3.1. Efficiency and Emissions
In order to achieve the constant BMEP in different mixtures, the throttle was opened more than at the stoichiometric condition and the amount of fuel was slightly reduced. As the volumetric efficiency increases, the efficiency (effective) improves. In addition to the increase in volumetric efficiency, pumping losses decreased in this process. When λ = 1.7 was selected instead of the stoichiometric mixture at BMEP of 3 bar, pumping losses were reduced by approximately 30–40%. In partial loads, fuels like CNG, which need more air in stoichiometric combustion and can operate with ultra-lean mixtures, have higher efficiency due to improvements in volumetric efficiency and pumping losses. This effect leads to a reduction in CO2 emissions, as well as a reduction in fuel consumption. According to the results obtained for both fuels, CNG has the same efficiency or is more efficient (4–6%) than gasoline at all operating points. In constant BMEP, the net indicated efficiency increased by 15% by using the ultra-lean mixture instead of the stoichiometric mixture. With the CNG, the engine can operate with a full open throttle in BMEP > 4.5 bar. This means that only the amount of fuel, such as in diesel engines, must be changed to achieve higher loads. Also, CO2 emissions decreased by approximately 12% compared to gasoline. Decrease of the CO2 was due to the increase in efficiency and the higher hydrogen/carbon (H/C) ratio of CNG. Theoretically, even if both fuels have the same efficiency in the stoichiometric mixture, when 1 mole of gasoline or CH4 is burned, CH4 produces about 10% less CO2.
Therefore, CO2 emission, which causes greenhouse gas effects, can be reduced by the usage of CNG. In addition, the Mexa 7500 device, which is used for emission measurement, can separately measure the CH4 concentration in THC. Approximately 90% of THC consists of CH4 at all operating points.
The main reason for the desire to operate with the lean mixture is to reduce NO emissions, even if the efficiency remains constant. In the experiment, only NO gas was measured. It was intended to keep the NO level below 300 ppm or 3 g/kWh in the ultra-lean mixture. As it is known, in-cylinder mean temperature decreases in lean mixtures. For this reason, the amount of NO, which exponentially depends on temperature, decreases even though there is excess oxygen in the mixture. Because of the difference in volumetric efficiency, it is more appropriate to use g/kWh instead of the ppm unit in order to compare the two fuels’ emissions. This is also necessary to compare with exhaust emission limits. Conversion of exhaust gases was applied based on the European Commission regulation [
22]. In addition, the NO correction coefficient, which is specified in the regulation and depends on the relative humidity, was taken into account according to the relevant directives. NO results at 1500 rpm are given in
Figure 2. NO was reduced to the desired values for BMEP of 3–5 bar and
λ ≥ 1.6 operating conditions.
NO values at 2000 rpm are shown in
Figure 3. In order to keep the same NO values at 1500 rpm, the relative air/fuel ratio of the lean mixture should be increased to 1.7. This condition shows up especially at a BMEP of 5 bar. In stoichiometric mixture, the spark advance is almost the same for both fuels such as 14° CA btdc (before top dead center) for gasoline and 15 btdc for CNG in this condition. Therefore, it is expected to have the same NO values. However, the CNG flame proceeds faster than gasoline during early flame development and first burning locations have higher temperatures [
23]. Due to this, despite almost the same lower heating value of mixture for both fuels, the CNG produces higher NO emission at about stoichiometric condition (
Figure 3). In addition, an increase of the burn duration in CNG also rose the NO formation at stoichiometric and some lean conditions. But, by increasing the relative air/fuel ratio to ultra-lean mixture, the spark advance was higher for gasoline. So, the gasoline engine produced more NO in (
λ = 1.3–1.6). This relation shows similar tendencies in both speeds. NO limit values for both fuels were provided under
λ ≥ 1.6 conditions. In order to achieve the NO limits, the engine was operated like that in leaner mixtures as the engine load (BMEP) increased. In the following sections, it was investigated that the cyclic variations are acceptable in the lean mixture where NO values were too low for both fuels.
3.3. Combustion Analysis
In the study, the spark advance was selected based on maximum engine torque. Except for stoichiometric mixture, the spark advance in the gasoline engine was higher than CNG. As could be seen from
Figure 5, differences between the spark advances increase by moving to the ultra-lean region. This difference is related to ignition limits and laminar flame speeds of fuels. In the experiments, the volumetric efficiency could be different for two fuels due to constant engine speed and BMEP. For this reason, the amount of residual gas, turbulence intensity, and temperature of the mixture at the moment of the ignition time have a direct effect on spark advance. By increasing the engine load, the difference between the spark advance for both fuels decreases. In the case of the stoichiometric mixture, the engine with both fuels was operated at the similar spark advance (14°–15° CA btdc). However, at a BMEP of 3 bar, spark advance in lean mixture increases rapidly for gasoline (
Figure 5). Ignition delay was also longer for gasoline due to igniting in colder conditions than CNG.
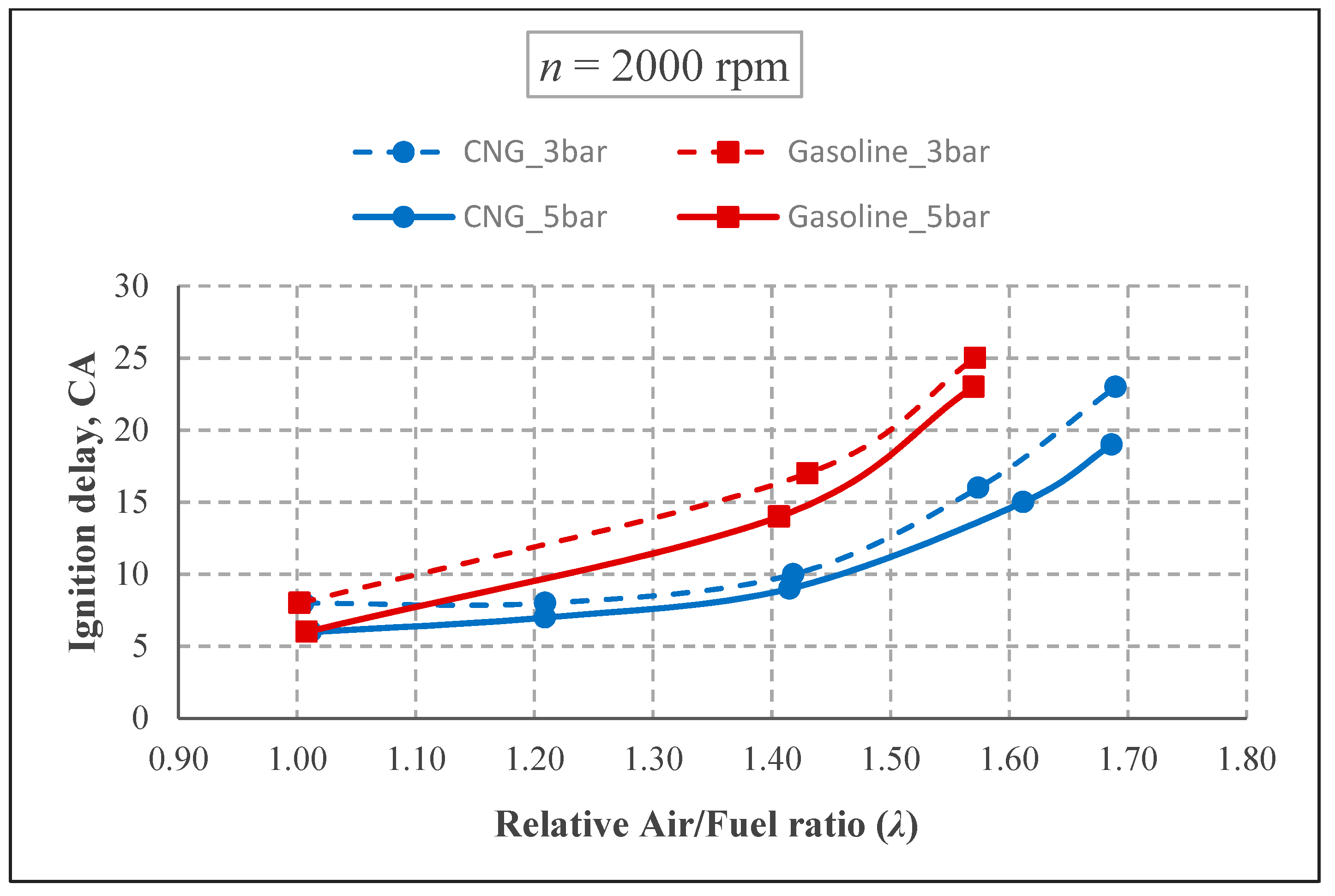
The spark advances for 2000 rpm condition are given in
Figure 6. As the speed rose, all the spark advances increased. The changes of spark advance were similar for both engine speeds. At a BMEP of 3 bar and 2000 rpm, the ignition advance for both fuels were 20° CA in the stoichiometric mixture, whereas in the lean mixture (
λ = 1.6) it was 34° and 42° CA for CNG and gasoline, respectively. It is declared in the literature that the gasoline needs a shorter spark advance than CNG in the same operating condition [
12,
14]. However, the spark advance values obtained in this study do not support these results. The main reasons for this difference arise from the different structure of experimental engines. The research engine used in this study was converted from a diesel engine to spark ignition. Therefore, the intake port geometry had been designed to create swirl air motion. In the classic gasoline engine, this design is different and more simple. In addition, the compression ratio selected was 12. Due to the piston design of the research engine, it allows the creation of different geometries on piston. These results were investigated in detail in the “ignition delay” part. In addition, the spark advance was checked on the data acquisition system by two different methods, one of them being ECU signal (5 Volt), and the other from a spark current adapter that mounted to a spark plug cable. There is a negligible delay between the values measured by these two methods (<0.3° CA).
The effect of different spark advance (SA) on the ignition delay and heat release was investigated for both fuels. It is necessary to determine the start of combustion to calculate the ignition delay time. In some of the studies, the ignition delay is assumed to be the time between the spark advance and the 5% MFB (mass fraction burned) location. In this study, the ignition delay is defined as the time between the spark advance and the start of sensible positive heat release. To achieve a positive heat release location, firstly, the heat release rate was calculated from in-cylinder pressure and volume at each crank angle step. Then, the integrated (cumulative) heat release was obtained from heat release rate data. As an overall approach, the heat release calculation starts a little before the spark advance in the case of using a pressure acquisition system, because these systems generally do not determine the start of combustion location. This start point (positive heat release location) for integrated heat release calculation is entered in the system by the user and it has to update for every change in the spark advance. It is difficult to estimate the correct start location of combustion due to ignition delay during the measurement.
In brief, to calculate the integrated heat release, the start point should be set at the moment that the heat transfer from the cylinder to the surroundings is equal or less than the heat release rate that was calculated from the pressure and volume information in the cylinder. In this study, to determine the combustion start point, the integrated heat release was calculated starting from any point during the compression stroke before spark advance. Then, the derivation of this curve was calculated. Finally, the start of combustion was determined as where the sign of derivative integrate heat release changes permanently before Top Dead Center (TDC). To do this recalculation method, a code was written to calculate the heat release rate by using an average p-V data obtained from 200 cycles by using the Rassweiler–Withrow method (Equation (1)).
In
Figure 7, the heat release rate and two different integrated heat releases were shown together. The blue line is related to the constant starting point and the orange shows the heat release related to the starting point obtained from the recalculation method.
Figure 7 shows the location of positive heat and integrated heat release in magnified view. Also, the offset position was demonstrated by an arrow. In calculation with a constant start point (blue line), even if the combustion starts in real time, it will be considered that the ignition has not started yet due to negative integrated heat release.
This problem has been removed by using a recalculation period, as described above. A heat transfer model such as Woschni can be applied on the heat release rate data to obtain integrated heat release directly. However, some assumptions are required to use a heat transfer model. Therefore, the recalculation method is more simple than a heat transfer model.
Ignition delay has been calculated after determination of the start of combustion. The time between the spark advance and start of combustion was defined as ignition delay (DI-SA). As shown in
Figure 8, ignition delay had the same tendency, similar to spark advance. The ignition delay time for gasoline in ultra-lean mixture is quiet longer due to approaching the ignition limits and laminar flame speed. This effect was seen in cycle to cycle variation as well.
By increasing the speed, the ignition delay has increased as expected, but the general tendency is similar to spark advance (
Figure 6 and
Figure 9). The ignition delay is similar for both fuels in the case of stoichiometric mixture with the same spark advance. In addition, this period for CNG is shorter than gasoline in lean mixture. For example, at a BMEP of 5 bar and 1500 rpm, the ignition delay duration was 5° CA for both fuels in the stoichiometric condition, whereas it was 11° CA and 16° CA for CNG and gasoline in lean mixture, respectively. However, this period is defined as longer for CNG in the stoichiometric conditions by some researchers [
6,
7,
12]. The main reason for this contrast is structural differences between the experimental engines. Pan et al. [
14] have used an engine with variable compression ratio and the results show that the ignition delay decreases as compression ratio increases. But, despite the increase of compression ratio, the ignition delay for CNG was longer than gasoline. The compression ratio is set on 12 for an Antor 3LD 450 research engine. This is a high value compared to other studies. Therefore, a decrease in ignition delay is not an unexpected situation. This issue is handled in burn duration part. The ignition delay period was defined as the time between the start of combustion location (sometimes SA) and 5% or 10% MBF and the start of combustion by some researchers [
13,
14]. The ignition delay time based on this definition is given in
Figure 10. This figure shows that the ignition delay time is less for CNG. So, both of the two definitions for ignition delay time have a negligible effect and do not change the general result in this study. The small difference between results obtained from two definitions is due to the different laminar flame speed of fuels. The combustion has started before while using 5% or 10% MBF definition. So, the ignition delay time of CNG is less than gasoline in the stoichiometric mixture due to higher laminar flame speed at low temperature and pressure conditions [
5,
6].
Heat release values were compared to gain an understanding about the combustion period of the fuels. Selected spark advance (SA) was the same for both fuels in the stoichiometric mixture condition. The heat release rates for both fuels with the same spark advance are shown in
Figure 11. It can be seen that the gasoline burned faster than CNG. However, the heat release rates are very similar for these fuels up to the top dead center. Actually, the laminar burning speeds of both fuels in the atmospheric condition are almost the same. After TDC, the gasoline heat release rate raised more rapidly. In this operating condition, the ignition delays of both fuels are again the same (
Figure 8). However, the laminar flame speeds of both fuels show different behavior under high pressure and temperature [
9,
24]. Consequently, during the combustion process, both fuels could be faster or slower than each other. Here, CNG has higher volumetric efficiency than gasoline due to the experimental conditions. Thus, the parameters like temperature and turbulence intensity have a positive effect on the heat release rate of CNG.
The ignition delay and burn duration depend on the heat release rate for both fuels. The turbulence has a major effect on the heat release rate. The test engine, which is used in some studies, is converted from gasoline base engine. Therefore, it is not designed to create high turbulence levels. The engine that was used in this study is converted from a diesel engine. The intake manifold geometry of this engine creates extra swirl motion. Also, in contrast to some other studies where the flat type piston has been used, there is a bowl-shaped combustion chamber on top of the piston in this study. Some researchers investigated the effect of combustion chamber geometries on burn parameters [
17]. Spark advance and ignition delay decreases as the reentrant level (piston diameter/bowl diameter) increases [
16]. Moreover, the reduction in bowl diameter increases the turbulence at the edge of the bowl and decreases the air motion around the spark plug. This is a positive condition for flame kernel formation process. Combustion takes place in two different stages due to the bowl. The combustion proceeds quickly in the bowl due to the high turbulence level. This is a decisive factor in early flame development period and burn duration [
25,
26].
As mentioned above, the combustion chamber shapes on the piston are effective on spark advance, ignition delay, and flame development period. However, the effect of the increase in turbulence intensity on CNG and gasoline was different in this study, because the spark advance and ignition delay time of CNG are higher in the experiments which used gasoline-based engines in the literature. In contrast, different results were obtained in the experiments which used diesel-based engines in this study. Brequigny et al. [
27] investigated the effects of Lewis number and Markstein length (
Lb) values on the laminar and turbulent flame velocities. Gasoline is more resistant to flame wrinkles due to its higher
Le and
Lb. Therefore, the increase in turbulence intensity is more effective on CNG, while it has a slower effect on gasoline. The high pressure and temperature in real engine operating conditions help to reduce this resistance for gasoline [
27]. In contrast to gasoline, the laminar flame speed of CNG decreases excessively with increasing temperature and pressure [
5]. As a result, increased turbulence intensity leads to rapid progression of CNG during ignition delay and early flame development. However, the burn speed of the CNG decreases due to the effect of high pressure on the laminar flame speed at the end of the combustion. This was seen more clearly in the stoichiometric mixture operating condition.
As previously mentioned, the heat release rate of gasoline is higher than CNG in stoichiometric mixture condition. But, in lean mixture, the relation between combustion duration slightly changes. In
Figure 12, the heat release rate of both fuels is given in ultra-lean mixture conditions. In this state, the spark advance must be different for fuels to have a constant break mean effective pressure. To have maximum break torque, the spark advance for CNG and gasoline is set to 27° CA and 37° CA btdc, respectively. Although gasoline was ignited 10 CA earlier than CNG, the heat release rate was not rapid as stoichiometric mixture. Furthermore, the maximum value of the integrated heat released was the same for CNG and gasoline. However, the integrated heat release curve followed a different path from stoichiometric conditions. Especially the rapid combustion of natural gas towards the end of combustion was the main difference between stoichiometric and ultra-lean conditions.
In the literature, the period between 5% and 90% MBF was used to compare the burn duration.
Figure 13 shows burn durations for CNG and gasoline at 1500 rpm. Evaluations about the combustion propagation period based on the heat release rate on the previous section were validated by the duration of combustion. In the region of
λ = 1 − 1.2, the burn duration of the gasoline was less than CNG. At a BMEP of 5 bar and 1500 rpm, burn duration in the stoichiometric mixture was approximately 24° CA and 27° CA for gasoline and CNG, respectively, whereas it was approximately 34° CA and 30° CA in the lean condition (
λ = 1.6). However, as shown in
Figure 13, as the mixture progresses from the stoichiometric conditions to the lean region, the burn duration decreases and then increases again. Normally, burn duration increases as the mixture becomes lean [
28]. This situation was observed clearly at 2000 rpm. In addition, GU21D and Kistler 6118CF-6CQ04 spark plug transducers were used at the same time to determine if there was an error caused by the transducers.
Although there was no change for the decrease of burn duration (stoichiometric to lean), it was found that there were significant differences between the 90% MBF locations that were calculated from different transducers. Generally, the location of 90% MBF occurs approximately 10° CA later according to spark plug transducers. Whereas, the 5%, 10%, 50%, and 70% MBF locations were extremely similar for both pressure transducers. Based on all these results, it was concluded that this unexpected drop tendency in burn duration was caused by a thermal shock effect on the pressure transducer. This effect became more apparent in the stoichiometric mixture due to high temperatures in the cylinder. The effect of the thermal shock on the transducer became more sensible during expansion stroke [
29,
30]. However, to compare gasoline and CNG, thermal shock has a negligible effect on transducers in the same relative air/fuel ratio. In order to see this effect, 5–80% MBF duration were examined under the same conditions. As shown in
Figure 14, as expected, the burn duration increases when the mixture becomes leaner, whereas considering the 5–90% MBF time, an inverse tendency was observed around the stoichiometric mixture (
Figure 13). The same explanation is acceptable for 2000 rpm (
Figure 15). For example, at a BMEP of 5 bar and 2000 rpm, burn duration in the stoichiometric mixture was approximately 17.5° CA and 18.5° CA, whereas for lean mixture, it was approximately 25.5° CA and 22.5° CA for gasoline and CNG, respectively.
Consequently, while the burn duration for gasoline is shorter in stoichiometric mixture, in lean mixture CNG burns faster than gasoline. Contrary to some studies, the increase in turbulence intensity has reduced this difference for CNG in the stoichiometric mixture. The increase in turbulence intensity was more effective on the burn duration of CNG. This change indirectly affects the ignition advance and delay duration. Namely, the ignition advance value that was required to obtain maximum engine torque was reduced. Therefore, the ignition delay duration reduced due to higher temperature at the time of ignition in the cylinder.