Improvement of Steam Injection Processes Through Nanotechnology: An Approach through in Situ Upgrading and Foam Injection
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
Porous Medium
2.2. Methods
2.2.1. Nanocatalyst Selection
2.2.2. Nanofluid Formulation for in Situ Upgrading
2.2.3. Selection of Foaming Nanofluid
2.2.4. Displacement Tests
2.2.5. Steam Injection Tests in the Presence and Absence of Nanofluids
2.2.6. Effluent Analysis
3. Results and Discussion
3.1. Selection of Nanocatalyst
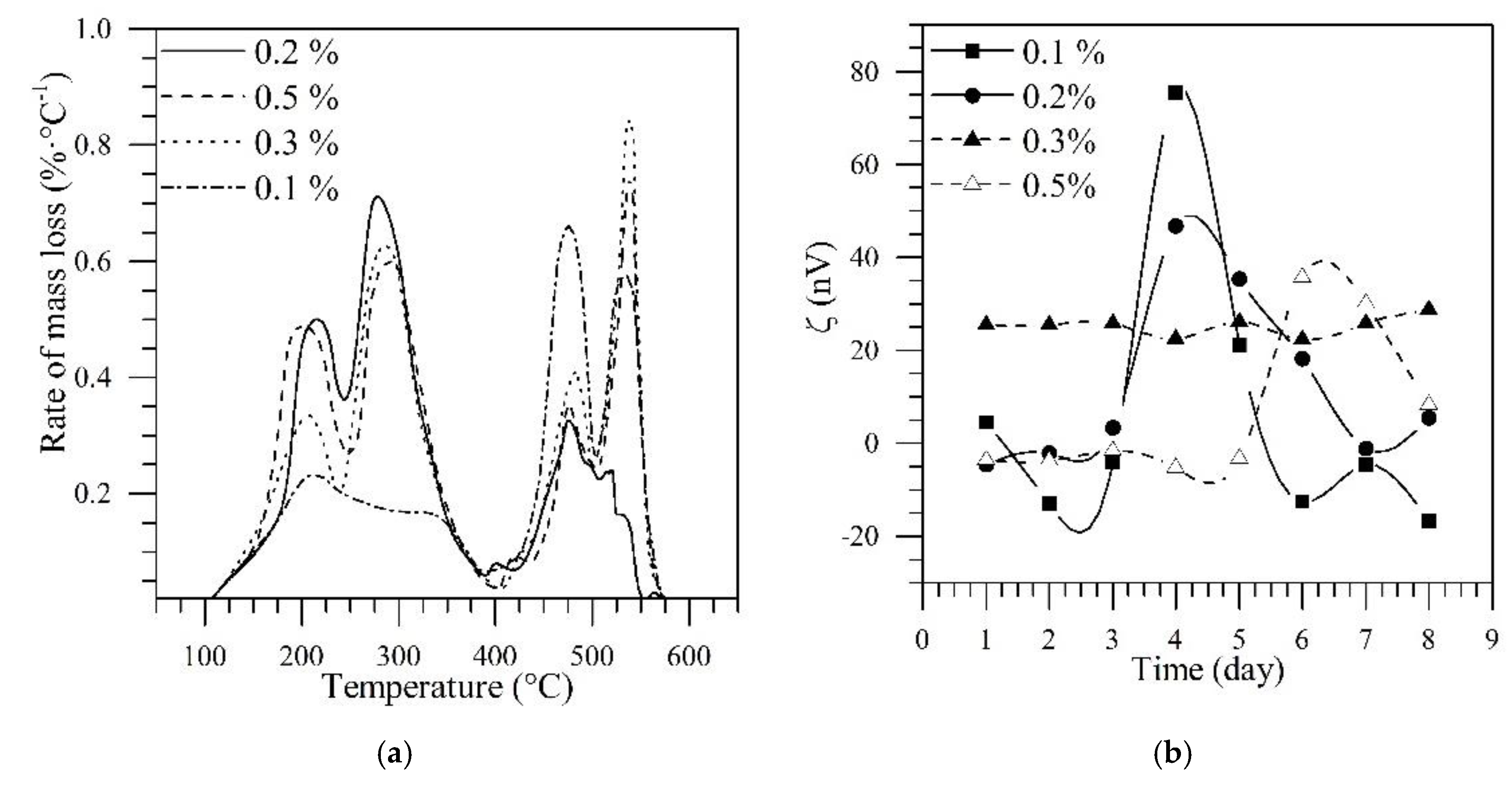
3.2. Design of Nanofluid for in Situ Upgrading
3.3. Selection of the Foaming Nanofluid
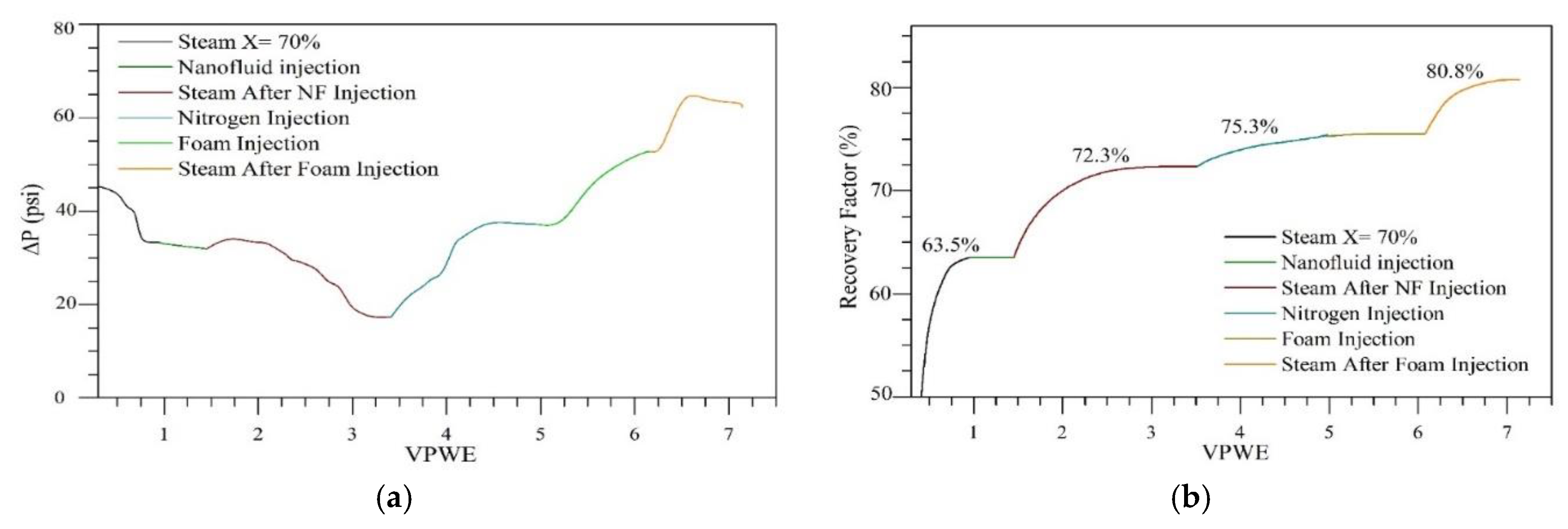
3.4. Displacement Tests
3.4.1. Steam Injection Process Assisted by Nanotechnology
3.4.2. Temperature Profile during Steam Injection Tests
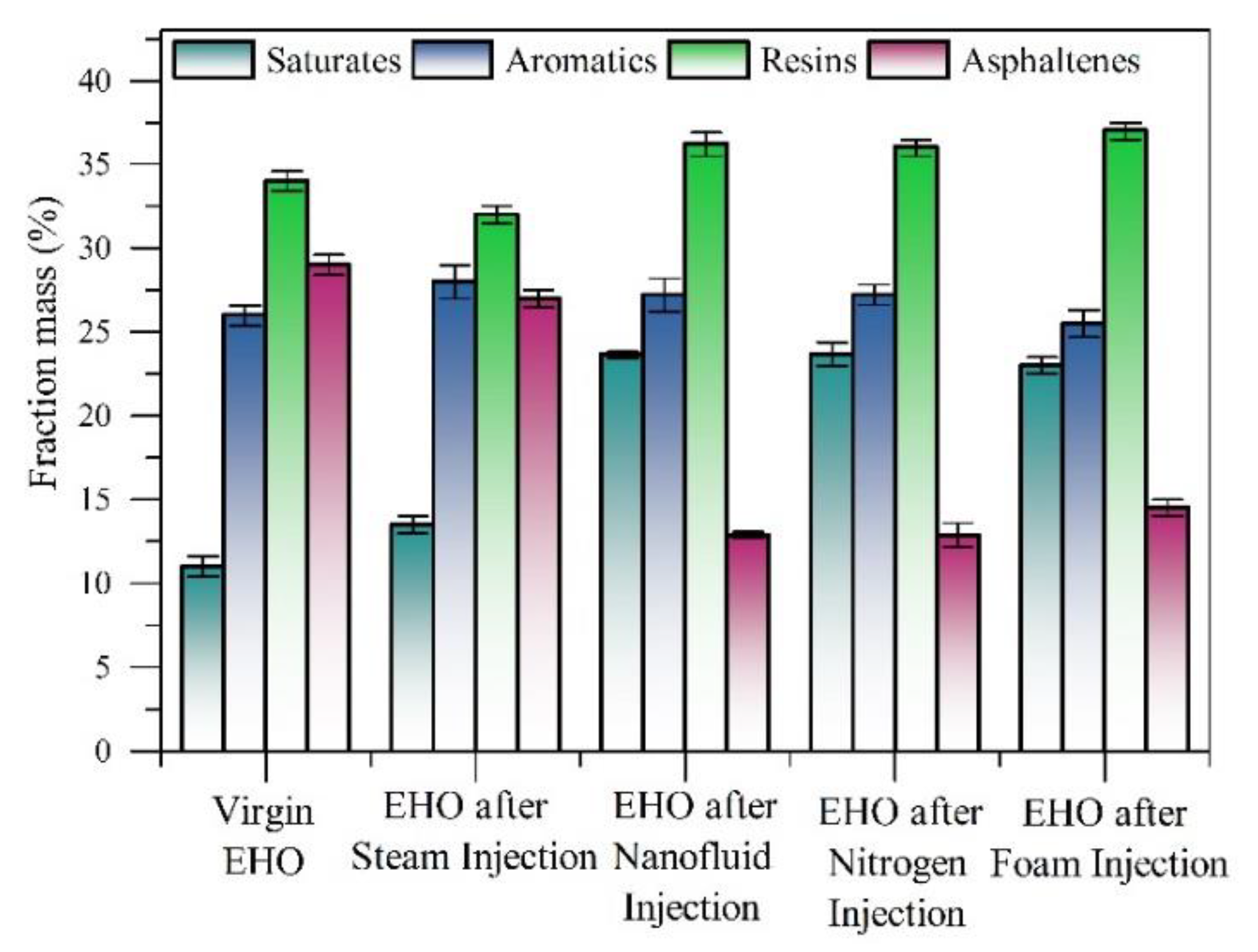
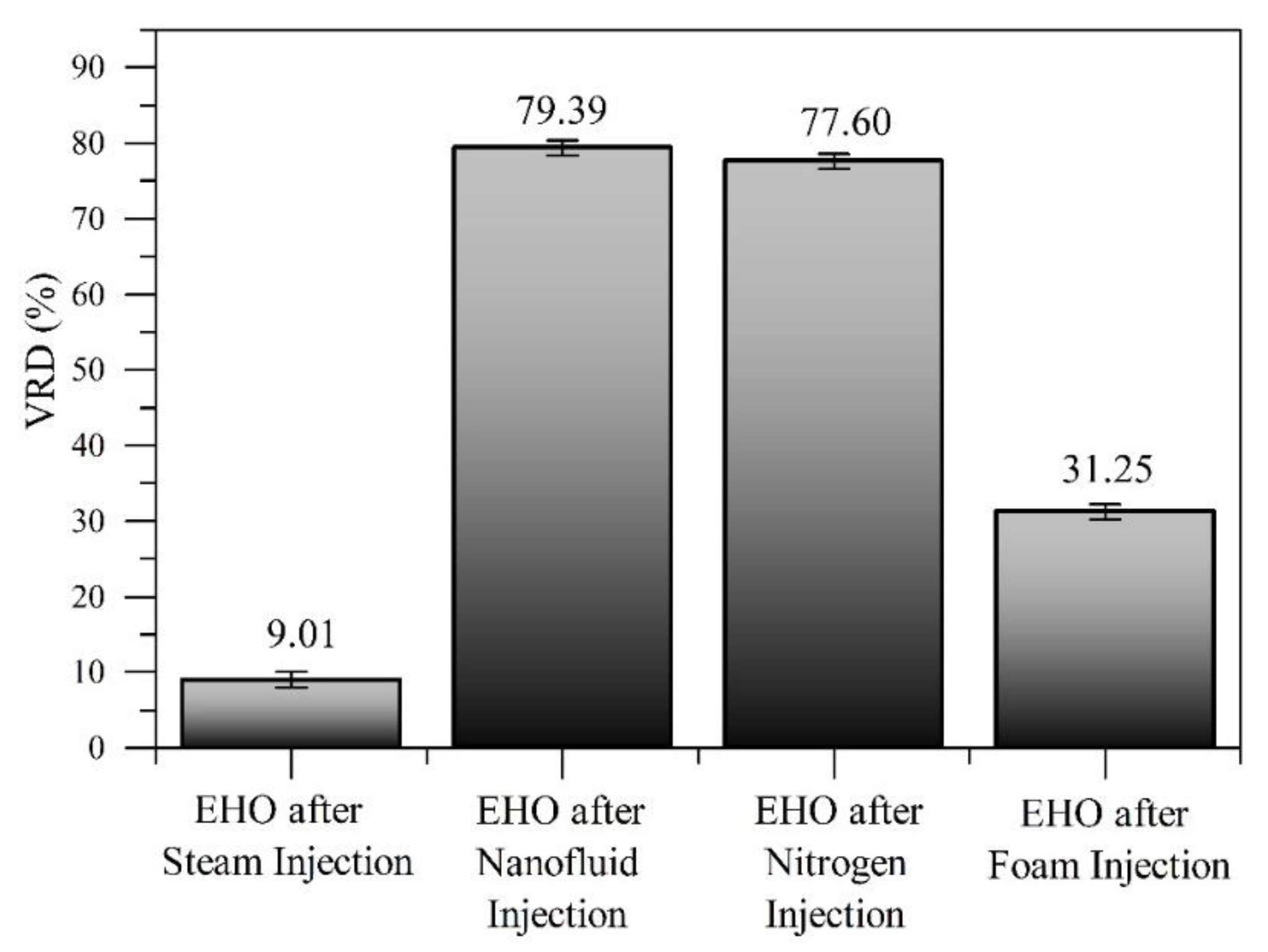
3.5. Crude Oil Characterization
3.5.1. API Gravity, Residue Conversion (R%) and SARA Content
3.5.2. Rheological Behavior
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Calemma, V.; Iwanski, P.; Nali, M.; Scotti, R.; Montanari, L. Structural characterization of asphaltenes of different origins. Energy Fuels 1995, 9, 225–230. [Google Scholar] [CrossRef]
- Boduszynski, M.M. Composition of heavy petroleums. 2. Molecular characterization. Energy Fuels 1988, 2, 597–613. [Google Scholar] [CrossRef]
- Medina, O.E.; Gallego, J.; Rodriguez, E.; Franco, C.A.; Cortés, F.B. Effect of Pressure on the Oxidation Kinetics of Asphaltenes. Energy Fuels 2019, 33, 10734–10744. [Google Scholar] [CrossRef]
- Tremblay, B.; Sedgwick, G.; Forshner, K. Simulation of cold production in heavy-oil reservoirs: Wormhole dynamics. SPE Reserv. Eng. 1997, 12, 110–117. [Google Scholar] [CrossRef]
- Langevin, D.; Poteau, S.; Hénaut, I.; Argillier, J. Crude oil emulsion properties and their application to heavy oil transportation. Oil Gas Sci. Technol. 2004, 59, 511–521. [Google Scholar] [CrossRef]
- Banerjee, D.K. Oil Sands, Heavy Oil & Bitumen: From Recovery to Refinery; PennWell Books: Tulsa, OK, USA, 2012. [Google Scholar]
- Acevedo, S.; Zuloaga, C.; Rodríguez, P. Aggregation—Dissociation studies of asphaltene solutions in resins performed using the combined freeze fracture—Transmission electron microscopy technique. Energy Fuels 2008, 22, 2332–2340. [Google Scholar] [CrossRef]
- Acevedo, S.; Méndez, B.; Rojas, A.; Layrisse, I.; Rivas, H. Asphaltenes and resins from the Orinoco basin. Fuel 1985, 64, 1741–1747. [Google Scholar] [CrossRef]
- Ancheyta, J.; Centeno, G.; Trejo, F.; Marroquin, G.; Garcia, J.; Tenorio, E.; Torres, A. Extraction and characterization of asphaltenes from different crude oils and solvents. Energy Fuels 2002, 16, 1121–1127. [Google Scholar] [CrossRef]
- Andersen, S.I.; Speight, J.G. Petroleum resins: Separation, character, and role in petroleum. Pet. Sci. Technol. 2001, 19, 1–34. [Google Scholar] [CrossRef]
- Bera, A.; Babadagli, T. Status of electromagnetic heating for enhanced heavy oil/bitumen recovery and future prospects: A review. Appl. Energy 2015, 151, 206–226. [Google Scholar] [CrossRef]
- Shah, A.; Fishwick, R.; Wood, J.; Leeke, G.; Rigby, S.; Greaves, M. A review of novel techniques for heavy oil and bitumen extraction and upgrading. Energy Environ. Sci. 2010, 3, 700–714. [Google Scholar] [CrossRef]
- McKay, A.S.; Nasr, T.N. Recovery of Bitumen or Heavy Oil in Situ by Injection of Hot Water of Low Quality Steam Plus Caustic and Carbon Dioxide. U.S. Patent No. 5,056,596, 15 October 1991. [Google Scholar]
- Jorshari, K.; O’Hara, B. A new SAGD-well-pair placement: A field case review. J. Can. Pet. Technol. 2013, 52, 12–19. [Google Scholar] [CrossRef]
- Kar, T.; Ovalles, C.; Rogel, E.; Vien, J.; Hascakir, B. The residual oil saturation determination for Steam Assisted Gravity Drainage (SAGD) and Solvent-SAGD. Fuel 2016, 172, 187–195. [Google Scholar] [CrossRef]
- Liu, H.; Cheng, L.; Huang, S.; Jia, P.; Chen, M. Evolution characteristics of SAGD steam chamber and its impacts on heavy oil production and heat consumption. Int. J. Heat Mass Transf. 2018, 121, 579–596. [Google Scholar] [CrossRef]
- Franco, C.; Cardona, L.; Lopera, S.; Mejía, J.; Cortés, F. Heavy oil upgrading and enhanced recovery in a continuous steam injection process assisted by nanoparticulated catalysts. In Proceedings of the SPE Improved Oil Recovery Conference, Tulsa, OK, USA, 11–13 April 2016. [Google Scholar]
- Luft, H.; Pelensky, P.; George, G. Development and operation of a new insulated concentric coiled tubing string for continuous steam injection in heavy oil production. In Proceedings of the SPE International Heavy Oil Symposium, Calgary, AB, Canada, 19–21 June 1995. [Google Scholar]
- Melcon, S. Oil Recovery by in Situ Combustion. U.S. Patent No. 3,165,154, 12 January 1965. [Google Scholar]
- Dabbous, M.K.; Fulton, P.F. Low-temperature-oxidation reaction kinetics and effects on the in-situ combustion process. Soc. Pet. Eng. J. 1974, 14, 253–262. [Google Scholar] [CrossRef]
- Jiang, S.; Liu, X.; Liu, Y.; Zhong, L. In situ upgrading heavy oil by aquathermolytic treatment under steam injection conditions. In Proceedings of the SPE International Symposium on Oilfield Chemistry, The Woodlands, TX, USA, 2–4 February 2005. [Google Scholar]
- Kapadia, P.R.; Kallos, M.S.; Gates, I.D. A review of pyrolysis, aquathermolysis, and oxidation of Athabasca bitumen. Fuel Process. Technol. 2015, 131, 270–289. [Google Scholar] [CrossRef]
- Ali, S. Current status of steam injection as a heavy oil recovery method. J. Can. Pet. Technol. 1974, 13, 54–68. [Google Scholar] [CrossRef]
- Sandiford, B.B. Gel and Method for Reducing Steam Channeling. U.S. Patent No. 4,665,986, 19 May 1987. [Google Scholar]
- Aljundi, I.H. Energy and exergy analysis of a steam power plant in Jordan. Appl. Therm. Eng. 2009, 29, 324–328. [Google Scholar] [CrossRef]
- Herranz, L.E.; Anderson, M.H.; Corradini, M.L. A diffusion layer model for steam condensation within the AP600 containment. Nucl. Eng. Des. 1998, 183, 133–150. [Google Scholar] [CrossRef]
- Nassar, N.N.; Hassan, A.; Pereira-Almao, P. Application of nanotechnology for heavy oil upgrading: Catalytic steam gasification/cracking of asphaltenes. Energy Fuels 2011, 25, 1566–1570. [Google Scholar] [CrossRef]
- Choi, S.U.; Eastman, J.A. Enhancing Thermal Conductivity of Fluids with Nanoparticles; Argonne National Lab.: Lemont, IL, USA, 1995. [Google Scholar]
- Li, W.; Ni, C.; Lin, H.; Huang, C.; Shah, S.I. Size dependence of thermal stability of TiO2 nanoparticles. J. Appl. Phys. 2004, 96, 6663–6668. [Google Scholar] [CrossRef]
- Hwang, Y.-j.; Lee, J.; Lee, C.; Jung, Y.; Cheong, S.; Lee, C.; Ku, B.; Jang, S. Stability and thermal conductivity characteristics of nanofluids. Thermochim. Acta 2007, 455, 70–74. [Google Scholar] [CrossRef]
- Adams, J.J. Asphaltene adsorption, a literature review. Energy Fuels 2014, 28, 2831–2856. [Google Scholar] [CrossRef]
- Franco, C.A.; Montoya, T.; Nassar, N.N.; Cortés, F.B. NiO and PdO Supported on Fumed Silica Nanoparticles for Adsorption and Catalytic Steam Gasification of Colombian n-C7 Asphaltenes. Handb. Oil Prod. Res. 2014, 26, 101–145. [Google Scholar]
- Cardona Rojas, L. Efecto de Nanopartículas en Procesos con Inyección de Vapor a Diferentes Calidades. Master’s Thesis, Universidad Nacional de Colombia-Sede Medellín, Antioquia, Colombia, March 2018. [Google Scholar]
- Nassar, N.N.; Franco, C.A.; Montoya, T.; Cortés, F.B.; Hassan, A. Effect of oxide support on Ni–Pd bimetallic nanocatalysts for steam gasification of n-C7 asphaltenes. Fuel 2015, 156, 110–120. [Google Scholar] [CrossRef]
- Medina, O.E.; Gallego, J.; Arias-Madrid, D.; Cortés, F.B.; Franco, C.A. Optimization of the Load of Transition Metal Oxides (Fe2O3, Co3O4, NiO and/or PdO) onto CeO2 Nanoparticles in Catalytic Steam Decomposition of n-C7 Asphaltenes at Low Temperatures. Nanomaterials 2019, 9, 401. [Google Scholar] [CrossRef]
- Cardona, L.; Arias-Madrid, D.; Cortés, F.; Lopera, S.; Franco, C. Heavy oil upgrading and enhanced recovery in a steam injection process assisted by NiO-and PdO-Functionalized SiO2 nanoparticulated catalysts. Catalysts 2018, 8, 132. [Google Scholar] [CrossRef]
- Badoga, S.; Sharma, R.V.; Dalai, A.K.; Adjaye, J. Hydrotreating of Heavy Gas Oil on Mesoporous Mixed Metal Oxides (M–Al2O3, M = TiO2, ZrO2, SnO2) Supported NiMo Catalysts: Influence of Surface Acidity. Ind. Eng. Chem. Res. 2014, 53, 18729–18739. [Google Scholar] [CrossRef]
- Gao, Y.; Ghorbanian, B.; Gargari, H.N.; Gao, W. Steam reforming of gaseous by-products from bitumen oil using various supported Ni-based catalysts. Pet. Sci. Technol. 2018, 36, 34–39. [Google Scholar] [CrossRef]
- Guo, K.; Zhang, Y.; Shi, Q.; Yu, Z. The effect of carbon-supported nickel nanoparticles in the reduction of carboxylic acids for in situ upgrading of heavy crude oil. Energy Fuels 2017, 31, 6045–6055. [Google Scholar] [CrossRef]
- Vignatti, C.I.; Avila, M.S.; Apesteguia, C.R.; Garetto, T.F. Study of the water-gas shift reaction over Pt supported on CeO2–ZrO2 mixed oxides. Catal. Today 2011, 171, 297–303. [Google Scholar] [CrossRef]
- Yi, S.; Babadagli, T.; Li, H.A. Use of nickel nanoparticles for promoting aquathermolysis reaction during cyclic steam stimulation. SPE J. 2018, 23, 145–156. [Google Scholar] [CrossRef]
- Afzal, S.; Nikookar, M.; Ehsani, M.R.; Roayaei, E. An experimental investigation of the catalytic effect of Fe2O3 nanoparticle on steam injection process of an Iranian reservoir. Iran. J. Oil Gas Sci. Technol. 2014, 3, 27–36. [Google Scholar]
- Rossen, W.R. Foams in enhanced oil recovery. Foam. Theory Meas. Appl. 1996, 57, 413–464. [Google Scholar]
- Hiraski, G.J. The steam-foam process. J. Pet. Technol. 1989, 41, 449–456. [Google Scholar] [CrossRef]
- Dilgren, R.E.; Owens, K.B. Steam-foaming surfactant mixtures which are tolerant of divalent ions. U.S. Patent No. 4,643,256, 17 February 1987. [Google Scholar]
- Sanders, A.; Dado, G.P.; Holland, B.; Dong, X.M.; Rojas, C.E. Steam Foam Methods for Steam-Assisted Gravity Drainage. U.S. Patent Application No. 15/503,293, 10 August 2017. [Google Scholar]
- Sun, Q.; Li, Z.; Wang, J.; Li, S.; Li, B.; Jiang, L.; Wang, H.; Lü, Q.; Zhang, C.; Liu, W. Aqueous foam stabilized by partially hydrophobic nanoparticles in the presence of surfactant. Colloids Surf. A Physicochem. Eng. Asp. 2015, 471, 54–64. [Google Scholar] [CrossRef]
- Pang, Z.; Liu, H.; Zhu, L. A laboratory study of enhancing heavy oil recovery with steam flooding by adding nitrogen foams. J. Pet. Sci. Eng. 2015, 128, 184–193. [Google Scholar] [CrossRef]
- Singh, R.; Mohanty, K.K. Synergistic stabilization of foams by a mixture of nanoparticles and surfactants. In Proceedings of the SPE Improved Oil Recovery Symposium, Tulsa, OK, USA, 12–16 April 2014. [Google Scholar]
- Nguyen, P.; Fadaei, H.; Sinton, D. Pore-scale assessment of nanoparticle-stabilized CO2 foam for enhanced oil recovery. Energy Fuels 2014, 28, 6221–6227. [Google Scholar] [CrossRef]
- Hurtado, Y.; Beltrán, C.; Zabala, R.D.; Lopera, S.H.; Franco, C.A.; Nassar, N.N.; Cortés, F.B. Effects of Surface Acidity and Polarity of SiO2 Nanoparticles on the Foam Stabilization Applied to Natural Gas Flooding in Tight Gas-Condensate Reservoirs. Energy Fuels 2018, 32, 5824–5833. [Google Scholar] [CrossRef]
- Hurtado, Y.; Franco, C.A.; Cortés, F.B. Mejoramiento de espumas de Nitrogéno por adición de nanopartículas parcialmente hidrofobicas. In Proceedings of the SPETC, Medellín, Colombia, 28 May 2018. [Google Scholar]
- Ostgard, D.; Kustov, L.; Poeppelmeier, K.R.; Sachtler, W. Comparison of Pt/KL catalysts prepared by ion exchange or incipient wetness impregnation. J. Catal. 1992, 133, 342–357. [Google Scholar] [CrossRef]
- Ramakrishnan, T.; Cappiello, A. A new technique to measure static and dynamic properties of a partially saturated porous medium. Chem. Eng. Sci. 1991, 46, 1157–1163. [Google Scholar] [CrossRef]
- Whitaker, S. Flow in porous media I: A theoretical derivation of Darcy’s law. Transp. Porous Media 1986, 1, 3–25. [Google Scholar] [CrossRef]
- Scheffe, H. The simplex-centroid design for experiments with mixtures. J. R. Stat. Soc. Ser. B 1963, 25, 235–251. [Google Scholar] [CrossRef]
- Guzmán, J.D.; Betancur, S.; Carrasco-Marín, F.; Franco, C.A.; Nassar, N.N.; Cortés, F.B. Importance of the adsorption method used for obtaining the nanoparticle dosage for asphaltene-related treatments. Energy Fuels 2016, 30, 2052–2059. [Google Scholar] [CrossRef]
- Mukherjee, S.; Paria, S. Preparation and stability of nanofluids—A Review. IOSR J. Mech. Civ. Eng. 2013, 9, 63–69. [Google Scholar] [CrossRef]
- Giraldo, J.; Benjumea, P.; Lopera, S.; Cortés, F.B.; Ruiz, M.A. Wettability alteration of sandstone cores by alumina-based nanofluids. Energy Fuels 2013, 27, 3659–3665. [Google Scholar] [CrossRef]
- Bansal, V.; Krishna, G.; Chopra, A.; Sarpal, A. Detailed hydrocarbon characterization of RFCC feed stocks by NMR spectroscopic techniques. Energy Fuels 2007, 21, 1024–1029. [Google Scholar] [CrossRef]
- Montes, D.; Orozco, W.; Taborda, E.A.; Franco, C.A.; Cortés, F.B. Development of Nanofluids for Perdurability in Viscosity Reduction of Extra-Heavy Oils. Energies 2019, 12, 1068. [Google Scholar] [CrossRef]
- Austrich, A.; Buenrostro-Gonzalez, E.; Lira-Galeana, C. ASTM D-5307 and ASTM D-7169 SIMDIS standards: A comparison and correlation of methods. Pet. Sci. Technol. 2015, 33, 657–663. [Google Scholar] [CrossRef]
- Hashemi, R.; Nassar, N.N.; Pereira-Almao, P. Transport behavior of multimetallic ultradispersed nanoparticles in an oil-sands-packed bed column at a high temperature and pressure. Energy Fuels 2012, 26, 1645–1655. [Google Scholar] [CrossRef]
- Brewer, L.; Wengert, P.R. Erratum to: Transition metal alloys of extraordinary stability; An example of generalized Lewis-acid-base interactions in metallic systems. Metall. Trans. 1973, 4, 83–104. [Google Scholar] [CrossRef]
- Medina, O.E.; Gallego, J.; Restrepo, L.G.; Cortés, F.B.; Franco, C.A. Influence of the Ce4+/Ce3+ Redox-Couple on the Cyclic Regeneration for Adsorptive and Catalytic Performance of NiO-PdO/CeO2±δ Nanoparticles for n-C7 Asphaltene Steam Gasification. Nanomaterials 2019, 9, 734. [Google Scholar] [CrossRef]
- Franco, C.A.; Nassar, N.N.; Montoya, T.; Ruíz, M.A.; Cortés, F.B. Influence of asphaltene aggregation on the adsorption and catalytic behavior of nanoparticles. Energy Fuels 2015, 29, 1610–1621. [Google Scholar] [CrossRef]
- Clogston, J.D.; Patri, A.K. Zeta potential measurement. In Characterization of Nanoparticles Intended for Drug Delivery; Springer: Berlin, Germany, 2011; pp. 63–70. [Google Scholar]
- Franco, C.A. Synthesis and Application of Supported Metallic and Multi-Metallic Oxides Nanoparticles for In-Situ Upgrading and Inhibition of Formation Damage; Universidad Nacional de Colombia-Sede Medellín: Antioquia, Colombia, 2015. [Google Scholar]
- Li, X.; Shi, L.; Li, H.; Liu, P.; Luo, J.; Yuan, Z. Experimental study on viscosity reducers for SAGD in developing extra-heavy oil reservoirs. J. Pet. Sci. Eng. 2018, 166, 25–32. [Google Scholar] [CrossRef]
- Ji, D.; Zhong, H.; Dong, M.; Chen, Z. Study of heat transfer by thermal expansion of connate water ahead of a steam chamber edge in the steam-assisted-gravity-drainage process. Fuel 2015, 150, 592–601. [Google Scholar] [CrossRef]
- Huang, S.; Cao, M.; Cheng, L. Experimental study on aquathermolysis of different viscosity heavy oil with superheated steam. Energy Fuels 2018, 32, 4850–4858. [Google Scholar] [CrossRef]
- Ageeb, A.A.; Al-siddig, M.H.; Nor-aldeen, M.R.; Soliman, M.S.; Ibrahim, I.H. The Influence of Steam Injection Volume on Sand and Oil Production in Cyclic Steam Stimulation (CSS) Wells. Ph.D. Thesis, Sudan University of Science and Technology, Khartoum, Sudan, 2017. [Google Scholar]
- Hamedi Shokrlu, Y.; Babadagli, T. Effects of nano-sized metals on viscosity reduction of heavy oil/bitumen during thermal applications. In Proceedings of the Canadian Unconventional Resources and International Petroleum Conference, Calgary, AB, Canada, 19–21 October 2010. [Google Scholar]
- Taborda, E.A.; Franco, C.A.; Ruiz, M.A.; Alvarado, V.; Cortés, F.B. Experimental and theoretical study of viscosity reduction in heavy crude oils by addition of nanoparticles. Energy Fuels 2017, 31, 1329–1338. [Google Scholar] [CrossRef]
- Cortés, F.B.; Montoya, T.; Acevedo, S.; Nassar, N.N.; Franco, C.A. Adsorption-desorption of n-c7 asphaltenes over micro-and nanoparticles of silica and its impact on wettability alteration. CTF Cienc. Tecnol. Futuro 2016, 6, 89–106. [Google Scholar] [CrossRef]
- Guerrini, L.; Jurasekova, Z.; Domingo, C.; Pérez-Méndez, M.; Leyton, P.; Campos-Vallette, M.; García-Ramos, J.V.; Sánchez-Cortés, S. Importance of metal–adsorbate interactions for the surface-enhanced Raman scattering of molecules adsorbed on plasmonic nanoparticles. Plasmonics 2007, 2, 147–156. [Google Scholar] [CrossRef]
- Taborda, E.A.; Franco, C.A.; Lopera, S.H.; Alvarado, V.; Cortés, F.B. Effect of nanoparticles/nanofluids on the rheology of heavy crude oil and its mobility on porous media at reservoir conditions. Fuel 2016, 184, 222–232. [Google Scholar] [CrossRef]
- Shokrlu, Y.H.; Babadagli, T. Transportation and interaction of nano and micro size metal particles injected to improve thermal recovery of heavy-oil. In Proceedings of the SPE Annual Technical Conference and Exhibition, Denver, CO, USA, 30 October–2 November 2011. [Google Scholar]
- Elahi, S.M.; Ahmadi Khoshooei, M.; Scott, C.E.; Chen, Z.; Pereira-Almao, P. In-situ upgrading of heavy oil using nano-catalysts: A computational fluid dynamics study of hydrogen and vacuum residue injection. Can. J. Chem. Eng. 2019, 97, 1352–1360. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, Y.; Chen, G.; Gai, Z. Application of nanoparticles in enhanced oil recovery: A critical review of recent progress. Energies 2017, 10, 345. [Google Scholar] [CrossRef]
- López, D.; Giraldo, L.J.; Salazar, J.P.; Zapata, D.M.; Ortega, D.C.; Franco, C.A.; Cortés, F.B. Metal Oxide Nanoparticles Supported on Macro-Mesoporous Aluminosilicates for Catalytic Steam Gasification of Heavy Oil Fractions for On-Site Upgrading. Catalysts 2017, 7, 319. [Google Scholar] [CrossRef]
- Hosseinpour, N.; Mortazavi, Y.; Bahramian, A.; Khodatars, L.; Khodadadi, A.A. Enhanced pyrolysis and oxidation of asphaltenes adsorbed onto transition metal oxides nanoparticles towards advanced in-situ combustion EOR processes by nanotechnology. Appl. Catal. A Gen. 2014, 477, 159–171. [Google Scholar] [CrossRef]
- Alamolhoda, S.; Vitale, G.; Hassan, A.; Nassar, N.N.; Almao, P.P. Synergetic effects of cerium and nickel in Ce-Ni-MFI catalysts on low-temperature water-gas shift reaction. Fuel 2019, 237, 361–372. [Google Scholar] [CrossRef]
- Nematollahi, B.; Rezaei, M.; Lay, E.N. Preparation of highly active and stable NiO–CeO2 nanocatalysts for CO selective methanation. Int. J. Hydrogen Energy 2015, 40, 8539–8547. [Google Scholar] [CrossRef]
- Khajenoori, M.; Rezaei, M.; Nematollahi, B. Preparation of noble metal nanocatalysts and their applications in catalytic partial oxidation of methane. J. Ind. Eng. Chem. 2013, 19, 981–986. [Google Scholar] [CrossRef]
- Liu, R.-J.; Crozier, P.; Smith, C.; Hucul, D.; Blackson, J.; Salaita, G. Metal sintering mechanisms and regeneration of palladium/alumina hydrogenation catalysts. Appl. Catal. A Gen. 2005, 282, 111–121. [Google Scholar] [CrossRef]
- Wrobel, G.; Sohier, M.; D’Huysser, A.; Bonnelle, J.; Marcq, J. Hydrogenation catalysts based on nickel and rare earth oxides: Part II: XRD, electron microscopy and XPS studies of the cerium-nickel-oxygen-hydrogen system. Appl. Catal. A Gen. 1993, 101, 73–93. [Google Scholar] [CrossRef]
- Wang, R.; Xu, H.; Liu, X.; Ge, Q.; Li, W. Role of redox couples of Rh0/Rhδ+ and Ce4+/Ce3+ in CH4/CO2 reforming over Rh–CeO2/Al2O3 catalyst. Appl. Catal. A Gen. 2006, 305, 204–210. [Google Scholar] [CrossRef]
- Vidal, H.; Kašpar, J.; Pijolat, M.; Colon, G.; Bernal, S.; Cordón, A.; Perrichon, V.; Fally, F. Redox behavior of CeO2–ZrO2 mixed oxides: I. Influence of redox treatments on high surface area catalysts. Appl. Catal. B Environ. 2000, 27, 49–63. [Google Scholar] [CrossRef]
- Hilaire, S.; Wang, X.; Luo, T.; Gorte, R.; Wagner, J. A comparative study of water-gas-shift reaction over ceria supported metallic catalysts. Appl. Catal. A Gen. 2001, 215, 271–278. [Google Scholar] [CrossRef]
- Jacobs, G.; Ricote, S.; Graham, U.M.; Patterson, P.M.; Davis, B.H. Low temperature water gas shift: Type and loading of metal impacts forward decomposition of pseudo-stabilized formate over metal/ceria catalysts. Catal. Today 2005, 106, 259–264. [Google Scholar] [CrossRef]
- Dragonetti, R.; Napolitano, M.; Di Filippo, S.; Romano, R. Modeling energy conversion in a tortuous stack for thermoacostic applications. Appl. Therm. Eng. 2016, 103, 233–242. [Google Scholar] [CrossRef]
- Zhu, Q.; Xuan, Y. Pore scale numerical simulation of heat transfer and flow in porous volumetric solar receivers. Appl. Therm. Eng. 2017, 120, 150–159. [Google Scholar] [CrossRef]
- Liu, H.; Cheng, L.; Du, Y.; Huang, Q.; Xiao, P. A heat and mass transfer coupling model for transition interface of expanding solvent steam-assisted gravity drainage. Spec. Top. Rev. Porous Media Int. J. 2017, 8. [Google Scholar] [CrossRef]
- Lee, C.; Park, C.; Park, S. Flow characteristics of steam and gas push in the presence of heat thief zones overlying oil sands deposits. Appl. Sci. 2017, 7, 919. [Google Scholar] [CrossRef]
- Iwamoto, M.; Yoda, Y.; Yamazoe, N.; Seiyama, T. Study of metal oxide catalysts by temperature programmed desorption. 4. Oxygen adsorption on various metal oxides. J. Phys. Chem. 1978, 82, 2564–2570. [Google Scholar] [CrossRef]
- Astruc, D. Nanoparticles and Catalysis; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Jana, N.R.; Wang, Z.; Pal, T. Redox catalytic properties of palladium nanoparticles: Surfactant and electron donor∓acceptor effects. Langmuir 2000, 16, 2457–2463. [Google Scholar] [CrossRef]
- Mansour, E.; Desouky, S.; El Aily, M.; Helmi, M. The effect of asphaltene content on predicting heavy dead oils viscosity: Experimental and modeling study. Fuel 2018, 212, 405–411. [Google Scholar] [CrossRef]
- Guo, K.; Li, H.; Yu, Z. In-situ heavy and extra-heavy oil recovery: A review. Fuel 2016, 185, 886–902. [Google Scholar] [CrossRef]
- Hosseinpour, M.; Fatemi, S.; Ahmadi, S.J. Catalytic cracking of petroleum vacuum residue in supercritical water media: Impact of α-Fe2O3 in the form of free nanoparticles and silica-supported granules. Fuel 2015, 159, 538–549. [Google Scholar] [CrossRef]
- Ternan, M. Catalytic hydrogenation and asphaltene conversion of Athabasca bitumen. Can. J. Chem. Eng. 1983, 61, 689–696. [Google Scholar] [CrossRef]
- Hamedi Shokrlu, Y.; Babadagli, T. In-situ upgrading of heavy oil/bitumen during steam injection by use of metal nanoparticles: A study on in-situ catalysis and catalyst transportation. SPE Reserv. Eval. Eng. 2013, 16, 333–344. [Google Scholar] [CrossRef]
- Hamedi Shokrlu, Y.; Babadagli, T. Kinetics of the in-situ upgrading of heavy oil by nickel nanoparticle catalysts and its effect on cyclic-steam-stimulation recovery factor. SPE Reserv. Eval. Eng. 2014, 17, 355–364. [Google Scholar] [CrossRef]
- Wang, X.; Rodriguez, J.A.; Hanson, J.C.; Gamarra, D.; Martínez-Arias, A.; Fernández-García, M. In situ studies of the active sites for the water gas shift reaction over Cu-CeO2 catalysts: Complex interaction between metallic copper and oxygen vacancies of ceria. J. Phys. Chem. B 2006, 110, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Diagne, C.; Idriss, H.; Kiennemann, A. Hydrogen production by ethanol reforming over Rh/CeO2–ZrO2 catalysts. Catal. Commun. 2002, 3, 565–571. [Google Scholar] [CrossRef]
- Khan, M.R. Rheological properties of heavy oils and heavy oil emulsions. Energy Sources 1996, 18, 385–391. [Google Scholar] [CrossRef]
- Santos, R.; Loh, W.; Bannwart, A.; Trevisan, O. An overview of heavy oil properties and its recovery and transportation methods. Braz. J. Chem. Eng. 2014, 31, 571–590. [Google Scholar] [CrossRef]
- Iskandar, F.; Dwinanto, E.; Abdullah, M.; Muraza, O. Viscosity reduction of heavy oil using nanocatalyst in aquathermolysis reaction. KONA Powder Part. J. 2016, 2016005. [Google Scholar] [CrossRef]
- Maity, S.; Ancheyta, J.; Marroquín, G. Catalytic aquathermolysis used for viscosity reduction of heavy crude oils: A review. Energy Fuels 2010, 24, 2809–2816. [Google Scholar] [CrossRef]
- Shokrlu, Y.H.; Babadagli, T. Viscosity reduction of heavy oil/bitumen using micro-and nano-metal particles during aqueous and non-aqueous thermal applications. J. Pet. Sci. Eng. 2014, 119, 210–220. [Google Scholar] [CrossRef]
- Desnoyer, A.N.; Love, J.A. Recent advances in well-defined, late transition metal complexes that make and/or break C–N, C–O and C–S bonds. Chem. Soc. Rev. 2017, 46, 197–238. [Google Scholar] [CrossRef] [PubMed]
- Tajmiri, M.; Ehsani, M.R. The Potential of ZnO Nanoparticles to Reduce Water Consuming in Iranian Heavy Oil Reservoir. J. Water Environ. Nanotechnol. 2016, 1, 84–90. [Google Scholar] [CrossRef]
- Al-Maamari, R.S.H.; Buckley, J.S. Asphaltene Precipitation and Alteration of Wetting: The Potential for Wettability Changes During Oil Production. SPE Reserv. Eval. Eng. 2003, 6, 210–214. [Google Scholar] [CrossRef]
- Gharfeh, S.; Yen, A.; Asomaning, S.; Blumer, D. Asphaltene Flocculation Onset Determinations for Heavy Crude Oil and Its Implications. Pet. Sci. Technol. 2004, 22, 1055–1072. [Google Scholar] [CrossRef]
- Oskui, G.; Reza, P.; Jumaa, M.A.; Folad, E.G.; Rashed, A.; Patil, S. Systematic approach for prevention and remediation of asphaltene problems during CO2/hydrocarbon injection project. In Proceedings of the Twenty-first International Offshore and Polar Engineering Conference, Maui, HI, USA, 19–24 June 2011. [Google Scholar]
- Kojima, T.; Tahara, K. Refinement and transportation of petroleum with hydrogen from renewable energy. Energy Convers. Manag. 2001, 42, 1839–1851. [Google Scholar] [CrossRef]
- Medina, O.E.; Olmos, C.; Lopera, S.H.; Cortés, F.B.; Franco, C.A. Nanotechnology Applied to Thermal Enhanced Oil Recovery Processes: A Review. Energies 2019, 12. Accepted. [Google Scholar]




| Nanoparticle | Hydrodynamic Diameter (nm) | SBET (m2·g−1) | Ni Average Particle Size (nm) | Pd Average Particle Size (nm) | Metal Dispersion (%) |
|---|---|---|---|---|---|
| CeNi0.89Pd1.1 | 22 | 65 | 3.53 | 6.61 | 36 |
| SPG12 | 64 | 35 | − | − | − |
| Nanoparticle (Mass Fraction %) | t1/2 (min) | t∞ (min) |
|---|---|---|
| 0 | 5 | 9 |
| 0.01 | 10 | 40 |
| 0.1 | 8 | 32 |
| 0.5 | 8 | 30 |
| 1 | 6 | 22 |
| Stage | Swr (%) | Sor (%) | Ultimate Oil Recovery (%) | Incremental Regarding Steam Injection (%) |
|---|---|---|---|---|
| Steam injection | 71.2 | 28.8 | 63.5 | − |
| Steam injection assisted by Nanocatalyst | 78.2 | 78.2 | 72.3 | 8.8 |
| Nitrogen injection | 21.8 | 21.8 | 75.3 | 11.8 |
| Steam injection assisted by foam | 80.6 | 80.6 | 80.8 | 17.3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medina, O.E.; Hurtado, Y.; Caro-Velez, C.; Cortés, F.B.; Riazi, M.; Lopera, S.H.; Franco, C.A. Improvement of Steam Injection Processes Through Nanotechnology: An Approach through in Situ Upgrading and Foam Injection. Energies 2019, 12, 4633. https://doi.org/10.3390/en12244633
Medina OE, Hurtado Y, Caro-Velez C, Cortés FB, Riazi M, Lopera SH, Franco CA. Improvement of Steam Injection Processes Through Nanotechnology: An Approach through in Situ Upgrading and Foam Injection. Energies. 2019; 12(24):4633. https://doi.org/10.3390/en12244633
Chicago/Turabian StyleMedina, Oscar E., Yira Hurtado, Cristina Caro-Velez, Farid B. Cortés, Masoud Riazi, Sergio H. Lopera, and Camilo A. Franco. 2019. "Improvement of Steam Injection Processes Through Nanotechnology: An Approach through in Situ Upgrading and Foam Injection" Energies 12, no. 24: 4633. https://doi.org/10.3390/en12244633
APA StyleMedina, O. E., Hurtado, Y., Caro-Velez, C., Cortés, F. B., Riazi, M., Lopera, S. H., & Franco, C. A. (2019). Improvement of Steam Injection Processes Through Nanotechnology: An Approach through in Situ Upgrading and Foam Injection. Energies, 12(24), 4633. https://doi.org/10.3390/en12244633








