Fabrication of Hierarchical NiMoO4/NiMoO4 Nanoflowers on Highly Conductive Flexible Nickel Foam Substrate as a Capacitive Electrode Material for Supercapacitors with Enhanced Electrochemical Performance
Abstract
1. Introduction
2. Experimental Methods
2.1. Chemicals and Materials
2.2. Preparation of the NF/NiMoO4 Nanoball Electrode on a Ni Foam Substrate
2.3. Preparation of the NF/NiMoO4/NiMoO4 Nanoflower Composite Electrode on a Ni Foam Substrate
2.4. Characterizations of Nanoballs and Nanoflowers
2.5. Electrochemical Measurements
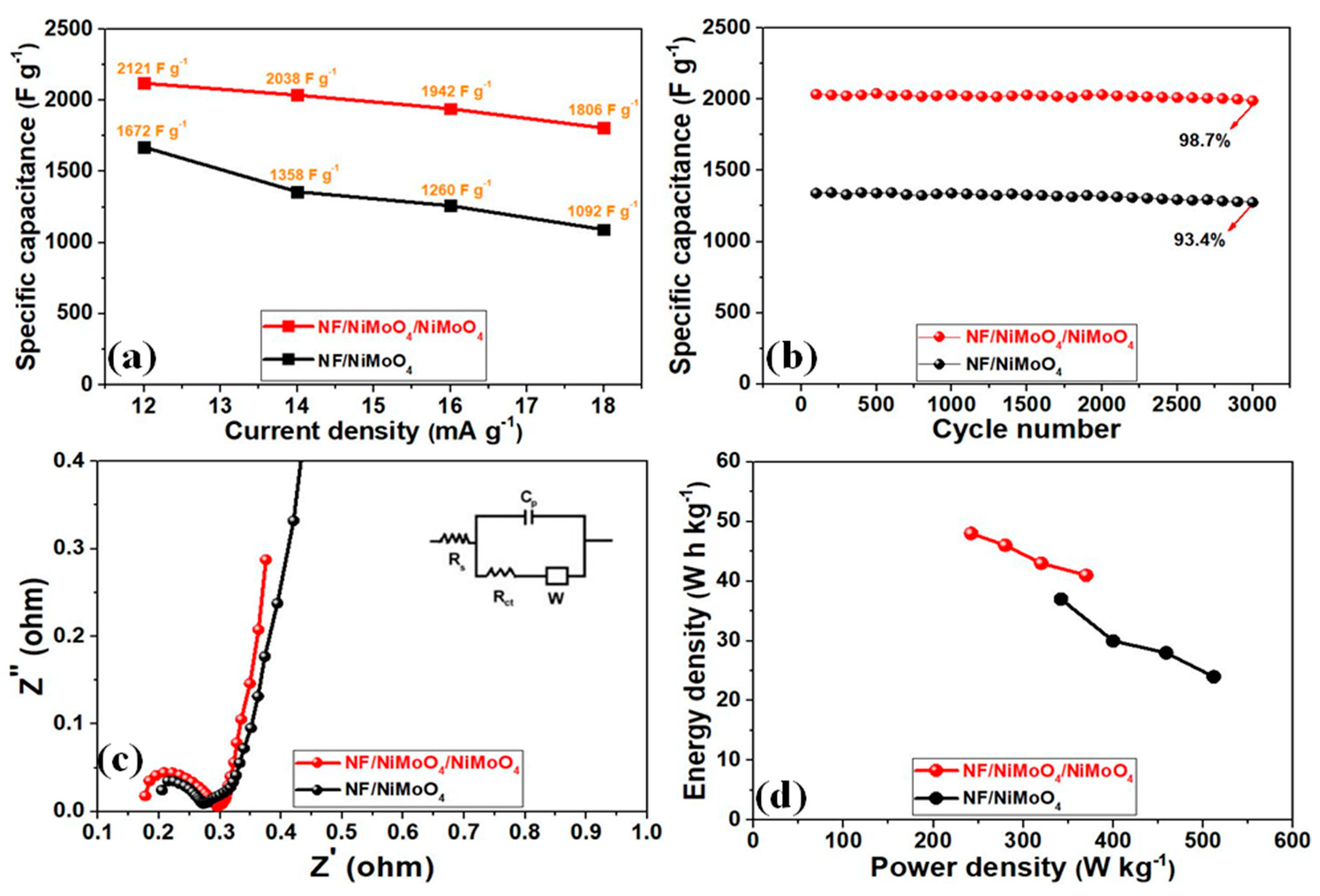
3. Results and Discussion
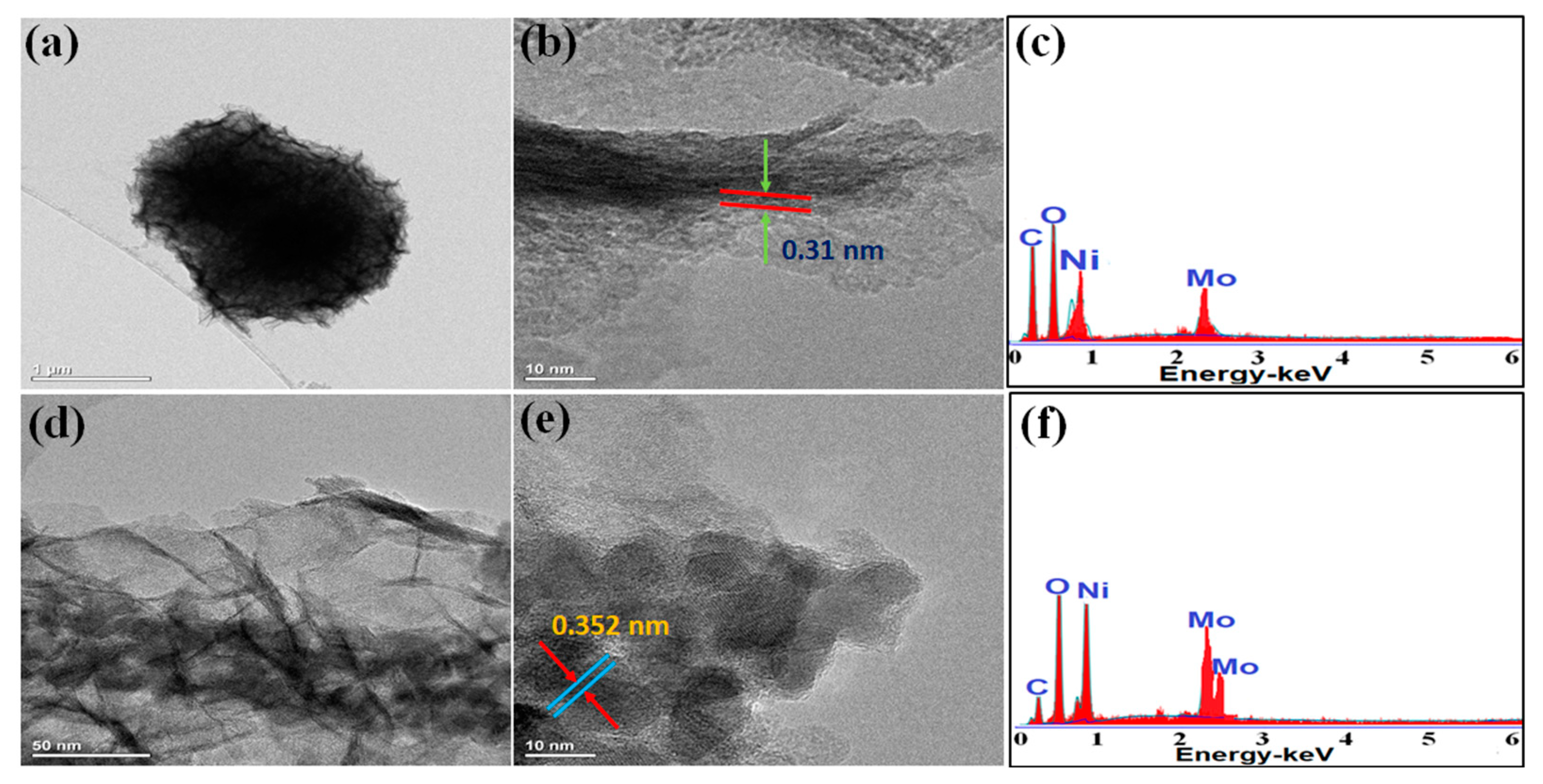
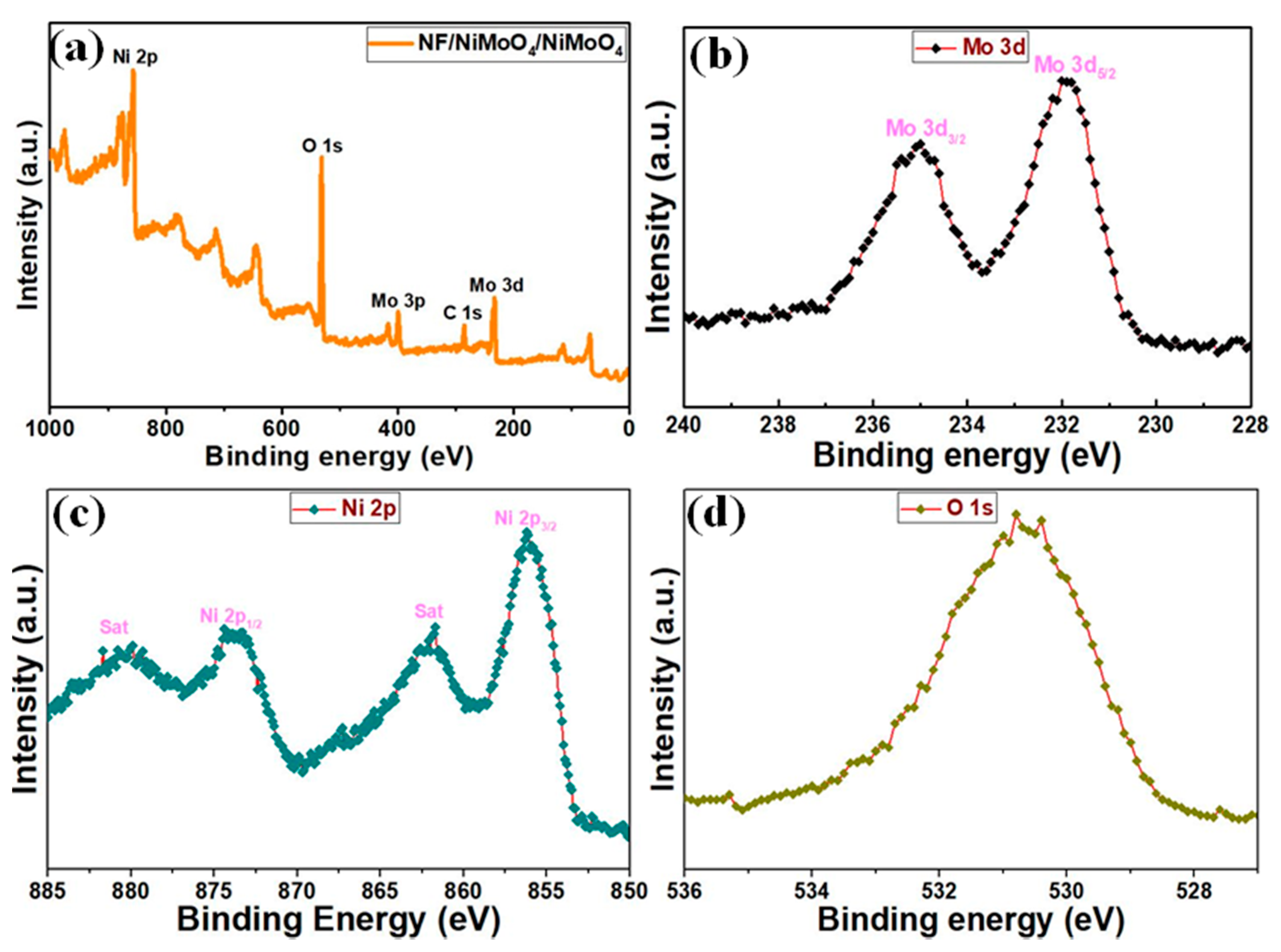
Preparation, Morphology, and Characterization of NF/NiMoO4 and NF/NiMoO4/NiMoO4
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, Z.C.; Xin, Y.Q.; Jia, H.L.; Wang, Z.; Sun, J.; Zhou, Q. Rational design of coaxial MWCNT-COOH@NiCo2S4 hybrid for supercapacitors. J. Mater. Sci. 2017, 52, 9661–9672. [Google Scholar] [CrossRef]
- Qin, G.; Zhang, H.Y.; Liao, H.Y.; Li, Z.H.; Tian, J.Y.; Lin, Y.X. Novel graphene nanosheet-wrapped polyaniline rectangular-like nanotubes for flexible all-solid-state supercapacitors. J. Mater. Sci. 2017, 52, 10981–10992. [Google Scholar] [CrossRef]
- Zhao, J.; Lai, H.; Lyu, Z.Y.; Jiang, Y.F.; Xie, K.; Wang, X.Z. Hydrophilic hierarchical nitrogen-doped carbon nanocages for ultrahigh supercapacitive performance. Adv. Mater. 2015, 27, 3541–3545. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, H.K.; Baek, E.; Pecht, M.; Lee, S.H.; Lee, Y.H. Improved performance of cylindrical hybrid supercapacitor using activated carbon/niobium doped hydrogen titanate. J. Power Sources 2016, 301, 348–354. [Google Scholar] [CrossRef]
- Fajardy, M.; Dowell, N.M. Can BECCS deliver sustainable and resource efficient negative emissions? Energy Environ. Sci. 2017, 10, 1389–1426. [Google Scholar] [CrossRef]
- Fares, R.L.; Webber, M.E. The impacts of storing solar energy in the home to reduce reliance on the utility. Nat. Energy 2017, 2, 17001. [Google Scholar] [CrossRef]
- Segura, E.; Morales, R.; Somolinos, J.A.; López, A. Techno-economics challenges of tidal energy conversion system: Current status and trends. Renew. Sustain. Energy Rev. 2017, 77, 536–550. [Google Scholar] [CrossRef]
- Wiser, R.; Jenni, K.; Seel, J.; Baker, E.; Hand, M.; Lantz, E.; Smith, A. Expert elicitations survey on future wind energy costs. Nat. Energy 2016, 1, 16135. [Google Scholar] [CrossRef]
- Yedluri, A.K.; Kim, H.-J. Wearable super-high specific performance supercapacitors using a honeycomb with folded silk-like composite of NiCo2O4 nanoplates decorated with NiMoO4 honeycombs on nickel foam. Dalton Trans. 2018, 47, 15545–15554. [Google Scholar] [CrossRef]
- Yedluri, A.K.; Kim, H.-J. Preparation and electrochemical performance of NiCo2O4@NiCo2O4 composite nanoplates for high performance supercapacitor applications. New J. Chem. 2018, 42, 19971–19978. [Google Scholar]
- Miller, J.R.; Simon, P. Electrochemical capacitors for energy management. Science 2008, 321, 651–652. [Google Scholar] [CrossRef]
- Bai, M.H.; Bian, L.J.; Song, Y.; Liu, X.X. Electrochemical code position of vanadium oxide and polypyrrole for high-performance supercapacitor with high working voltage. ACS Appl. Mater. Interface 2014, 6, 12656–12664. [Google Scholar] [CrossRef] [PubMed]
- Kumar, Y.A.; Rao, S.S.; Punnoose, D.; Tulasivarma, C.V.; Gopi, C.V.V.M.; Prabakar, K.; Kim, H.-J. Influence of solvents in the preparation of cobalt sulfide for supercapacitors. R Soc. Open Sci. 2017, 4, 170427. [Google Scholar] [CrossRef]
- Lukatskaya, M.R.; Mashtalir, O.; Ren, C.E.; Agnese, Y.D.; Rozier, P.; Taberna, P.L.; Naguib, M.; Simon, P.; Barsoum, M.W.; Gogotsi, Y. Cation intercalation and high volumetric capacitance of two-dimensional titanium carbide. Science 2013, 341, 1502–1505. [Google Scholar] [CrossRef]
- Guo, D.; Zhang, P.; Zhang, H.; Yu, X.; Zhu, J.; Li, Q.; Wang, T. NiMoO4 nanowires supported on Ni foam as novel advanced electrodes for supercapacitors. J. Mater. Chem. A 2013, 1, 9024–9027. [Google Scholar] [CrossRef]
- Kumar, Y.A.; Kim, H.-J. Effect of time on hierarchical corn skeleton-like composite of CoO@ZnO as capacitive electrode material for high specific performance supercapacitors. Energies 2018, 11, 3285. [Google Scholar] [CrossRef]
- Yu, X.; Lu, B.; Xu, Z. Super long-life supercapacitors based on the construction of nanohoneycomb-like strongly coupled CoMoO4-3D graphene hybrid electrodes. Adv. Mater. 2014, 26, 1044–1051. [Google Scholar] [CrossRef]
- Wu, H.; Lou, Z.; Yang, H.; Shen, G. A flexible spiral-type supercapacitor based on ZnCo2O4 nanorod electrodes. Nanoscale 2015, 7, 1921–1926. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Deng, Y.; Hu, Z.; Liu, Y.; Yao, M.; Liu, P. Seaurchin-like hierarchical NiCo2O4@NiMoO4 core-shell nanomaterials for high performance supercapacitors. Phys. Chem. Chem. Phys. 2014, 16, 23451–23460. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Jiang, J.; Zhang, L.; Qi, T.; Xia, D.; Wan, H. Facilely synthesized porous NiCo2O4 flowerlike nanostructure for high-rate supercapacitors. J. Power Sources 2014, 248, 28–36. [Google Scholar] [CrossRef]
- Wei, T.Y.; Chen, C.H.; Chien, H.C.; Lu, S.Y.; Hu, C.C. A cost-effective supercapacitor material of ultrahigh specific capacitances: spinel nickel cobaltite aerogels from an epoxide-driven sol gel process. Adv. Mater. 2010, 22, 347–351. [Google Scholar] [CrossRef] [PubMed]
- Cai, D.; Liu, B.; Wang, D.; Liu, Y.; Wang, L.; Li, H.; Wang, Y.; Wang, C.; Li, Q.; Wang, T. Enhanced performance of supercapacitors with ultrathin mesoporous NiMoO4 nanosheets. Electrochim. Acta 2014, 125, 294–301. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, B.; Zhai, Z.; Liu, X.; Yang, B.; Liu, L.; Jiang, X. A general strategy toward the rational synthesis of metal tungstate nanostructures using plasma electrolytic oxidation method. Appl. Surf. Sci. 2015, 356, 273–281. [Google Scholar] [CrossRef]
- Zhang, G.; Lou, X.W. Controlled Growth of NiCo2O4 Nanorods and Ultrathin Nanosheets on Carbon Nanofibers for High-performance Supercapacitors. Sci. Rep. 2013, 3, 1470. [Google Scholar] [CrossRef] [PubMed]
- Senthilkumar, B.; Sankar, K.V.; Selvan, R.K.; Danielle, M.; Manickam, M. Nano α-NiMoO4 as a new electrode for electrochemical supercapacitors. RSC Adv. 2013, 3, 352. [Google Scholar] [CrossRef]
- Shen, L.; Che, Q.; Li, H.; Zhang, X. Mesoporous NiCo2O4 nanowire arrays grown on carbon textiles as binder-free flexible electrodes for energy storage. Adv. Funt. Mater. 2014, 24, 2630–2637. [Google Scholar] [CrossRef]
- Mai, L.-Q.; Yang, F.; Zhao, Y.-L.; Xu, X.; Xu, L.; Luo, Y.-Z. Hierarchical MnMoO4/CoMoO4 heterostructured nanowires with enhanced supercapacitor performance. Nat. Commun. 2011, 2, 381. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Xu, J.; Wang, L.; Guo, D.; Mao, M.; Ma, J.; Wang, T. Growth of NiCo2O4@MnMOO4 nanocolumn arrays with superior pseudocapacitor properties. ACS Appl. Mater. Interfaces 2016, 8, 8568–8575. [Google Scholar] [CrossRef]
- Cheng, J.; Lu, Y.; Qiu, K.; Yan, H.; Xu, J.; Han, L.; Liu, X.; Luo, J.; Kim, J.-K.; Luo, Y. Hierarchical core/shell NiCo2O4@NiCo2O4 nanocactus arrays with dual functionalities for high performance supercapacitors and Li-ion batteries. Sci. Rep. 2015, 5, 12099. [Google Scholar] [CrossRef]
- Xu, Z.W.; Li, Z.; Tan, X.H.; Holt, C.M.B.; Zhang, L.; Amirkhiz, B.S. Supercapacitive carbon nanotube – cobalt molybdate nanocomposites prepared via solvent free microwave synthesis. RSC Adv. 2012, 2, 2753–2755. [Google Scholar] [CrossRef]
- Gobal, F.; Faraji, M. Preparation and electrochemical performances of nanoporous/cracked cobalt oxide layer for supercapacitors. Appl. Phys. A 2014, 117, 2087–20194. [Google Scholar] [CrossRef]
- Meng, X.; Sun, H.; Zhu, J.; Bi, H.; Han, Q.; Liu, X.; Wang, X. Graphene-based cobalt sulfide composite hydrogel with enhanced electrochemical properties for supercapcitors. New J. Chem. 2016, 40, 2843–2849. [Google Scholar] [CrossRef]
- Purushothaman, K.K.; Cuba, M.; Muralidharan, G. Supercapacitor behavior of α-MnMoO4 nanorods on different electrolytes. Mater. Res. Bull. 2012, 47, 3348–3351. [Google Scholar] [CrossRef]
- Park, K.S.; Seo, S.D.; Shim, H.W.; Kim, D.W. Electrochemical performance of NixCo1-xMoO4(0≦x≦1) nanocire anodes for lithium-ion batteries. Nanoscale Res. Lett. 2012, 7, 35–41. [Google Scholar] [CrossRef]
- Xiao, W.; Chen, J.S.; Li, M.C.; Xu, R.; Lou, X.W. Synthesis, characterization, and lithium storage capability of AMoO4(A = Ni, Co) nanorods. Chem. Mater. 2010, 22, 746–754. [Google Scholar] [CrossRef]
- Chavan, H.S.; Hou, B.; Ahmed, A.T.A.; Jo, Y.; Cho, S.; Kim, J.; Pawar, S.M.; Cha, S.N.; Inamdar, A.I.; Im, H.; Kim, H. Nanoflake NiMoO4 based smart supercapacitor for intelligent power balance monitoring. Sol. Energy Mater. Sol. Cells 2018, 185, 166–173. [Google Scholar] [CrossRef]
- Yedluri, A.K.; Kim, H.-J. Enhanced electrochemical performance of nanoplate nickel cobaltite (NiCo2O4) supercapacitor applications. RSC Adv. 2019, 9, 1115. [Google Scholar] [CrossRef]
- Zhou, Y.; Song, J.; Li, H.; Feng, X.; Huang, Z.; Chen, S.; Ma, Y.; Wang, L.; Yan, X. The synthesis of shape-controlled α-MoO3/graphene nanocomposites for high performance supercapacitors. New J. Chem. 2015, 39, 8780–8786. [Google Scholar] [CrossRef]
- Lin, L.; Liu, T.; Liu, J.; Sun, R.; Hao, J.; Ji, K.; Wang, Z. Facile synthesis of groove-like NiMoO4 hollow nanorods for high-performance supercapacitors. Appl. Surface Sci. 2016, 360, 234–239. [Google Scholar] [CrossRef]
- Xia, X.F.; Lei, W.; Hao, Q.L. One-step synthesis of CoMoO4/graphene composites with enhanced electrochemical properties for supercapacitors. Electrochim. Acta 2013, 99, 253–261. [Google Scholar] [CrossRef]
- Yan, J.; Fan, Z.J.; Sun, W.; Ning, G.Q.; Wei, T.; Zhang, Q. Advanced asymmetric supercpacitors based on Ni(OH)2/graphene and porous graphene electrodes with high energy density. Adv. Funct. Mater. 2012, 22, 2632. [Google Scholar] [CrossRef]
- Roginskaya, Y.E.; Morozova, O.; Lubnin, E.; Ulitina, Y.E.; Lopukhova, G.; Trasatti, S. Characterization of bulk and surface composition of CoxNi1-xOy mixed oxides for electrocatalysis. Langmuir 1997, 13, 4621–4627. [Google Scholar] [CrossRef]
- Marco, J.; Gancedo, J.; Gracia, M.; Gautier, J.; RÍos, E.; Berry, F.J. Characterization of the nickel cobaltite, prepared by several methods: an XRD, XANES, and XPS study. Solid State Chem. 2000, 153, 74–81. [Google Scholar] [CrossRef]
- Yao, M.; Hu, Z.; Liu, Y.; Liu, P. Design and synthesis of hierarchical NiCo2S4@NiMoO4 core/shell nanospheres for high-performance supercapacitors. New J. Chem. 2015, 39, 8430. [Google Scholar] [CrossRef]
- Hong, W.; Wang, J.Q.; Gong, P.W.; Sun, J.F.; Niu, L.Y.; Yang, Z.G.; Wang, Z.F.; Yang, S.R. Rational construction of three dimensional hybrid Co3O4@NiMoO4 nanosheets array for energy storage application. J. Power Sources 2014, 270, 516. [Google Scholar] [CrossRef]
- Liu, M.C.; Kong, L.B.; Lu, C.; Ma, X.J.; Li, X.M.; Luo, Y.C.; Kang, L. Design and synthesis of CoMoO4-NiMoO4.xH2O bundles with improved electrochemical properties for supercapacitors. J. Mater. Chem. A 2013, 1, 1380. [Google Scholar] [CrossRef]
- Cai, D.P.; Wang, D.D.; Liu, B.; Wang, L.L.; Liu, Y.; Li, H.; Wang, Y.R.; Li, Q.H.; Wang, T.H. Three dimensional Co3O4@NiMoO4 core/shell nanowire arrays on Ni foam for electrochemical energy storage. ACS Appl. Mater. Interfaces 2014, 6, 5050–5055. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liu, Y.; Huang, Z.; Ren, L.; Qi, X.; Wei, X.; Zhong, J. Facile hydrothermal synthesis of NiMoO4@CoMoO4 hierarchical nanospheres for supercapacitor applications. J. Phys. Chem. Phys. 2015, 17, 20795–20804. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Zhang, W.; Xiang, J.; Xu, H.; Li, G.; Huang, Y. Hierarchical core-shell NiCo2O4@NiMoO4 nanowires grown on carbon cloth as integrated electrode for high-performance supercapacitors. Sci. Rep. 2016, 6, 31465. [Google Scholar] [CrossRef]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yedluri, A.K.; Anitha, T.; Kim, H.-J. Fabrication of Hierarchical NiMoO4/NiMoO4 Nanoflowers on Highly Conductive Flexible Nickel Foam Substrate as a Capacitive Electrode Material for Supercapacitors with Enhanced Electrochemical Performance. Energies 2019, 12, 1143. https://doi.org/10.3390/en12061143
Yedluri AK, Anitha T, Kim H-J. Fabrication of Hierarchical NiMoO4/NiMoO4 Nanoflowers on Highly Conductive Flexible Nickel Foam Substrate as a Capacitive Electrode Material for Supercapacitors with Enhanced Electrochemical Performance. Energies. 2019; 12(6):1143. https://doi.org/10.3390/en12061143
Chicago/Turabian StyleYedluri, Anil Kumar, Tarugu Anitha, and Hee-Je Kim. 2019. "Fabrication of Hierarchical NiMoO4/NiMoO4 Nanoflowers on Highly Conductive Flexible Nickel Foam Substrate as a Capacitive Electrode Material for Supercapacitors with Enhanced Electrochemical Performance" Energies 12, no. 6: 1143. https://doi.org/10.3390/en12061143
APA StyleYedluri, A. K., Anitha, T., & Kim, H.-J. (2019). Fabrication of Hierarchical NiMoO4/NiMoO4 Nanoflowers on Highly Conductive Flexible Nickel Foam Substrate as a Capacitive Electrode Material for Supercapacitors with Enhanced Electrochemical Performance. Energies, 12(6), 1143. https://doi.org/10.3390/en12061143






