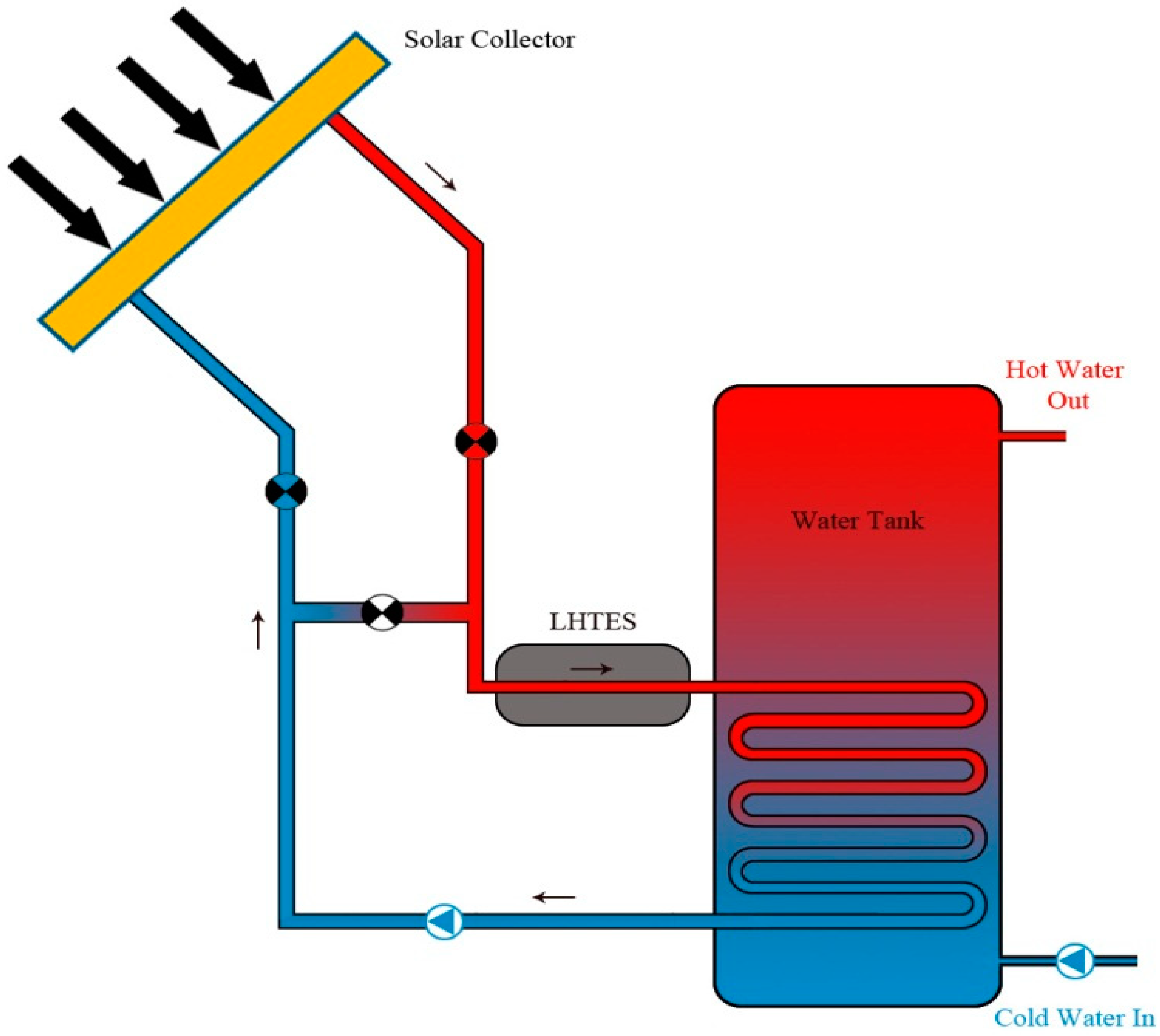
4. PCMs Solar Water Heating System
5. PCMs Solar Cookers
6. PCMs Solar Dryers
7. Conclusions and Recommendations
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CLHS | cascade latent thermal energy storage |
| CST | concentrated solar thermal |
| DSG | direct steam generation |
| ETSC | evacuated tube solar collector |
| LHTES | latent heat thermal energy storage |
| LPG | liquid petroleum gas |
| PCM | phase change material |
| SEGS | solar electricity generating systems |
| TES | thermal energy storage |
References
- Mofijur, M.; Masjuki, H.H.; Kalam, M.A.; Atabani, A.E.; Fattah, I.M.R.; Mobarak, H.M. Comparative evaluation of performance and emission characteristics of Moringa oleifera and Palm oil based biodiesel in a diesel engine. Ind. Crops Prod. 2014, 53, 78–84. [Google Scholar] [CrossRef]
- Mofijur, M.; Masjuki, H.H.; Kalam, M.A.; Hazrat, M.A.; Liaquat, A.M.; Shahabuddin, M.; Varman, M. Prospects of biodiesel from Jatropha in Malaysia. Renew. Sustain. Energy Rev. 2012, 16, 5007–5020. [Google Scholar] [CrossRef]
- Mofijur, M.; Masjuki, H.H.; Kalam, M.A.; Atabani, A.E. Evaluation of biodiesel blending, engine performance and emissions characteristics of Jatropha curcas methyl ester: Malaysian perspective. Energy 2013, 55, 879–887. [Google Scholar] [CrossRef]
- Norhasyima, R.S.; Mahlia, T.M.I. Advances in CO2 utilization technology: A patent landscape review. J. CO2 Util. 2018, 26, 323–335. [Google Scholar] [CrossRef]
- Mofijur, M.; Atabani, A.E.; Masjuki, H.H.; Kalam, M.A.; Masum, B.M. A study on the effects of promising edible and non-edible biodiesel feedstocks on engine performance and emissions production: A comparative evaluation. Renew. Sustain. Energy Rev. 2013, 23, 391–404. [Google Scholar] [CrossRef]
- Ismail, M.S.; Moghavvemi, M.; Mahlia, T.M.I. Characterization of PV panel and global optimization of its model parameters using genetic algorithm. Energy Convers. Manag. 2013, 73, 10–25. [Google Scholar] [CrossRef]
- IEA. World energy balances: Overview 2018. Int. Energy Agency. France. Available online: https://webstore.iea.org/world-energy-balances-2018 (accessed on 30 May 2019).
- Mazandarani, A.; Mahlia, T.M.I.; Chong, W.T.; Moghavvemi, M. Fuel consumption and emission prediction by Iranian power plants until 2025. Renew. Sustain. Energy Rev. 2011, 15, 1575–1592. [Google Scholar] [CrossRef]
- Mohammadnejad, M.; Ghazvini, M.; Mahlia, T.M.I.; Andriyana, A. A review on energy scenario and sustainable energy in Iran. Renew. Sustain. Energy Rev. 2011, 15, 4652–4658. [Google Scholar] [CrossRef]
- Ong, H.C.; Mahlia, T.M.I.; Masjuki, H.H. A review on emissions and mitigation strategies for road transport in Malaysia. Renew. Sustain. Energy Rev. 2011, 15, 3516–3522. [Google Scholar] [CrossRef]
- Dharma, S.; Masjuki, H.H.; Ong, H.C.; Sebayang, A.H.; Silitonga, A.S.; Kusumo, F.; Mahlia, T.M.I. Optimization of biodiesel production process for mixed Jatropha curcas-Ceiba pentandra biodiesel using response surface methodology. Energy Convers. Manag. 2016, 115, 178–190. [Google Scholar] [CrossRef]
- Ong, H.C.; Masjuki, H.H.; Mahlia, T.M.I.; Silitonga, A.S.; Chong, W.T.; Leong, K.Y. Optimization of biodiesel production and engine performance from high free fatty acid Calophyllum inophyllum oil in CI diesel engine. Energy Convers. Manag. 2014, 81, 30–40. [Google Scholar] [CrossRef]
- Silitonga, A.S.; Atabani, A.E.; Mahlia, T.M.I.; Masjuki, H.H.; Badruddin, I.A.; Mekhilef, S. A review on prospect of Jatropha curcas for biodiesel in Indonesia. Renew. Sustain. Energy Rev. 2011, 15, 3733–3756. [Google Scholar] [CrossRef]
- Silitonga, A.S.; Masjuki, H.H.; Ong, H.C.; Sebayang, A.H.; Dharma, S.; Kusumo, F.; Siswantoro, J.; Milano, J.; Daud, K.; Mahlia, T.M.I.; et al. Evaluation of the engine performance and exhaust emissions of biodiesel-bioethanol-diesel blends using kernel-based extreme learning machine. Energy 2018, 159, 1075–1087. [Google Scholar] [CrossRef]
- Uddin, M.N.; Techato, K.; Taweekun, J.; Rahman, M.M.; Rasul, M.G.; Mahlia, T.M.I.; Ashrafur, S.M. An Overview of Recent Developments in Biomass Pyrolysis Technologies. Energies 2018, 11, 3115. [Google Scholar] [CrossRef]
- Coh, B.H.H.; Ong, H.C.; Cheah, M.Y.; Chen, W.H.; Yu, K.L.; Mahlia, T.M.I. Sustainability of direct biodiesel synthesis from microalgae biomass: A critical review. Renew. Sustain. Energy Rev. 2019, 107, 59–74. [Google Scholar]
- Domański, R.; Jaworski, M.; Rebow, M. Thermal energy storage problems. Available online: http://papers.itc.pw.edu.pl/index.php/JPT/article/viewFile/146/260 (accessed on 30 May 2019).
- Fauzi, H.; Metselaar, H.S.C.; Mahlia, T.M.I.; Silakhori, M.; Ong, H.C. Thermal characteristic reliability of fatty acid binary mixtures as phase change materials (PCMs) for thermal energy storage applications. Appl. Therm. Eng. 2015, 80, 127–131. [Google Scholar] [CrossRef]
- Hendra, R.; Hamdani; Mahlia, T.M.I.; Masjuki, H.H. Thermal and melting heat transfer characteristics in a latent heat storage system using Mikro. Appl. Therm. Eng. 2005, 25, 1503–1515. [Google Scholar] [CrossRef]
- Mahlia, T.M.I.; Saktisandan, T.J.; Jannifar, A.; Hasan, M.H.; Matseelar, H.S.C. A review of available methods and development on energy storage; technology update. Renew. Sustain. Energy Rev. 2014, 33, 532–545. [Google Scholar] [CrossRef]
- Akhiani, A.R.; Mehrali, M.; Latibari, S.T.; Mehrali, M.; Mahlia, T.M.I.; Sadeghinezhad, E.; Metselaar, H.S.C. One-Step Preparation of Form-Stable Phase Change Material through Self-Assembly of Fatty Acid and Graphene. J. Phys. Chem. C 2015, 119, 22787–22796. [Google Scholar] [CrossRef]
- Milano, J.; Ong, H.C.; Masjuki, H.H.; Silitonga, A.S.; Chen, W.H.; Kusumo, F.; Dharma, S.; Sebayang, A.H. Optimization of biodiesel production by microwave irradiation-assisted transesterification for waste cooking oil-Calophyllum inophyllum oil via response surface methodology. Energy Convers. Manag. 2018, 158, 400–415. [Google Scholar] [CrossRef]
- Dincer, I.; Rosen, M. Thermal Energy Storage: Systems and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2010. [Google Scholar]
- Evans, A.; Strezov, V.; Evans, T.J. Assessment of utility energy storage options for increased renewable energy penetration. Renew. Sustain. Energy Rev. 2012, 16, 4141–4147. [Google Scholar] [CrossRef]
- Lanahan, M.; Tabares-Velasco, P. Seasonal thermal-energy storage: A critical review on BTES systems, modeling, and system design for higher system efficiency. Energies 2017, 10, 743. [Google Scholar] [CrossRef]
- Kalaiselvam, S.; Parameshwaran, R. Thermal Energy Storage Technologies for Sustainability: Systems Design, Assessment and Applications; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Nordell, B.; Grein, M.; Kharseh, M. Large-scale utilisation of renewable energy requires energy storage. In Proceedings of the International Conference of Renewable Energys and Suistainable Development, Tlemcen, Algeria, 21–24 May 2007; pp. 21–24. [Google Scholar]
- Sibbet, B.; McClenahan, D. Seasonal Borehole Thermal Energy Storage—Guidelines for Design & Construction. Available online: http://task45.iea-shc.org/data/sites/1/publications/IEA-SHC-T45.B.3.1-TECH-Seasonal-storages-Borehole-Guidelines.pdf (accessed on 3 June 2019).
- Noël, J.A.; Kahwaji, S.; Desgrosseilliers, L.; Groulx, D.; White, M.A. Chapter 13—Phase Change Materials. In Storing Energy; Letcher, T.M., Ed.; Elsevier: Oxford, UK, 2016; pp. 249–272. [Google Scholar] [CrossRef]
- Silakhori, M.; Fauzi, H.; Mahmoudian, M.R.; Metselaar, H.S.C.; Mahlia, T.M.I.; Khanlou, H.M. Preparation and thermal properties of form-stable phase change materials composed of palmitic acid/polypyrrole/graphene nanoplatelets. Energy Build. 2015, 99, 189–195. [Google Scholar] [CrossRef]
- Latibari, S.T.; Mehrali, M.; Mehrali, M.; Mahlia, T.M.I.; Metselaar, H.S.C. Synthesis, characterization and thermal properties of nanoencapsulated phase change materials via sol-gel method. Energy 2013, 61, 664–672. [Google Scholar] [CrossRef]
- Silakhori, M.; Jafarian, M.; Arjomandi, M.; Nathan, G.J. Comparing the thermodynamic potential of alternative liquid metal oxides for the storage of solar thermal energy. Sol. Energy 2017, 157, 251–258. [Google Scholar] [CrossRef]
- Silakhori, M.; Jafarian, M.; Arjomandi, M.; Nathan, G.J. Experimental assessment of copper oxide for liquid chemical looping for thermal energy storage. J. Energy Storage 2019, 21, 216–221. [Google Scholar] [CrossRef]
- Mehrali, M.; Latibari, S.T.; Mehrali, M.; Mahlia, T.M.I.; Metselaar, H.S.C.; Naghavi, M.S.; Sadeghinezhad, E.; Akhiani, A.R. Preparation and characterization of palmitic acid/graphene nanoplatelets composite with remarkable thermal conductivity as a novel shape-stabilized phase change material. Appl. Therm. Eng. 2013, 61, 633–640. [Google Scholar] [CrossRef]
- Van Helden, W.; Yamaha, M.; Rathgeber, C.; Hauer, A.; Huaylla, F.; Le Pierrès, N.; Stutz, B.; Mette, B.; Dolado, P.; Lazaro, A.; et al. IEA SHC Task 42/ECES Annex 29—Working Group B: Applications of Compact Thermal Energy Storage. Energy Proc. 2016, 91, 231–245. [Google Scholar] [CrossRef]
- Sala, J.M. 20—Thermal energy storage (TES) systems for cogeneration and trigeneration systems A2—Cabeza, Luisa, F. In Advances in Thermal Energy Storage Systems; Woodhead Publishing: Amsterdam, The Netherlands, 2015; pp. 493–509. [Google Scholar] [CrossRef]
- Zhang, P.; Meng, Z.N.; Zhu, H.; Wang, Y.L.; Peng, S.P. Melting heat transfer characteristics of a composite phase change material fabricated by paraffin and metal foam. Appl. Energy 2017, 185, 1971–1983. [Google Scholar] [CrossRef]
- Boy, E.; Boss, R.; Lutz, M. A collector storage module withy integrated phase change material. Proc. ISES Pergamon Press Hambg. 1987, 3672–3680. [Google Scholar] [CrossRef]
- Hepbasli, A.; Alsuhaibani, Z. A key review on present status and future directions of solar energy studies and applications in Saudi Arabia. Renew. Sustain. Energy Rev. 2011, 15, 5021–5050. [Google Scholar] [CrossRef]
- Tyagi, V.V.; Kaushik, S.C.; Tyagi, S.K.; Akiyama, T. Development of phase change materials based microencapsulated technology for buildings: A review. Renew. Sustain. Energy Rev. 2011, 15, 1373–1391. [Google Scholar] [CrossRef]
- Verma, P.; Varun; Singal, S.K. Review of mathematical modeling on latent heat thermal energy storage systems using phase-change material. Renew. Sustain. Energy Rev. 2008, 12, 999–1031. [Google Scholar] [CrossRef]
- Zalba, B.; Marín, J.M.; Cabeza, L.F.; Mehling, H. Review on thermal energy storage with phase change: Materials, heat transfer analysis and applications. Appl. Therm. Eng. 2003, 23, 251–283. [Google Scholar] [CrossRef]
- Zhao, C.Y.; Zhang, G.H. Review on microencapsulated phase change materials (MEPCMs): Fabrication, characterization and applications. Renew. Sustain. Energy Rev. 2011, 15, 3813–3832. [Google Scholar] [CrossRef]
- Zhou, D.; Zhao, C.Y.; Tian, Y. Review on thermal energy storage with phase change materials (PCMs) in building applications. Appl. Energy 2012, 92, 593–605. [Google Scholar] [CrossRef] [Green Version]
- Zhu, N.; Ma, Z.; Wang, S. Dynamic characteristics and energy performance of buildings using phase change materials: A review. Energy Convers. Manag. 2009, 50, 3169–3181. [Google Scholar] [CrossRef]
- Aditya, L.; Mahlia, T.M.I.; Rismanchi, B.; Ng, H.M.; Hasan, M.H.; Metselaar, H.S.C.; Muraza, O.; Aditiya, H.B. A review on insulation materials for energy conservation in buildings. Renew. Sustain. Energy Rev. 2017, 73, 1352–1365. [Google Scholar] [CrossRef]
- Mehrali, M.; Latibari, S.T.; Mehrali, M.; Mahlia, T.M.I.; Metselaar, H.S.C. Effect of carbon nanospheres on shape stabilization and thermal behavior of phase change materials for thermal energy storage. Energy Convers. Manag. 2014, 88, 206–213. [Google Scholar] [CrossRef]
- Silakhori, M.; Metselaar, H.S.C.; Mahlia, T.M.I.; Fauzi, H.; Baradaran, S.; Naghavi, M.S. Palmitic acid/polypyrrole composites as form-stable phase change materials for thermal energy storage. Energy Convers. Manag. 2014, 80, 491–497. [Google Scholar] [CrossRef]
- Amin, M.; Putra, N.; Kosasih, E.A.; Prawiro, E.; Luanto, R.A.; Mahlia, T.M.I. Thermal properties of beeswax/graphene phase change material as energy storage for building applications. Appl. Therm. Eng. 2017, 112, 273–280. [Google Scholar] [CrossRef]
- Abhat, A. Low temperature latent heat thermal energy storage: Heat storage materials. Sol. Energy 1983, 30, 313–332. [Google Scholar] [CrossRef]
- Fallahi, A.; Guldentops, G.; Tao, M.; Granados-Focil, S.; Van Dessel, S. Review on solid-solid phase change materials for thermal energy storage: Molecular structure and thermal properties. Appl. Therm. Eng. 2017, 127, 1427–1441. [Google Scholar] [CrossRef]
- Sharma, A.; Tyagi, V.V.; Chen, C.R.; Buddhi, D. Review on thermal energy storage with phase change materials and applications. Renew. Sustain. Energy Rev. 2009, 13, 318–345. [Google Scholar] [CrossRef]
- Hamdani; Thaib, R.; Irwansyah; Dailami; Mahlia, T.M.I. Experimental investigation on melting heat transfer of paraffin wax-Al2O3 storage system. Int. J. Appl. Eng. Res. 2014, 9, 17903–17910. [Google Scholar]
- Silakhori, M.; Metselaar, H.S.C.; Mahlia, T.M.I.; Fauzi, H. Preparation and characterisation of microencapsulated paraffin wax with polyaniline-based polymer shells for thermal energy storage. Mater. Res. Innov. 2014, 18, S6–S480. [Google Scholar] [CrossRef]
- Silakhori, M.; Naghavi, M.S.; Metselaar, H.S.C.; Mahlia, T.M.I.; Fauzi, H.; Mehrali, M. Accelerated thermal cycling test of microencapsulated paraffin wax/polyaniline made by simple preparation method for solar thermal energy storage. Materials 2013, 6, 1608–1620. [Google Scholar] [CrossRef]
- Yasin, M.M.; Yusaf, T.; Mamat, R.; Yusop, A.F. Characterization of a diesel engine operating with a small proportion of methanol as a fuel additive in biodiesel blend. Appl. Energy 2014, 114, 865–873. [Google Scholar] [CrossRef]
- Huang, Y.-H.; Wu, J.-H. Analysis of biodiesel promotion in Taiwan. Renew. Sustain. Energy Rev. 2008, 12, 1176–1186. [Google Scholar] [CrossRef]
- Xie, N.; Huang, Z.; Luo, Z.; Gao, X.; Fang, Y.; Zhang, Z. Inorganic Salt Hydrate for Thermal Energy Storage. Appl. Sci. 2017, 7, 1317. [Google Scholar] [CrossRef]
- Oró, E.; de Gracia, A.; Castell, A.; Farid, M.M.; Cabeza, L.F. Review on phase change materials (PCMs) for cold thermal energy storage applications. Appl. Energy 2012, 99, 513–533. [Google Scholar] [CrossRef]
- Bland, A.; Khzouz, M.; Statheros, T.; Gkanas, E. PCMs for residential building applications: A short review focused on disadvantages and proposals for future development. Buildings 2017, 7, 78. [Google Scholar] [CrossRef]
- Fan, Y.; Zhang, X.; Wang, X.; Li, J.; Zhu, Q. Super-cooling prevention of microencapsulated phase change material. Thermochim. Acta 2004, 413, 1–6. [Google Scholar] [CrossRef]
- Yamagishi, Y.; Sugeno, T.; Ishige, T.; Takeuchi, H.; Pyatenko, A.T. An evaluation of microencapsulated PCM for use in cold energy transportation medium. In Proceedings of the 31st Intersociety Energy Conversion Engineering Conference, Washington, DC, USA, 11–16 August 1996; pp. 2077–2083. [Google Scholar]
- Jin, Y.; Wan, Q.; Ding, Y. PCMs heat transfer performance enhancement with expanded graphite and its thermal stability. Proc. Eng. 2015, 102, 1877–1884. [Google Scholar] [CrossRef]
- Letcher, T.M.; Law, R.; Reay, D. Storing Energy: With Special Reference to Renewable Energy Sources; Elsevier: Oxford, UK, 2016. [Google Scholar]
- Sittisart, P.; Farid, M.M. Fire retardants for phase change materials. Appl. Energy 2011, 88, 3140–3145. [Google Scholar] [CrossRef]
- Song, G.; Ma, S.; Tang, G.; Yin, Z.; Wang, X. Preparation and characterization of flame retardant form-stable phase change materials composed by EPDM, paraffin and nano magnesium hydroxide. Energy 2010, 35, 2179–2183. [Google Scholar] [CrossRef]
- Lin, Y.; Alva, G.; Fang, G. Review on thermal performances and applications of thermal energy storage systems with inorganic phase change materials. Energy 2018, 165, 685–708. [Google Scholar] [CrossRef]
- Mohamed, S.A.; Al-Sulaiman, F.A.; Ibrahim, N.I.; Zahir, M.H.; Al-Ahmed, A.; Saidur, R.; Yılbaş, B.S.; Sahin, A.Z. A review on current status and challenges of inorganic phase change materials for thermal energy storage systems. Renew. Sustain. Energy Rev. 2017, 70, 1072–1089. [Google Scholar] [CrossRef]
- Montes, M.J.; Abanades, A.; Martinez-Val, J.M. Performance of a direct steam generation solar thermal power plant for electricity production as a function of the solar multiple. Sol. Energy 2009, 83, 679–689. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.D.; Zhao, L. Analysis of zeotropic mixtures used in low-temperature solar Rankine cycles for power generation. Sol. Energy 2009, 83, 605–613. [Google Scholar] [CrossRef]
- Chong, W.T.; Naghavi, M.S.; Poh, S.C.; Mahlia, T.M.I.; Pan, K.C. Techno-economic analysis of a wind-solar hybrid renewable energy system with rainwater collection feature for urban high-rise application. Appl. Energy 2011, 88, 4067–4077. [Google Scholar] [CrossRef]
- Ismail, M.S.; Moghavvemi, M.; Mahlia, T.M.I. Techno-economic analysis of an optimized photovoltaic and diesel generator hybrid power system for remote houses in a tropical climate. Energy Convers. Manag. 2013, 69, 163–173. [Google Scholar] [CrossRef]
- Gil, A.; Medrano, M.; Martorell, I.; Lazaro, A.; Dolado, P.; Zalba, B.; Cabeza, L.F. State of the art on high temperature thermal energy storage for power generation. Part 1—Concepts, materials and modellization. Renew. Sustain. Energy Rev. 2010, 14, 31–55. [Google Scholar] [CrossRef]
- Stutz, B.; Le Pierres, N.; Kuznik, F.; Johannes, K.; Palomo Del Barrio, E.; Bédécarrats, J.-P.; Gibout, S.; Marty, P.; Zalewski, L.; Soto, J.; et al. Storage of thermal solar energy. Comptes Rendus Phys. 2017, 18, 401–414. [Google Scholar] [CrossRef]
- Michels, H.; Pitz-Paal, R. Cascaded latent heat storage for parabolic trough solar power plants. Sol. Energy 2007, 81, 829–837. [Google Scholar] [CrossRef]
- Hunold, D. Zur Auslegung und Konstruktion von Thermischen Energiespeichern Mit Einem Fest/Flüssig Phasenwechsel des Speichermaterials für Parabolrinnen-Solarkraftwerke; VDI-Verl: Dusseldorf, Germany, 1994. [Google Scholar]
- Hunold, D.; Ratzesberger, R.; Tamme, R. Heat Transfer Mechanism in Latent-Heat Thermal Energy Storage for Medium Temperature Application. In Proceedings of the 6th International Symposium on Solar Thermal Concentrating Technologies, Mojacar, Spain, 28 September–2 October 1992. [Google Scholar]
- Hunold, D.; Tamme, R. Thermal Energy Storage at Medium and High Temperatures for Solar Power Plants, Forschungsverbund Sonnenenergie. Forschungsverbund Sonnenenergie Topics 1994, 93, 94. [Google Scholar]
- SunShot, E.E. US Department of Energy, 2012; NREL Report No. BK5200-47927. DOE/GO–102012-3037; SunShot Vision Study: Washington, DC, USA, 2012. [Google Scholar]
- Turchi, C.S.; Ma, Z.; Neises, T.W.; Wagner, M.J. Thermodynamic study of advanced supercritical carbon dioxide power cycles for concentrating solar power systems. J. Sol. Energy Eng. 2013, 135, 041007. [Google Scholar] [CrossRef]
- Regin, A.F.; Solanki, S.; Saini, J. Heat transfer characteristics of thermal energy storage system using PCM capsules: A review. Renew. Sustain. Energy Rev. 2008, 12, 2438–2458. [Google Scholar] [CrossRef]
- Agyenim, F.; Eames, P.; Smyth, M. Heat transfer enhancement in medium temperature thermal energy storage system using a multitube heat transfer array. Renew. Energy 2010, 35, 198–207. [Google Scholar] [CrossRef]
- Pirasaci, T.; Goswami, D.Y. Influence of design on performance of a latent heat storage system for a direct steam generation power plant. Appl. Energy 2016, 162, 644–652. [Google Scholar] [CrossRef] [Green Version]
- Valenzuela, L. Thermal energy storage concepts for direct steam generation (DSG) solar plants. In Advances in Concentrating Solar Thermal Research and Technology; Elsevier: Amsterdam, The Netherlands, 2017; pp. 269–289. [Google Scholar]
- Dinter, F.; Geyer, M.; Tamme, R. Thermal Energy Storage for Commercial Application (TESCA), a Feasibility Study on Economic Storage Systems; Springer: Berlin/Heidelberg, Germany, 1991. [Google Scholar]
- Ratzesberger, R.; Beine, B.; Hahne, E. Regeneratoren mit Beton und Phasenwechselmaterial als Speichermasse. VDI Berichte-Verein Deutscher Ingenieure 1994, 1168, 467. [Google Scholar]
- Pandey, A.K.; Hossain, M.S.; Tyagi, V.V.; Abd Rahim, N.; Selvaraj, J.A.L.; Sari, A. Novel approaches and recent developments on potential applications of phase change materials in solar energy. Renew. Sustain. Energy Rev. 2018, 82, 281–323. [Google Scholar] [CrossRef]
- Zauner, C.; Hengstberger, F.; Mörzinger, B.; Hofmann, R.; Walter, H. Experimental characterization and simulation of a hybrid sensible-latent heat storage. Appl. Energy 2017, 189, 506–519. [Google Scholar] [CrossRef]
- Zanganeh, G.; Commerford, M.; Haselbacher, A.; Pedretti, A.; Steinfeld, A. Stabilization of the outflow temperature of a packed-bed thermal energy storage by combining rocks with phase change materials. Appl. Therm. Eng. 2014, 70, 316–320. [Google Scholar] [CrossRef]
- Pelay, U.; Luo, L.; Fan, Y.; Stitou, D.; Rood, M. Thermal energy storage systems for concentrated solar power plants. Renew. Sustain. Energy Rev. 2017, 79, 82–100. [Google Scholar] [CrossRef]
- Jaisankar, S.; Ananth, J.; Thulasi, S.; Jayasuthakar, S.T.; Sheeba, K.N. A comprehensive review on solar water heaters. Renew. Sustain. Energy Rev. 2011, 15, 3045–3050. [Google Scholar] [CrossRef]
- Mehrali, M.; Ghatkesar, M.K.; Pecnik, R. Full-spectrum volumetric solar thermal conversion via graphene/silver hybrid plasmonic nanofluids. Appl. Energy 2018, 224, 103–115. [Google Scholar] [CrossRef]
- Shukla, A.; Buddhi, D.; Sawhney, R.L. Solar water heaters with phase change material thermal energy storage medium: A review. Renew. Sustain. Energy Rev. 2009, 13, 2119–2125. [Google Scholar] [CrossRef]
- Bhatia, S.C. 4—Solar thermal energy. In Advanced Renewable Energy Systems; Bhatia, S.C., Ed.; Woodhead Publishing India: Delhi, India, 2014; pp. 94–143. [Google Scholar] [CrossRef]
- Chaabane, M.; Mhiri, H.; Bournot, P. Thermal performance of an integrated collector storage solar water heater (ICSSWH) with phase change materials (PCM). Energy Convers. Manag. 2014, 78, 897–903. [Google Scholar] [CrossRef]
- Prakash, J.; Garg, H.; Datta, G. A solar water heater with a built-in latent heat storage. Energy Convers. Manag. 1985, 25, 51–56. [Google Scholar] [CrossRef]
- Bansal, N.; Buddhi, D. An analytical study of a latent heat storage system in a cylinder. Energy Convers. Manag. 1992, 33, 235–242. [Google Scholar] [CrossRef]
- Porteiro, J.; Míguez, J.L.; Crespo, B.; De Lara, J.; Pousada, J.M. On the Behavior of Different PCMs in a Hot Water Storage Tank against Thermal Demands. Materials 2016, 9, 213. [Google Scholar] [CrossRef] [PubMed]
- Benmoussa, F.; Benzaoui, A.; Benmoussa, H. Thermal behavior of latent thermal energy storage unit using two phase change materials: Effects of HTF inlet temperature. Case Stud. Therm. Eng. 2017, 10, 475–483. [Google Scholar] [CrossRef]
- Kaygusuz, K. Experimental and theoretical investigation of latent heat storage for water based solar heating systems. Energy Convers. Manag. 1995, 36, 315–323. [Google Scholar] [CrossRef]
- Rabin, Y.; Bar-Niv, I.; Korin, E.; Mikic, B. Integrated solar collector storage system based on a salt-hydrate phase-change material. Sol. Energy 1995, 55, 435–444. [Google Scholar] [CrossRef]
- Sharma, A.; Pradhan, N.; Kumar, B. Performance evaluation of a solar water heater having built in latent heat storage unit, IEA, ECESIA Annex 17. Advanced thermal energy storage through phase change materials and chemical reactions—feasibility studies and demonstration projects. In Proceedings of the 4th workshop, Indore, India, 21–24 March 2003; pp. 109–115. [Google Scholar]
- Mettawee, E.B.S.; Assassa, G.M.R. Experimental study of a compact PCM solar collector. Energy 2006, 31, 2958–2968. [Google Scholar] [CrossRef]
- Cabeza, L.F.; Ibanez, M.; Sole, C.; Roca, J.; Nogués, M. Experimentation with a water tank including a PCM module. Sol. Energy Mater. Sol. Cells 2006, 90, 1273–1282. [Google Scholar] [CrossRef]
- Kumar, B. Design, Development and Performance Evaluation of a Latent Heat Storage Unit for Evening and Morning Hot Water Using a Box Type Solar Collector; Project Report, M. Tech. (Energy Management); School of Energy and Environmental Studies, Devi Ahilya University: Indore, India, 2001. [Google Scholar]
- Shukla, A. Heat Transfer Studies on Phase Change Materials and Their Utilization in Solar Water Heaters. Ph.D. Thesis, School of Energy and Environmental Studies, Devi Ahilya University, Indore, India, 2006. [Google Scholar]
- Hasan, A. Phase change material energy storage system employing palmitic acid. Sol. Energy 1994, 52, 143–154. [Google Scholar] [CrossRef]
- Hasan, A. Thermal energy storage system with stearic acid as phase change material. Energy Convers. Manag. 1994, 35, 843–856. [Google Scholar] [CrossRef]
- Hasan, A.; Sayigh, A. Some fatty acids as phase-change thermal energy storage materials. Renew. Energy 1994, 4, 69–76. [Google Scholar] [CrossRef]
- Tiwari, G.; Rai, S.; Ram, S.; Singh, M. Performance prediction of PCCM collection-cum-storage water heater: Quasi-steady state solution. Energy Convers. Manag. 1988, 28, 219–223. [Google Scholar] [CrossRef]
- Ling, D.; Mo, G.; Jiao, Q.; Wei, J.; Wang, X. Research on Solar Heating System with Phase Change Thermal Energy Storage. Energy Procedia 2016, 91, 415–420. [Google Scholar] [CrossRef] [Green Version]
- Tayeb, A.M. A simulation model for a phase-change energy storage system: Experimental and verification. Energy Convers. Manag. 1993, 34, 243–250. [Google Scholar] [CrossRef]
- Font, J.; Muntasell, J.; Cardoner, F. Preliminary study of a heat storage unit using a solid-solid transition. Sol. Energy Mater. Sol. Cells 1994, 33, 169–176. [Google Scholar] [CrossRef]
- Bhargava, A.K. A solar water heater based on phase-changing material. Appl. Energy 1983, 14, 197–209. [Google Scholar] [CrossRef]
- Canbazoglu, S.; Sahinaslan, A.; Ekmekyapar, A.; Aksoy, Y.G.; Akarsu, F. Enhancement of solar thermal energy storage performance using sodium thiosulfate pentahydrate of a conventional solar water-heating system. Energy Build. 2005, 37, 235–242. [Google Scholar] [CrossRef]
- Mahfuz, M.H.; Anisur, M.R.; Kibria, M.A.; Saidur, R.; Metselaar, I.H.S.C. Performance investigation of thermal energy storage system with Phase Change Material (PCM) for solar water heating application. Int. Commun. Heat Mass Transf. 2014, 57, 132–139. [Google Scholar] [CrossRef]
- Hussein, H.; El-Ghetany, H.; Nada, S. Experimental investigation of novel indirect solar cooker with indoor PCM thermal storage and cooking unit. Energy Convers. Manag. 2008, 49, 2237–2246. [Google Scholar] [CrossRef]
- Cuce, E.; Cuce, P.M. A comprehensive review on solar cookers. Appl. Energy 2013, 102, 1399–1421. [Google Scholar] [CrossRef]
- Ozoegwu, C.G.; Mgbemene, C.A.; Ozor, P.A. The status of solar energy integration and policy in Nigeria. Renew. Sustain. Energy Rev. 2017, 70, 457–471. [Google Scholar] [CrossRef]
- Yettou, F.; Azoui, B.; Malek, A.; Gama, A.; Panwar, N.L. Solar cooker realizations in actual use: An overview. Renew. Sustain. Energy Rev. 2014, 37, 288–306. [Google Scholar] [CrossRef]
- Otte, P.P. Solar cooking in Mozambique—An investigation of end-user’s needs for the design of solar cookers. Energy Policy 2014, 74, 366–375. [Google Scholar] [CrossRef]
- Kumaresan, G.; Santosh, R.; Raju, G.; Velraj, R. Experimental and numerical investigation of solar flat plate cooking unit for domestic applications. Energy 2018, 157, 436–447. [Google Scholar] [CrossRef]
- Buddhi, D.; Sahoo, L. Solar cooker with latent heat storage: Design and experimental testing. Energy Convers. Manag. 1997, 38, 493–498. [Google Scholar] [CrossRef]
- Zhao, J.; Ji, Y.; Yuan, Y.; Zhang, Z.; Lu, J. Energy-Saving Analysis of Solar Heating System with PCM Storage Tank. Energies 2018, 11, 237. [Google Scholar] [CrossRef]
- Domanski, R.; El-Sebaii, A.; Jaworski, M. Cooking during off-sunshine hours using PCMs as storage media. Energy 1995, 20, 607–616. [Google Scholar] [CrossRef]
- Sharma, S.; Buddhi, D.; Sawhney, R.; Sharma, A. Design, development and performance evaluation of a latent heat storage unit for evening cooking in a solar cooker. Energy Convers. Manag. 2000, 41, 1497–1508. [Google Scholar] [CrossRef]
- Sharma, S.D.; Iwata, T.; Kitano, H.; Sagara, K. Thermal performance of a solar cooker based on an evacuated tube solar collector with a PCM storage unit. Sol. Energy 2005, 78, 416–426. [Google Scholar] [CrossRef]
- Kenisarin, M.; Mahkamov, K. Solar energy storage using phase change materials. Renew. Sustain. Energy Rev. 2007, 11, 1913–1965. [Google Scholar] [CrossRef]
- Muthusivagami, R.M.; Velraj, R.; Sethumadhavan, R. Solar cookers with and without thermal storage—A review. Renew. Sustain. Energy Rev. 2010, 14, 691–701. [Google Scholar] [CrossRef]
- Indora, S.; Kandpal, T.C. Institutional cooking with solar energy: A review. Renew. Sustain. Energy Rev. 2018, 84, 131–154. [Google Scholar] [CrossRef]
- Munir, A.; Hensel, O.; Scheffler, W. Design principle and calculations of a Scheffler fixed focus concentrator for medium temperature applications. Sol. Energy 2010, 84, 1490–1502. [Google Scholar] [CrossRef]
- Saxena, A.; Pandey, S.P.; Srivastav, G. A thermodynamic review on solar box type cookers. Renew. Sustain. Energy Rev. 2011, 15, 3301–3318. [Google Scholar] [CrossRef]
- Nkhonjera, L.; Bello-Ochende, T.; John, G.; King’ondu, C.K. A review of thermal energy storage designs, heat storage materials and cooking performance of solar cookers with heat storage. Renew. Sustain. Energy Rev. 2017, 75, 157–167. [Google Scholar] [CrossRef]
- Leon, M.A.; Kumar, S.; Bhattacharya, S. A comprehensive procedure for performance evaluation of solar food dryers. Renew. Sustain. Energy Rev. 2002, 6, 367–393. [Google Scholar] [CrossRef]
- Kant, K.; Shukla, A.; Sharma, A.; Kumar, A.; Jain, A. Thermal energy storage based solar drying systems: A review. Innov. Food Sci. Emerg. Technol. 2016, 34, 86–99. [Google Scholar] [CrossRef]
- Raponi, F.; Moscetti, R.; Monarca, D.; Colantoni, A.; Massantini, R. Monitoring and optimization of the process of drying fruits and vegetables using computer vision: A Review. Sustainability 2017, 9, 2009. [Google Scholar] [CrossRef]
- Sharma, V.K.; Colangelo, A.; Spagna, G. Experimental investigation of different solar dryers suitable for fruit and vegetable drying. Renew. Energy 1995, 6, 413–424. [Google Scholar] [CrossRef]
- Shalaby, S.M.; Bek, M.A.; El-Sebaii, A.A. Solar dryers with PCM as energy storage medium: A review. Renew. Sustain. Energy Rev. 2014, 33, 110–116. [Google Scholar] [CrossRef]
- Devahastin, S.; Pitaksuriyarat, S. Use of latent heat storage to conserve energy during drying and its effect on drying kinetics of a food product. Appl. Therm. Eng. 2006, 26, 1705–1713. [Google Scholar] [CrossRef]
- Bal, L.M.; Satya, S.; Naik, S.N. Solar dryer with thermal energy storage systems for drying agricultural food products: A review. Renew. Sustain. Energy Rev. 2010, 14, 2298–2314. [Google Scholar] [CrossRef]
- Lamidi, R.O.; Jiang, L.; Pathare, P.B.; Wang, Y.D.; Roskilly, A.P. Recent advances in sustainable drying of agricultural produce: A review. Appl. Energy 2019, 233, 367–385. [Google Scholar] [CrossRef]
- Alva, G.; Lin, Y.; Fang, G. An overview of thermal energy storage systems. Energy 2018, 144, 341–378. [Google Scholar] [CrossRef]
- Shanmugam, V.; Natarajan, E. Experimental study of regenerative desiccant integrated solar dryer with and without reflective mirror. Appl. Therm. Eng. 2007, 27, 1543–1551. [Google Scholar] [CrossRef]
- Shringi, V.; Kothari, S.; Panwar, N.L. Experimental investigation of drying of garlic clove in solar dryer using phase change material as energy storage. J. Therm. Anal. Calorim. 2014, 118, 533–539. [Google Scholar] [CrossRef]
- Jain, D.; Tewari, P. Performance of indirect through pass natural convective solar crop dryer with phase change thermal energy storage. Renew. Energy 2015, 80, 244–250. [Google Scholar] [CrossRef]
- Sain, P.; Songara, V.; Karir, R.; Balan, N. Natural convection type solar dryer with latent heat storage. In Proceedings of the 2013 International Conference on Renewable Energy and Sustainable Energy (ICRESE), Coimbatore, India, 5–6 December 2013; pp. 9–14. [Google Scholar]
- Dina, S.F.; Ambarita, H.; Napitupulu, F.H.; Kawai, H. Study on effectiveness of continuous solar dryer integrated with desiccant thermal storage for drying cocoa beans. Case Stud. Therm. Eng. 2015, 5, 32–40. [Google Scholar] [CrossRef] [Green Version]
- Reyes, A.; Mahn, A.; Vásquez, F. Mushrooms dehydration in a hybrid-solar dryer, using a phase change material. Energy Convers. Manag. 2014, 83, 241–248. [Google Scholar] [CrossRef]







| Type of Materials | Pros | Cons |
|---|---|---|
| Organics PCMs |
|
|
| Inorganic PCMs |
|
|
| Eutectics |
|
|
| Authors | Theoretical/Experimental Description | Results |
|---|---|---|
| Prakesh et al. [96] | Studied an integrated storage type water heater, where a layer of PCM is placed at the base of the heater. | The water is heated during the day and the heat is transferred to the PCM below it, which melts as latent heat accumulates. When sunshine is unavailable, the hot water is replaced with cold water, which collects thermal energy from the PCM, in which phases are changed to solid from a liquid. Ineffective transfer of thermal energy between PCM and the water means the system is not as efficient. |
| Bansal and Budi [97] | Suggested a cylindrical storage unit in the closed-loop with a flat plate collector for its discharging and charging mode. The PCMs used are paraffin wax (p-116) and stearic acid. | Calculations for the fluid temperature and its interface moving boundary were made. |
| Porteiro, Míguez [98] | A thermal analysis is performed to check the thermal properties of each PCM. | A temperature recovery is seen; the energy due to the water temperature, PCM and the thermal loss to the ambient environment are observed. |
| Benmoussa, Benzaoui [99] | A numerical study of the thermal behavior of a shell-and-tube latent thermal energy storage (LTES) unit using two-phase change materials (PCMs). | All heat transfer fluid inlet temperature and melting rate of PCM are varied. Also, a variation in the HTF inlet temperature significantly affects the temperature evolution of PCMs. |
| Kamiz Kayguz et al. [100] | Theoretical and experimental study of the performance of phase change energy storage materials for the solar heater unit. The PCM used is CaCl2.6H2O. A comparison study of heat storage performance for PCM-based, water-based and rock-based system was also conducted. | The solar heating system with Na2SO4.10H2O has more F values compared to CaCl2.6H2O. The thermal properties of the PCM are not reduced during the operation. |
| Rabin et al. [101] | Studied a solar thermal collector with thermal energy storage using salt hydrate as PCM and used for heating. | The results of the study show the correlation between transition temperature and thickness of the salt hydrate PCM layer and the effect on the thermal performance during the PCM charging process. |
| Sharma et al. [102] | Designed, developed and performance evaluated a latent heat storage unit using a box-type solar thermal collector, which can be used during the evening and morning where hot water is needed. The PCM used is paraffin wax. | It was found that the storage unit performed well in keeping the hot water within the desired temperature range. |
| Mattewa and Assassa [103] | Investigated the thermal performance of a compact PCM solar collector utilizing storage of energy in term of latent heat. | The charging process, the average heat transfer coefficient increases sharply with increasing the molten layer thickness, as the natural convection grows stronger. In the discharge process, the useful heat gain was found to increase as the water mass flow rate increases. |
| Cabeza et al. [104] | Tested PCM behaviour in real conditions at the University of Lleida by constructing a solar pilot plant. The solar pilot plant is designed to work continuously either with a solar energy system, or an electrical heater. | In order to use numerous cylinders at the top of the water tank, the PCM module geometry is adopted. |
| Kumar et al. [105] | Designed, developed, and evaluated a latent heat storage system to be used on-demand when warm water is needed, in which the thermal energy is collected by using a box-type solar collector. The system comprised of three finned heat exchangers and the PCM used paraffin wax with a melting point at 54 °C to store heat. | The results show that that storage unit in the heat storage system performed well in keeping the hot water within the desired temperature range. For the experiments, 15 L and 20 L of water were used. |
| Shukla [106] | Devised two solar water heaters in which the heat storage material is paraffin. One system had tank-type storage and the other system had incorporated storage type with a reflector. | These systems are capable of providing hot water during the day and night on a daily cycle basis. These two systems have an efficiency of 45% and 60%, respectively. |
| Hasan et al. [107,108,109] | Analyzed domestic water heater using fatty acids as PCM. | The study found that the best and most promising PCMs are myristic acid, palmitic acid, and stearic acid, all having melting temperatures between 50–70 °C, which are suitable for heating water. |
| Tiwari et al. [110] | Analyzed the effect of running water flow within a parallel plate on a solid-solid PCM interface to be used as a water heater. To reduce heat dissipated during nighttime, movable insulation is provided to the system. | They found that the hot water can be maintained at a high temperature all the time and increasing the melted region of PCM will reduce water temperature fluctuations. |
| Ling, Mo [111] | Studied the energy and thermal efficiency of the system, the energy consumption for room heating and the solar fraction. | The heating efficiency of the system would be 31.7% and the solar fraction would be 83.6% while the average temperature indoors was 14.9 °C and outdoors was −1.5 °C. |
| Boy et al. [38] | Suggested a salt hydrate PCM-based integrated collector storage system to provide instantaneous hot water. | Demonstrated that by incorporating an appropriate PCM the system’s efficiency could be raised considerably. However, the system is expected to have a high cost because the salt hydrate PCM is contained in a specially corrugated fin heat exchanger. |
| Tayeb [112] | Developed and investigated a system with Na2SO4.10H2O as PCM used for the domestic water heater. | The results are then used as a comparison with the simulation model, which provides the ideal inlet water flow rate required to keep the water temperature constant at the outlet flow. |
| Font et al. [113] | Researched a preliminary study using solid-solid PCM in designing domestic water heater device. Simulation with numerical values was utilized using a one-directional model and used to confirm the findings of the experiment. | The agreement of both simulation and experimental results reveals that this model can investigate heat transfer within PCMs and further optimize the water heater device design. |
| Bhargava [114] | Theoretically investigated a water heater using solar energy with PCM. | The results showed that when the thermal conductivity of solid-solid PCM is increased, outlet water temperature and the efficiency of the system during the evening hours will also increase. |
| Canbazoglu et al. [115] | Analyzed and compared conventional solar water heater with PCM-powered solar water heating. Polyethylene bottles were filled with approximately 180 kg of PCM and the bottles were left to set in the tank in three rows. | The temperature of the water is found to be constant at 46 °C throughout the night until morning, while the hot water does not change at all. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mofijur, M.; Mahlia, T.M.I.; Silitonga, A.S.; Ong, H.C.; Silakhori, M.; Hasan, M.H.; Putra, N.; Rahman, S.M.A. Phase Change Materials (PCM) for Solar Energy Usages and Storage: An Overview. Energies 2019, 12, 3167. https://doi.org/10.3390/en12163167
Mofijur M, Mahlia TMI, Silitonga AS, Ong HC, Silakhori M, Hasan MH, Putra N, Rahman SMA. Phase Change Materials (PCM) for Solar Energy Usages and Storage: An Overview. Energies. 2019; 12(16):3167. https://doi.org/10.3390/en12163167
Chicago/Turabian StyleMofijur, M., Teuku Meurah Indra Mahlia, Arridina Susan Silitonga, Hwai Chyuan Ong, Mahyar Silakhori, Muhammad Heikal Hasan, Nandy Putra, and S.M. Ashrafur Rahman. 2019. "Phase Change Materials (PCM) for Solar Energy Usages and Storage: An Overview" Energies 12, no. 16: 3167. https://doi.org/10.3390/en12163167
APA StyleMofijur, M., Mahlia, T. M. I., Silitonga, A. S., Ong, H. C., Silakhori, M., Hasan, M. H., Putra, N., & Rahman, S. M. A. (2019). Phase Change Materials (PCM) for Solar Energy Usages and Storage: An Overview. Energies, 12(16), 3167. https://doi.org/10.3390/en12163167











