Supercritical CO2 Exposure-Induced Surface Property, Pore Structure, and Adsorption Capacity Alterations in Various Rank Coals
Abstract
:1. Introduction
2. Materials and Methods
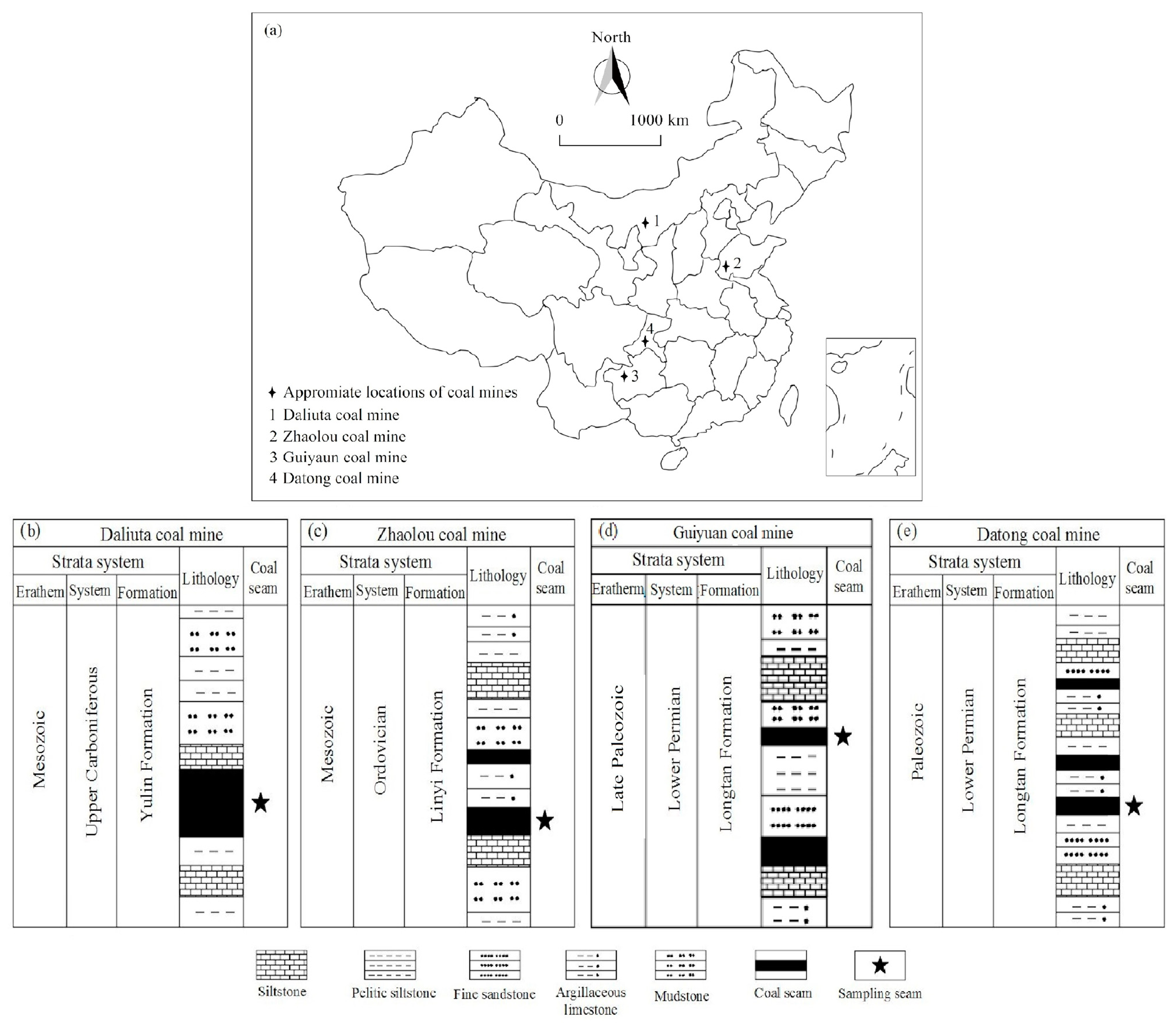
2.1. Sample Collection and Preparation
2.2. Exposure of Coal to ScCO2
2.3. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
2.4. Low-Pressure N2 and CO2 Isotherm Measurements
2.5. High-Pressure CO2 Isotherm Measurements
3. Results and Discussion
3.1. Effect of ScCO2 Exposure on Coal Surface Property
3.2. Effect of ScCO2 Exposure on Coal Pore Structure
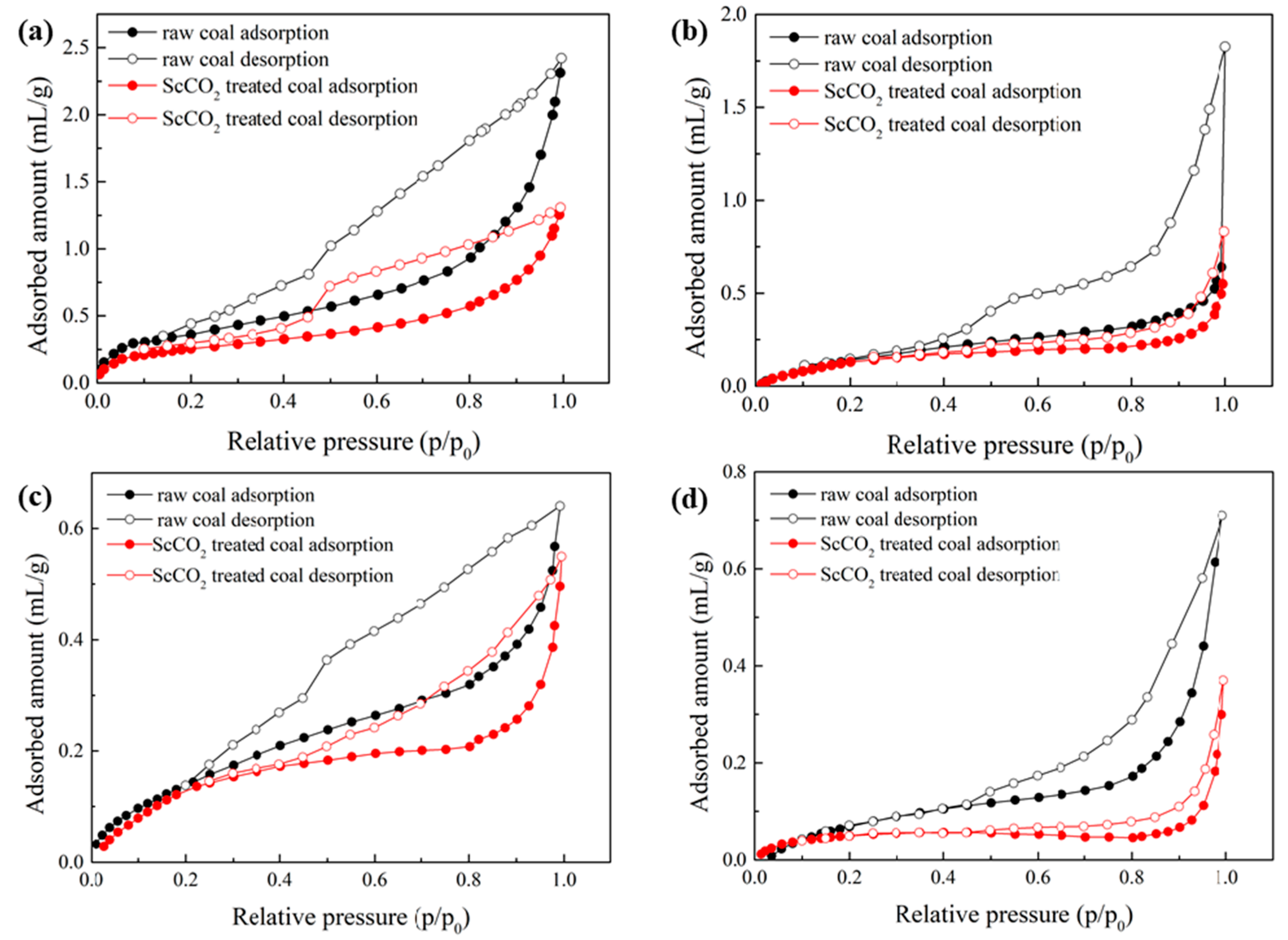
3.2.1. Low-Pressure N2 and CO2 Sorption Isotherms
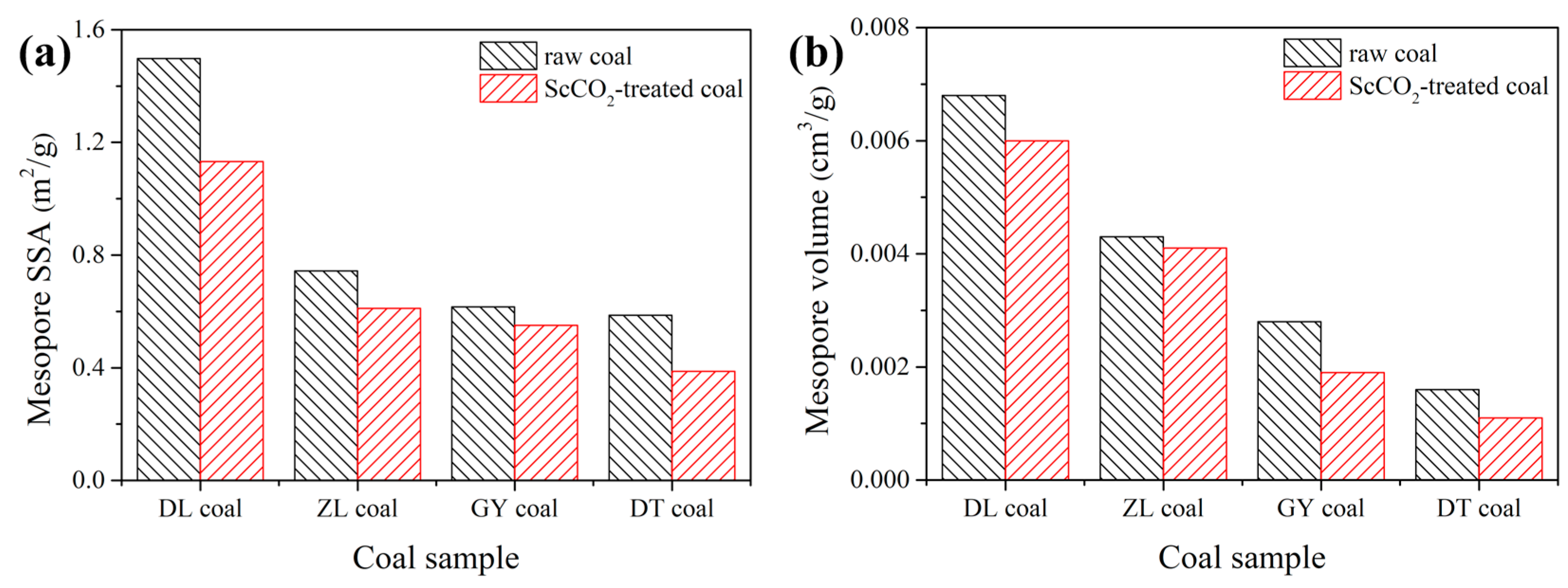
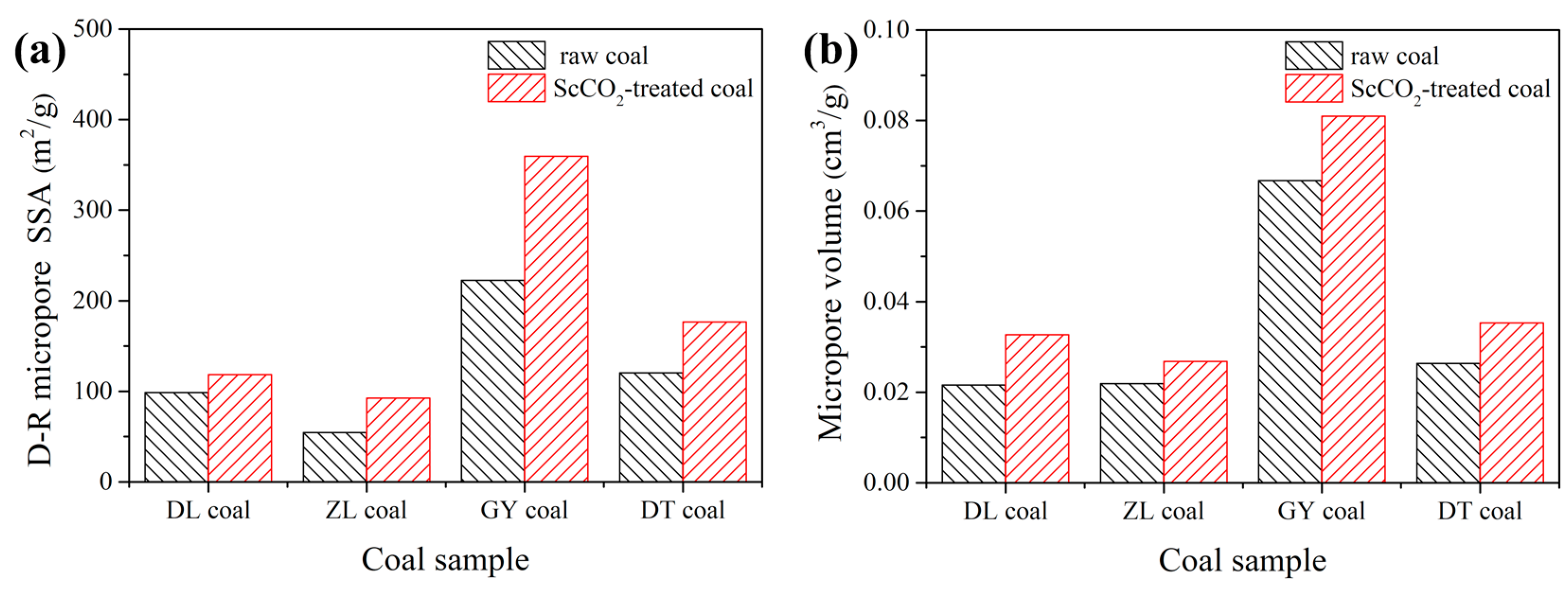
3.2.2. Pore Properties Determined by Low-Pressure N2 and CO2 Sorption
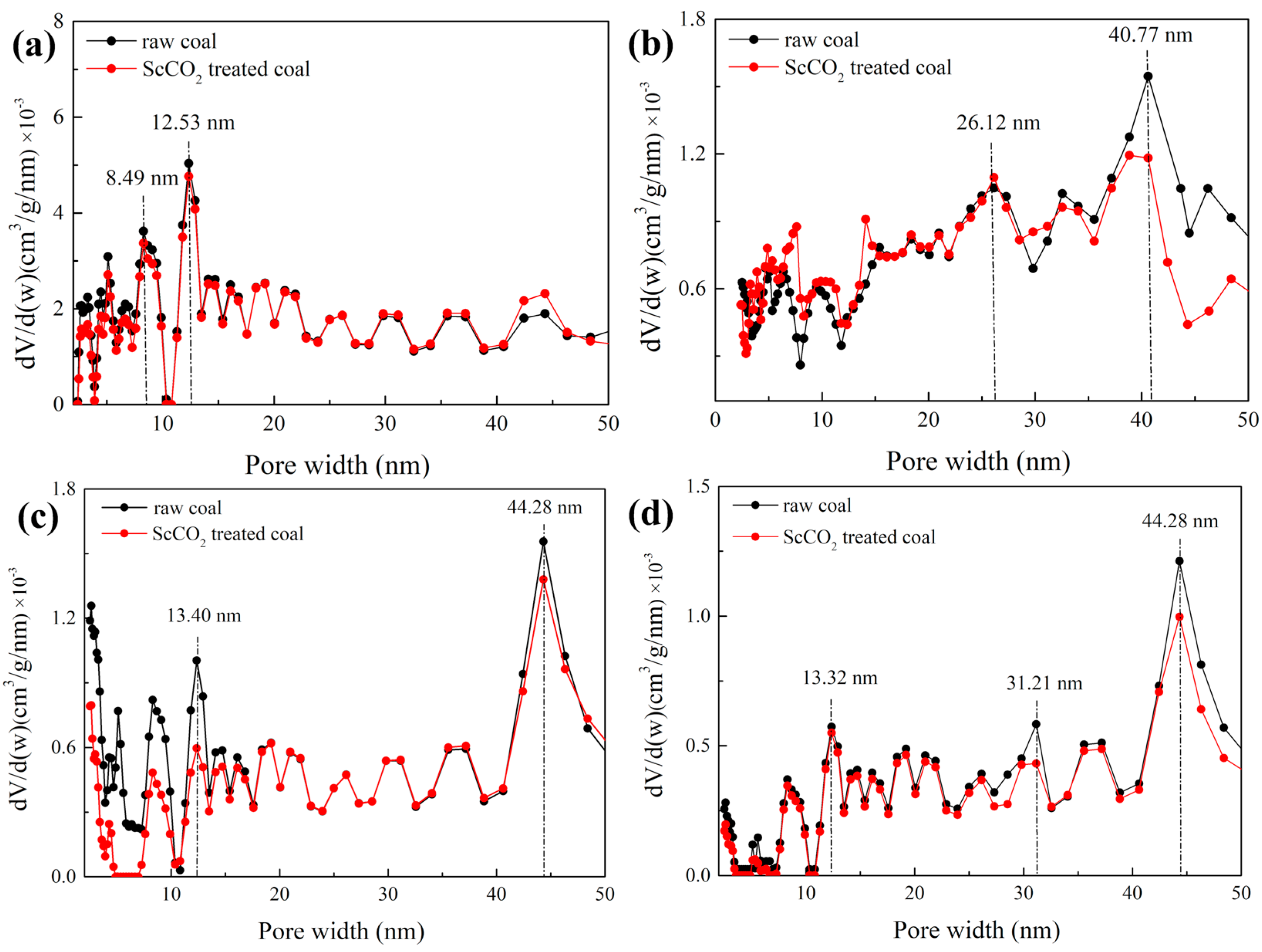
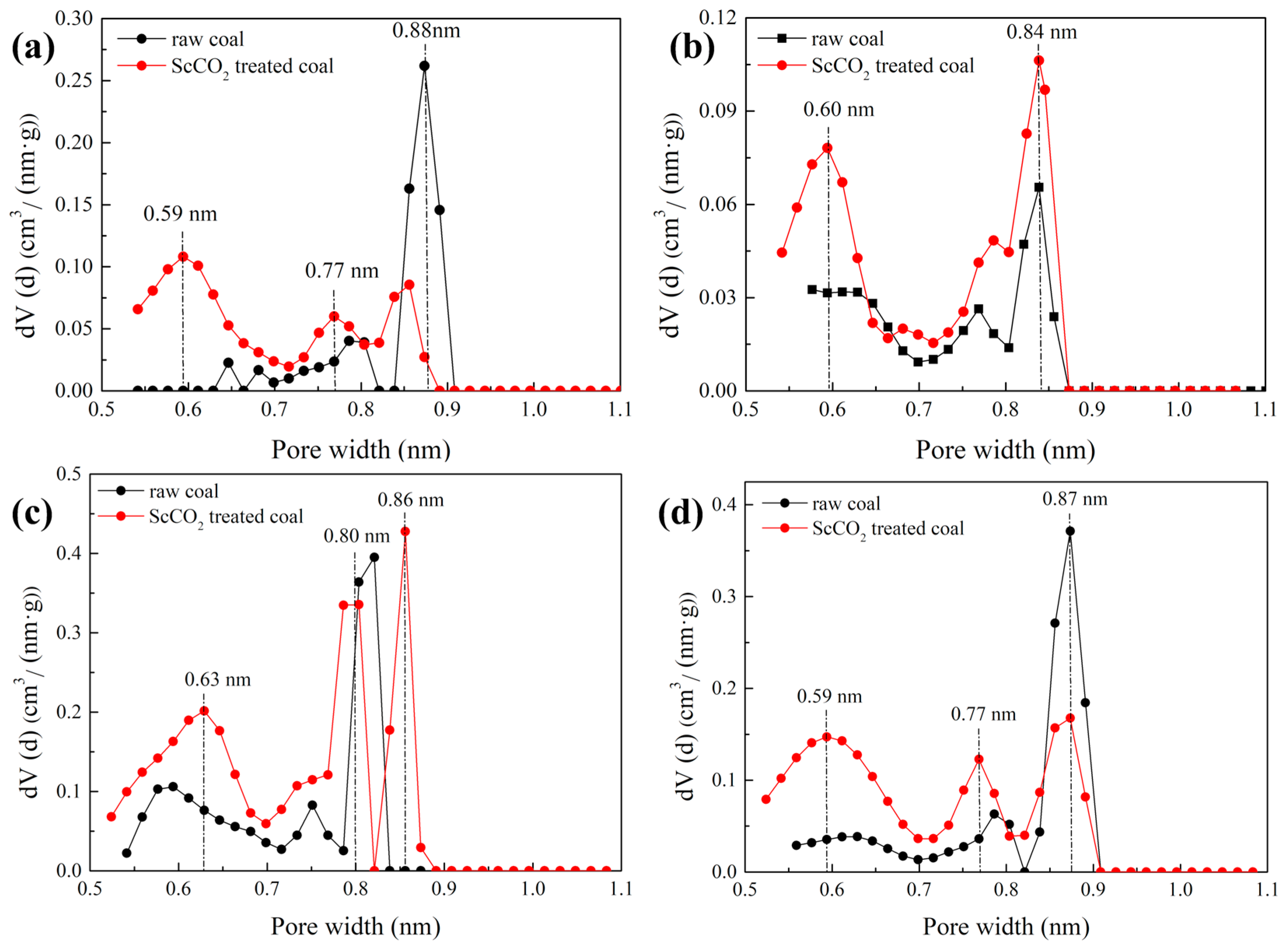
3.2.3. Pore Size Distribution Determined by Low-Pressure N2 and CO2 Sorption
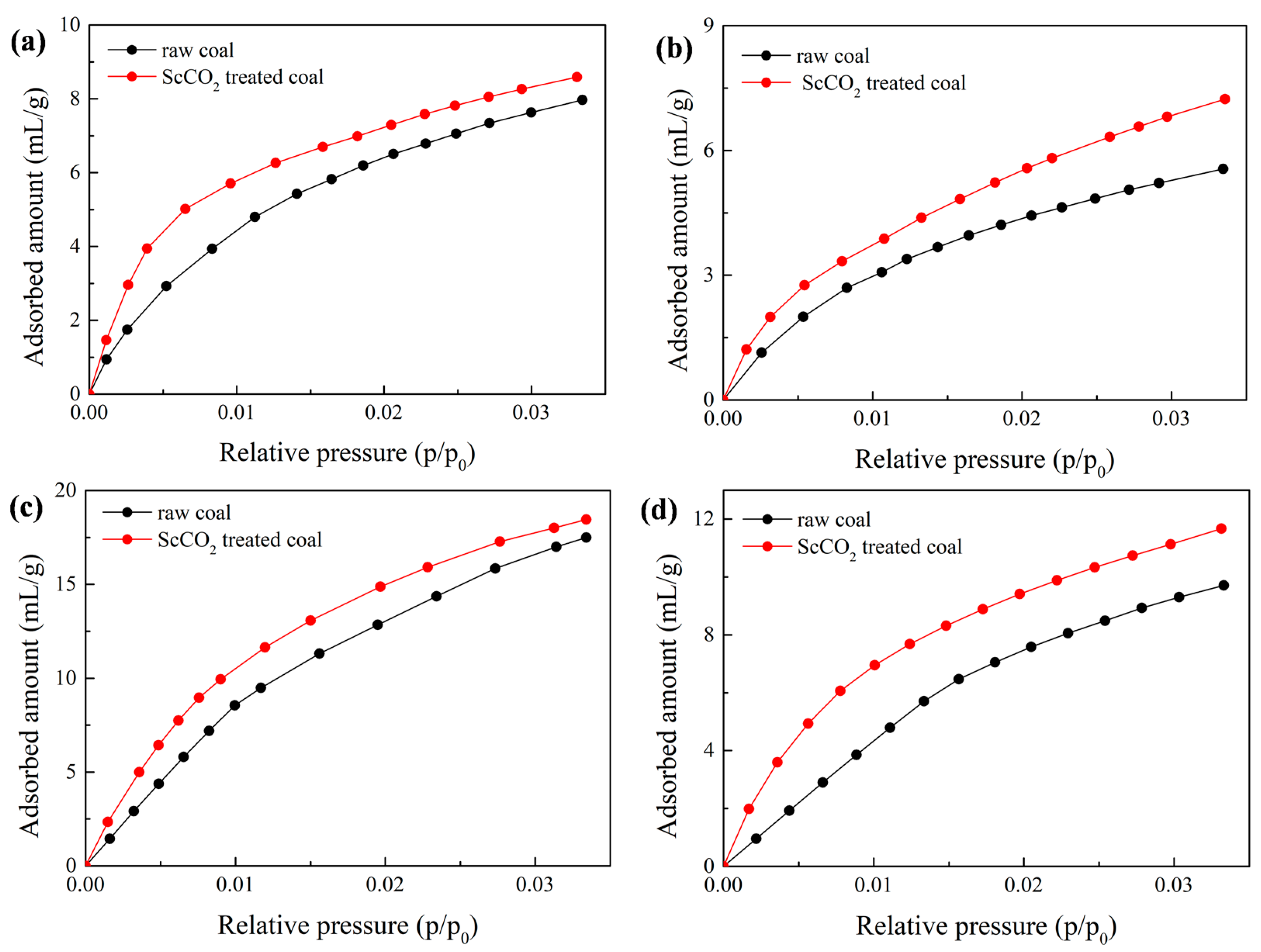
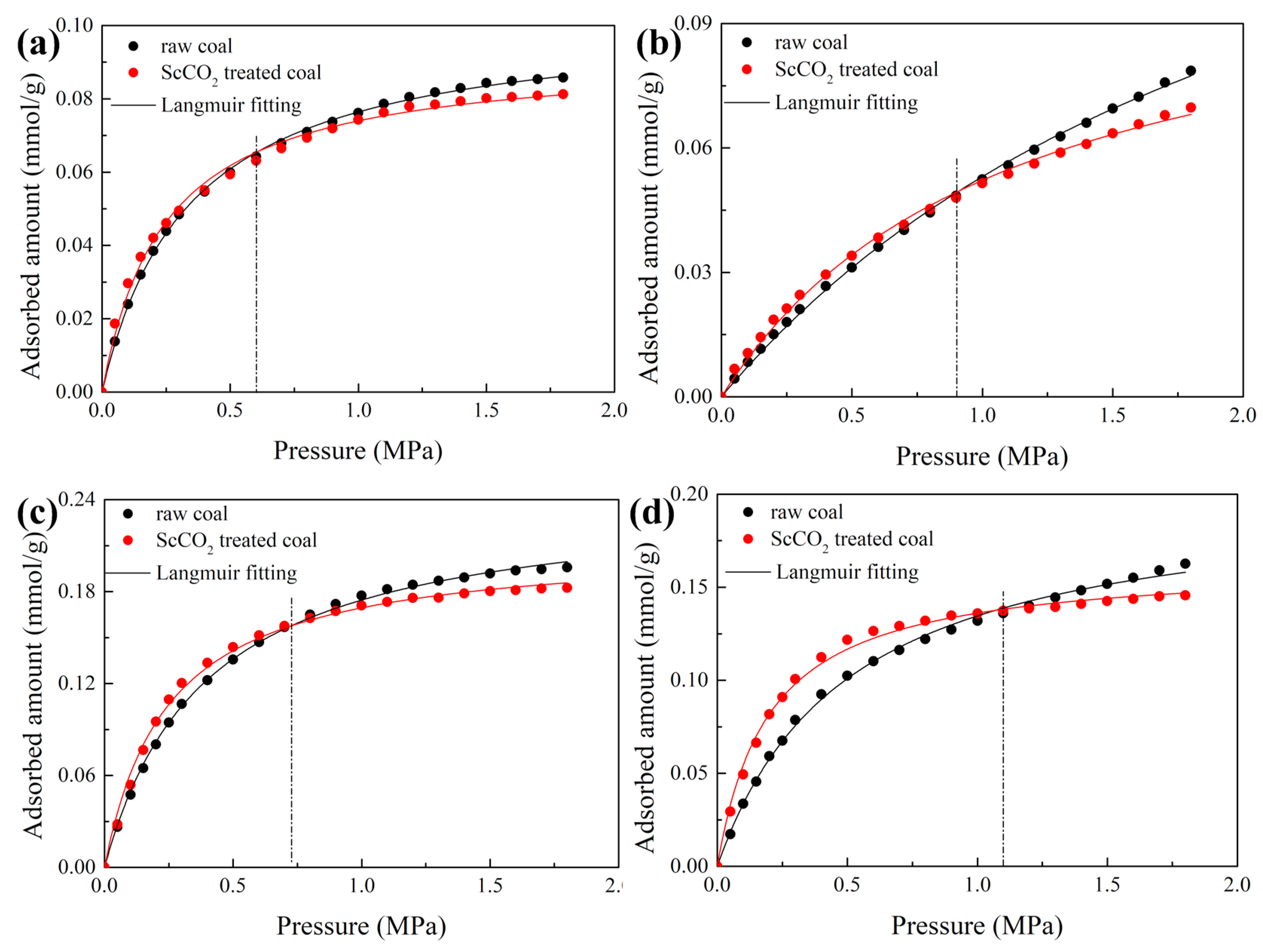
3.3. Effect of Sc-CO2 Exposure on High-Pressure CO2 Adsorption
3.4. Implications for CO2 Sequestration in Coal Seams for Coal Seam Gas (CSG) Enhancement
4. Conclusions
- (1)
- For various rank coals, the density of the surface functional groups and inorganic corresponds decreased after ScCO2 exposure due to the dissolution and extraction effect of ScCO2;
- (2)
- The interaction with ScCO2 has a minimal influence on pore shape of various rank coals. The micropore SSA and pore volume were increased while values for mesopore decreased;
- (3)
- The combined effects of surface property and pore structure alterations lead to a higher CO2 adsorption capacity at a low pressure but a lower CO2 adsorption capacity at a high pressure;
- (4)
- The adsorption isotherms of the raw and ScCO2-treated coals can be well described by Langmuir model. The monolayer capacity of the various rank coals decreased after exposure to ScCO2, indicating an elimination of the adsorption sites with ScCO2 exposure.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Leung, D.Y.C.; Caramanna, G.; Maroto-Valer, M.M. An overview of current status of carbon dioxide capture and storage technologies. Renew. Sustain. Energy Rev. 2014, 39, 426–443. [Google Scholar] [CrossRef] [Green Version]
- Peter, M.; Li, Z.; Maximilian, R.; Gkiokchan, M.; Yuan, W.; Christian, S.; Martin, R.; Detlef, S. Carbon capture for CO2 emission reduction in the cement industry in Germany. Energies 2019, 12, 2432. [Google Scholar]
- Du, X.; Gu, M.; Liu, Z.; Zhao, Y.; Sun, F.; Wu, T. Enhanced shale gas recovery by the injection of CO2, N2 and CO2/N2 mixture gases. Energy Fuels 2019, 33, 5091–5111. [Google Scholar] [CrossRef]
- White, C.M.; Smith, D.H.; Jones, K.L.; Goodman, A.L.; Jikich, S.A.; LaCount, R.B.; DuBose, S.B.; Ozdemir, E.; Morsi, B.I.; Schroeder, K.T. Sequestration of carbon dioxide in coal with enhanced coalbed methane recovery—A review. Energy Fuels 2005, 19, 659–724. [Google Scholar] [CrossRef]
- Busch, A.; Gensterblum, Y. CBM and CO2-ECBM related sorption processes in coal: A review. Int. J. Coal. Geol. 2011, 87, 49–71. [Google Scholar] [CrossRef]
- Perera, M.S.A. A comprehensive overview of CO2 flow behaviour in deep coal seams. Energies 2018, 11, 906. [Google Scholar] [CrossRef]
- Gan, H.; Nandi, S.P.; Walker, P.L., Jr. Nature of the porosity in American coals. Fuel 1972, 51, 272–277. [Google Scholar] [CrossRef]
- Li, Q.; Lin, B.; Wang, K.; Zhao, M.; Ruan, M. Surface properties of pulverized coal and its effects on coal mine methane adsorption behaviors under ambient conditions. Powder Technol. 2015, 270, 278–286. [Google Scholar] [CrossRef]
- Wang, F.; Cheng, Y.; Lu, S.; Jin, K.; Zhao, W. Influence of coalification on the pore characteristics of middle-high rank coal. Energy Fuels 2014, 28, 5729–5736. [Google Scholar] [CrossRef]
- Jian, K.; Fu, X.; Ding, Y.; Wang, H.; Li, T. Characteristics of pores and methane adsorption of low-rank coal in China. J. Nat. Gas Sci. Eng. 2015, 27, 207–218. [Google Scholar] [CrossRef]
- Okolo, G.N.; Everson, R.C.; Neomagus, H.W.J.P.; Roberts, M.J.; Sakurovs, R. Comparing the porosity and surface areas of coal as measured by gas adsorption, mercury intrusion and SAXS techniques. Fuel 2015, 141, 293–304. [Google Scholar] [CrossRef]
- Pini, R.; Ottiger, S.; Storti, G.; Mazzotti, M. Pure and competitive adsorption of CO2, CH4 and N2 on coal for ECBM. Energy Procedia 2009, 1, 1705–1710. [Google Scholar] [CrossRef]
- Jasinge, D.; Ranjith, P.G.; Choi, X.; Fernando, J. Investigation of the influence of coal swelling on permeability characteristics using natural brown coal and reconstituted brown coal specimens. Energy 2012, 39, 303–309. [Google Scholar] [CrossRef]
- Day, S.; Fry, R.; Sakurovs, R. Swelling of Australian coals in supercritical CO2. Int. J. Coal Geol. 2008, 74, 41–52. [Google Scholar] [CrossRef]
- Vandamme, M.; Brochard, L.; Lecampion, B.; Coussy, O. Adsorption and strain: The CO2-induced swelling of coal. J. Mech. Phys. Solids 2010, 58, 1489–1505. [Google Scholar] [CrossRef]
- Pluymakers, A.; Liu, J.; Kohler, F.; Renard, F.; Dysthe, D. A high resolution interferometric method to measure local swelling due to CO2 exposure in coal and shale. Int. J. Coal Geol. 2018, 187, 131–142. [Google Scholar] [CrossRef]
- Perera, M.S.A.; Ranjith, P.G.; Choi, S.K.; Bouazza, A.; Kodikara, J.; Airey, D. A review of coal properties pertinent to carbon dioxide sequestration in coal seams: With special reference to Victorian Brown coals. Environ. Earth Sci. 2011, 64, 223–235. [Google Scholar] [CrossRef]
- Ranathunga, A.S.; Perera, M.S.A.; Ranjith, P.G.; Rathnaweera, T.D.; Zhang, X.G. Effect of coal rank on CO2 adsorption induced coal matrix swelling with different CO2 properties and reservoir depths. Energy Fuels 2017, 31, 5297–5305. [Google Scholar] [CrossRef]
- Vishal, V.; Singh, T.N. A laboratory investigation of permeability of coal to supercritical CO2. Geotech. Geol. Eng. 2015, 33, 1009–1016. [Google Scholar] [CrossRef]
- Perera, M.S.A.; Ranjith, P.G.; Choi, S.K.; Airey, D. The effects of sub-critical and super-critical carbon dioxide adsorption-induced coal matrix swelling on the permeability of naturally fractured black coal. Energy 2011, 36, 6442–6450. [Google Scholar] [CrossRef]
- Hol, S.; Spiers, C.J.; Peach, C.J. Microfracturing of coal due to interaction with CO2 under unconfined conditions. Fuel 2012, 97, 569–584. [Google Scholar] [CrossRef]
- Clarksona, C.R.; Bustin, R.M. The Effect of pore structure and gas pressure upon the transport properties of coal: A laboratory and modeling study.1: Isotherms and pore volume distributions. Fuel 1999, 78, 1333–1344. [Google Scholar] [CrossRef]
- Larsen, J.W. The effects of dissolved CO2 on coal structure and properties. Int. J. Coal Geol. 2004, 57, 63–70. [Google Scholar] [CrossRef]
- Goodman, A.L.; Favors, R.N.; Larsen, J.W. Argonne coal structure rearrangement caused by sorption of CO2. Energy Fuel 2006, 20, 2537–2543. [Google Scholar] [CrossRef]
- Kolak, J.J.; Burruss, R.C. Geochemical investigation of the potential for mobilizing non-methane hydrocarbons during carbon dioxide storage in deep coal beds. Energy Fuels 2006, 20, 566–574. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, Z.; Lu, Y.; Choi, S.K. Surface properties and pore structure of anthracite, bituminous coal and lignite. Energies 2018, 11, 1502. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, D.; Wang, H.; Jiang, W.; Wu, X.; Yang, J.; Huo, P. Influence of CO2 exposure on high-pressure methane and CO2 adsorption on various rank coals: Implications for CO2 sequestration in coal seams. Energy Fuels 2015, 29, 3785–3795. [Google Scholar] [CrossRef]
- Li, H.; Chang, Q.; Dai, Z.; Chen, X.; Wang, F. Upgrading effects of supercritical carbon dioxide extraction on physicochemical characteristics of Chinese low-rank coals. Energy Fuels 2017, 31, 13305–13316. [Google Scholar] [CrossRef]
- Emmett, P.H.; Dewitt, T.W. The low temperature adsorption of Nitrogen, Oxygen, Argon, Hydrogen, N–Butane and carbon dioxide on porous glass and on Partially Dehydrated Chabazite. J. Am. Chem. Soc. 1943, 65, 617–621. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Mouscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Mastalerz, M.; Drobniak, A.; Rupp, J. Meso- and micropore characteristics of coal lithotypes: Implications for CO2 adsorption. Energy Fuels 2008, 22, 4049–4061. [Google Scholar] [CrossRef]
- Boer, J.H.D.; Lippens, B.C. Studies on pore systems in catalysts II. The shapes of pores in aluminum oxide systems. J. Catal. 1964, 3, 38–43. [Google Scholar]
- Zhang, D.; Gu, L.; Li, S.; Lian, P.; Tao, J. Interactions of supercritical CO2 with coal. Energy Fuels 2013, 27, 387–393. [Google Scholar] [CrossRef]
- Kutchko, B.G.; Goodman, A.L.; Rosenbaum, E.; Natesakhawat, S.; Wagner, K. Characterization of coal before and after supercritical CO2 exposure via feature relocation using field-emission scanning electron microscopy. Fuel 2013, 107, 777–786. [Google Scholar] [CrossRef]
- Zhao, J.; Xu, H.; Tang, D.; Mathews, J.; Li, S.; Tao, S. A comparative evaluation of coal specific surface area by CO2 and N2 adsorption and its influence on CH4 adsorption capacity at different pore sizes. Fuel 2016, 183, 420–431. [Google Scholar] [CrossRef]
- Do, D.D.; Do, H.D. Appropriate volumes for adsorption isotherm studies: The absolute void volume, accessible pore volume and enclosing particle volume. J. Colloid Interface Sci. 2007, 316, 317–330. [Google Scholar] [CrossRef]
- Gathitu, B.B.; Chen, W.; Mcclure, M. Effects of coal interaction with supercritical CO2: Physical structure. Ind. Eng. Chem. Res. 2009, 48, 5024–5034. [Google Scholar] [CrossRef]
- Pan, Z.; Niu, Q.; Wang, K.; Shi, X.; Li, M. The closed pores of tectonically deformed coal studied by small-angle X-ray scattering and liquid nitrogen adsorption. Micropor. Mesopor. Mater. 2016, 224, 245–252. [Google Scholar] [CrossRef]
- Chen, Y.; Mastalerz, M.; Schimmelmann, A. Characterization of chemical functional groups in macerals across different coal ranks via micro-FTIR spectroscopy. Int. J. Coal Geol. 2012, 104, 22–33. [Google Scholar] [CrossRef]
- Huang, X.; Chu, W.; Sun, W.; Jiang, C.; Feng, Y.; Xue, Y. Investigation of oxygen-containing group promotion effect on CO2–coal interaction by density functional theory. Appl. Surf. Sci. 2014, 299, 162–169. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Zhang, K.; Cheng, Y.; Jin, K.; Guo, H.; Li, W. Effects of supercritical CO2 fluids on pore morphology of coal: Implications for CO2 geological sequestration. Energy Fuels 2017, 31, 4731–4741. [Google Scholar] [CrossRef]



| Sample | Ro max (%) | Proximate Analysis (wt %) | Ultimate Analysis (wt % daf) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Cfix | Vdaf | Aad | M | C | H | N | O | ||
| DL coal | 0.42 | 53.55 | 36.96 | 19.42 | 2.46 | 72.71 | 4.95 | 1.19 | 20.50 |
| ZL coal | 0.81 | 65.10 | 28.71 | 16.24 | 1.50 | 82.54 | 4.57 | 1.08 | 11.03 |
| GY coal | 1.14 | 78.34 | 6.14 | 10.52 | 2.08 | 86.92 | 4.01 | 1.20 | 6.03 |
| DT coal | 1.86 | 73.85 | 12.81 | 13.34 | 1.96 | 90.11 | 3.79 | 0.96 | 2.05 |
| Sample | State | N2 Adsorption | CO2 Adsorption | ||||
|---|---|---|---|---|---|---|---|
| SBET/m2·g−1 | Vt/cm3·g−1 | D/nm | SD-R/m2·g−1 | SD-A/m2·g−1 | Vmic/cm3·g−1 | ||
| DL coal | raw coal | 1.498 | 0.0068 | 18.16 | 98.71 | 61.05 | 0.0216 |
| ScCO2-treated | 1.132 | 0.0060 | 21.20 | 118.5 | 84.18 | 0.0327 | |
| ZL coal | raw coal | 0.744 | 0.0043 | 23.12 | 54.54 | 45.77 | 0.0219 |
| ScCO2-treated | 0.611 | 0.0041 | 26.84 | 92.65 | 65.10 | 0.0268 | |
| GY coal | raw coal | 0.616 | 0.0028 | 18.19 | 222.4 | 160.9 | 0.0667 |
| ScCO2-treated | 0.551 | 0.0019 | 13.79 | 359.4 | 205.1 | 0.0810 | |
| DT coal | raw coal | 0.586 | 0.0016 | 10.92 | 120.4 | 74.34 | 0.0264 |
| ScCO2-treated | 0.387 | 0.0011 | 11.37 | 176.6 | 100.6 | 0.0353 | |
| Sample | State | nm/mmol·g−1 | B | R2 |
|---|---|---|---|---|
| DL coal | raw coal | 0.102 | 2.923 | 0.9993 |
| ScCO2-treated | 0.092 | 4.056 | 0.9954 | |
| ZL coal | raw coal | 0.181 | 0.416 | 0.9991 |
| ScCO2-treated | 0.110 | 0.906 | 0.9975 | |
| GY coal | raw coal | 0.243 | 2.538 | 0.9990 |
| ScCO2-treated | 0.211 | 4.050 | 0.9964 | |
| DT coal | raw coal | 0.202 | 1.994 | 0.9981 |
| ScCO2-treated | 0.163 | 5.029 | 0.9960 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Zhang, Z.; Liu, X.; Wu, T.; Du, X. Supercritical CO2 Exposure-Induced Surface Property, Pore Structure, and Adsorption Capacity Alterations in Various Rank Coals. Energies 2019, 12, 3294. https://doi.org/10.3390/en12173294
Liu Z, Zhang Z, Liu X, Wu T, Du X. Supercritical CO2 Exposure-Induced Surface Property, Pore Structure, and Adsorption Capacity Alterations in Various Rank Coals. Energies. 2019; 12(17):3294. https://doi.org/10.3390/en12173294
Chicago/Turabian StyleLiu, Zhenjian, Zhenyu Zhang, Xiaoqian Liu, Tengfei Wu, and Xidong Du. 2019. "Supercritical CO2 Exposure-Induced Surface Property, Pore Structure, and Adsorption Capacity Alterations in Various Rank Coals" Energies 12, no. 17: 3294. https://doi.org/10.3390/en12173294





