Investigation of Laser Power Output and Its Effect on Raman Spectrum for Marine Metal Corrosion Cleaning
Abstract
:1. Introduction
2. Image Feature and Raman Spectrum Analysis Methodologies
2.1. Image Feature Analysis Methodology
2.2. Raman Spectrum Analysis Methodology
3. Experiments and Results
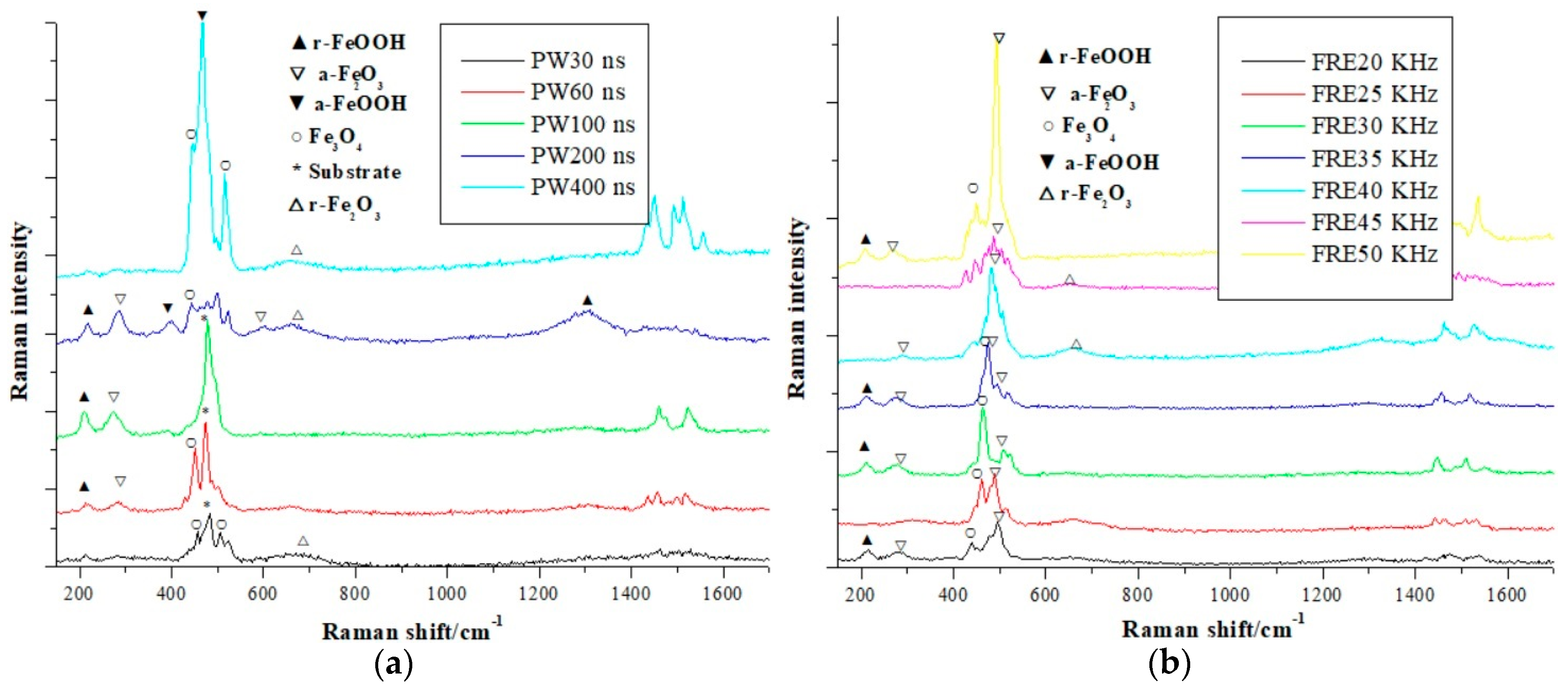
3.1. Experimental System and Data
3.2. Results of a Corrosive Degree Evaluation
3.3. Results of Raman Spectrum Data Processing
3.4. Results of Laser Power Output and its Effect on the Raman Spectrum
3.5. Discussions
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kumar, A.; Biswas, D.J. Particulate size and shape effects in laser cleaning of heavy metal oxide loose contamination off clad surface. Opt. Laser Technol. 2018, 106, 286–293. [Google Scholar] [CrossRef]
- See, T.L.; Metsios, I.; Qian, D.; Antar, M.; Marimuthu, S. Feasibility study and demonstration of cleaning with laser adaptively by novel use of sensors. Procedia CIRP 2018, 74, 376–380. [Google Scholar] [CrossRef]
- Senesi, G.S.; Allegretta, I.; Porfido, C.; Pascale, O.D.; Terzano, R. Application of micro X-ray fluorescence and micro computed tomography to the study of laser cleaning efficiency on limestone monuments covered by black crusts. Talanta 2018, 178, 419–425. [Google Scholar] [CrossRef]
- Liu, H.; Ma, Y.; Wang, W.; Yan, B. Non-destructive testing and assembly quality evaluation of IFOG optical path. J. Optoelectron Adv. Mater. 2019, 3–4, 171–179. [Google Scholar]
- Li, J.; Liu, H.; Wu, W.; Yang, S.; Sheng, L. Corrosion assessment of carbon steel using texture and color features. In Proceedings of the IEEE 3rd International Conference on Electronic Information Technology and Computer Engineering, Xiamen, China, 18–20 October 2019; pp. 799–803. [Google Scholar]
- Lee, W.-J.; Lee, S.-W. Improved spatiotemporal noise reduction for very low-light environments. IEEE T. Circuits-II 2016, 63, 888–892. [Google Scholar] [CrossRef]
- Li, Q.; Lu, H.; Cui, J.; An, M.; Li, D. Electrodeposition of nanocrystalline zinc on steel for enhanced resistance to corrosive wear. Surf. Coat. Technol. 2016, 304, 567–573. [Google Scholar] [CrossRef]
- Genna, S.; Lambiase, F.; Leone, C. Effect of laser cleaning in laser assisted joining of CFRP and PC sheets. Compos. Part B-Eng. 2018, 145, 206–214. [Google Scholar] [CrossRef]
- Zou, J.; Chen, W.; Wan, F.; Fan, Z.; Du, L. Raman spectral characteristics of oil-paper insulation and its application to ageing stage assessment of oil-immersed transformers. Energies 2016, 9, 946. [Google Scholar] [CrossRef] [Green Version]
- Beeskow-Strauch, B.; Schicks, J.M. The driving forces of guest substitution in gas hydrates—A laser Raman study on CH4-CO2 exchange in the presence of impurities. Energies 2012, 5, 420–437. [Google Scholar] [CrossRef] [Green Version]
- Uhlmann, E.; Pontes, R.P.; Laghmouchi, A.; Bergmann, A. Concept of sustainable data for a selective laser melting machine. Procedia Manuf. 2018, 21, 655–662. [Google Scholar] [CrossRef]
- Bonhoff, T.; Poppe, M.; Stollenwerk, J.; Schleifenbaum, J.H.; Loosen, P. Multi-physical analysis of thermos-optical effects for different selective laser melting (SLM) scanning strategies. Procedia CIRP 2018, 74, 97–101. [Google Scholar] [CrossRef]
- Ao, S.; Luo, Z.; Zhao, N.; Bu, X.; Li, Y. Microphone arrays for acoustic detection during laser welding of cold-rolled steel strip. In Proceedings of the International Conference on Intelligent System Design and Engineering Application, Changsha, China, 13–14 October 2010; pp. 935–939. [Google Scholar]
- Xiong, H.; Zhang, Y.; Chen, X.-W. Data-dependent kernel machines for microarray data classification. IEEE ACM Trans. Comput. Biol. Bioinforma. 2007, 4, 583–595. [Google Scholar] [CrossRef]
- Zhan, H.; Luo, Z.; He, Z.; Lu, K. Tamura coarseness for evaluating OTH radar image and its application in RFI suppression. In Proceedings of the IEEE Radar Conference, Boston, MA, USA, 22–26 April 2019; pp. 1–6. [Google Scholar]
- Yun, T.; Shu, H. Ultrasound image segmentation by spectral clustering algorithm based on the Curvelet and GLCM features. In Proceedings of the IEEE International Conference on Electrical and Control Engineering, Yichang, China, 16–18 September 2011; pp. 920–923. [Google Scholar]
- Asery, R.; Sunkaria, R.K.; Sharma, L.D.; Kumar, A. Fog detection using GLCM based features and SVM. In Proceedings of the Conference on Advances in Signal Processing, Pune, India, 9–11 June 2016; pp. 72–76. [Google Scholar]
- Umaselvi, M.; Kumar, S.S.; Athithya, M. Color based urban and agricultural land classification by GLCM texture features. In Proceedings of the IET Chennai 3rd International on Sustainable Energy and Intelligent Systems, Tiruchengode, India, 27–29 December 2012; pp. 1–4. [Google Scholar]
- Liang, X.; Li, L.; Cheng, G.; Gao, L. Underdeveloped village extraction from high spatial resolution optical image based on GLCM textures and fuzzy classification. In Proceedings of the 3rd International Workshop on Earth Observation and Remote Sensing Applications, Changsha, China, 11–14 June 2014; pp. 370–373. [Google Scholar]
- Yaraghi, N.; Tabesh, P.; Guan, P.; Zhuang, J. Comparison of AHP and Monte Carlo AHP under different levels of uncertainty. IEEE Trans. Eng. Manag. 2015, 62, 122–132. [Google Scholar] [CrossRef]
- Palomar, T.; Oujja, M.; Llorente, I.; Barat, B.R.; Canamares, M.V.; Cano, E.; Castillejo, M. Evaluation of laser cleaning for the restoration of tarnished silver artifacts. Appl. Surf. Sci. 2016, 387, 118–127. [Google Scholar] [CrossRef] [Green Version]
- Pelosi, C.; Calienno, L.; Fodaro, D.; Borrelli, E.; Rubino, A.R.; Sforzini, L.; Monaco, A.L. An integrated approach to the conservation of a wooden sculpture representing Saint Joseph by the workshop of Ignaz Gunther (1727-1775): Analysis, laser cleaning and 3D documentation. J. Cult. Herit. 2016, 17, 114–122. [Google Scholar] [CrossRef]
- Bloisia, F.; Baroneb, A.C.; Vicaria, L. Dry laser cleaning of mechanically thin films. Appl. Surf. Sci. 2004, 238, 121–124. [Google Scholar] [CrossRef]
- Sabour, M.; Dezvareh, G.; Bazzazzadeh, R. Corrosion prediction using the weight loss model in the sewer pipes made from sulfur and cement concretes and Response Surface Methodology (RSM). Constr. Build Mater. 2019, 199, 40–49. [Google Scholar] [CrossRef]
- Liu, L.; Tan, E.; Cai, Z.; Zhen, Y.; Yin, X. An integrated coating inspection system for marine and offshore corrosion management. In Proceedings of the International Conference on Control, Automation, Robotics and Vision, Singapore, 18–21 November 2018; pp. 1531–1536. [Google Scholar]
- Schulze, H.G.; Foist, R.B.; Okuda, K.; Ivanov, A.; Turner, R.F.B. A small-window moving average-based fully automated baseline estimation method for Raman spectra. Appl. Spectrosc. 2012, 66, 757–764. [Google Scholar] [CrossRef]
- Schettino, B.M.; Duque, C.A.; Silveira, P.M. Current-transformer saturation detection using Savitzky-Golay filter. IEEE Trans. Power Deliv. 2016, 31, 1400–1401. [Google Scholar] [CrossRef]
- Gu, Z.; Chen, W.; Du, L.; Zou, J.; Long, Z. Analysis of methyl ethyl ketone dissolved in transformer oil using laser Raman spectroscopy. In Proceedings of the IEEE International Conference on High Voltage Engineering and Application, Chengdu, China, 19–22 September 2016; pp. 1–4. [Google Scholar]
- Liu, H.; Lu, H. IQ evaluation based adaptive wavelet denoising and enhancement for a VRTAN system. In Proceedings of the IEEE/RSJ International Conference on Intelligent Robots and Systems, Nice, France, 22–26 September 2008; pp. 594–599. [Google Scholar]
- Du, G.; Bi, S.; Xiao, Y.; Li, W. The compliance control study of Chinese chess robot in Cartesian coordinate system. In Proceedings of the International Conference on Advanced Mechatronic Systems, Luoyang, China, 25–27 September 2013; pp. 31–35. [Google Scholar]
- Yang, C.; Hu, H.; Zhang, H. Modeling AOD-driven laser microvia drilling with machine learning approaches. J. Manuf. Process. 2018, 34, 555–565. [Google Scholar] [CrossRef]
- Li, J.; Liu, J.; Jin, R.; Tang, Y.; Yue, S.; Wang, L.; Long, X.; Zhang, G.; Meng, Q.; Li, R. Quantitative measurement of artemisinin content in Chinese traditional compound medicine by Raman spectroscopy. Spectrosc. Spect. Anal. 2019, 39, 2403–2408. [Google Scholar]
- Faria, D.L.A.D.; Silva, S.V.; Oliveira, M.T.D. Raman microspectroscopy of some iron oxides and oxyhydroxides. J. Raman Spectrosc. 1997, 28, 873–878. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, D. Corrosion behavior of Q235 carbon steel in air-saturated seawater containing Thalassospira sp. Corros. Sci. 2019, 148, 71–82. [Google Scholar] [CrossRef]
- Wang, X.; Melchers, R.E. Corrosion of carbon steel in presence of mixed deposits under stagnant seawater conditions. J. Loss Prevent. Proc. 2017, 45, 29–42. [Google Scholar] [CrossRef]
- Leng, X.; Yue, S.; Weng, Y.; Song, K.; Shi, Q. Effects of finite laser pulse width on two-dimensional electronic spectroscopy. Chem. Phys. Lett. 2017, 667, 79–86. [Google Scholar] [CrossRef]
- Bruncko, J.; Sutta, P.; Netrvalova, M.; Michalka, M.; Vincze, A. Pulsed laser deposition of Ga doped ZnO films—Influence of deposition temperature and laser pulse frequency on structural, optical and electrical properties. Vacuum 2019, 159, 134–140. [Google Scholar] [CrossRef]
- Tserevelakisa, G.J.; Pozo-Antonioab, G.S.; Siozosa, P.; Rivasb, T.; Poulia, P.; Zacharakisa, G. On-line photoacoustic monitoring of laser cleaning on stone: Evaluation of cleaning effectiveness and detection of potential damage to the substrate. J. Cult. Herit. 2019, 35, 108–115. [Google Scholar] [CrossRef]
- Pan, A.; Chiussi, S.; González, P.; Serra, J.; León, B. Comparative evaluation of UV–vis–IR Nd:YAG laser cleaning of beeswax layers on granite substrates. Appl. Surf. Sci. 2011, 257, 5484–5490. [Google Scholar] [CrossRef]
- Marimuthu, S.; Mhich, A.; Molchan, I.S.; Whitehead, D.; Wang, Z.; Mativenga, P.; Li, L.; Liu, Z.; Grafton-Reed, C.; Cheetham, S.; et al. Numerical simulation of excimer laser cleaning of film and particle contaminants. J. Heat Transf. 2013, 135, 121301-1–121301-12. [Google Scholar] [CrossRef]
- Lei, Z.; Sun, H.; Chen, Y.; Tian, Z. Elimination of rusting layer from high-strength steel surface using different laser cleaning methods. Chin. J. Lasers 2019, 46, 0702003-1–0702003-6. [Google Scholar]
- Yue, L.; Wang, Z.; Li, L. Modeling and simulation of laser cleaning of tapered micro-slots with different temporal pulses. Opt. Laser Technol. 2013, 45, 533–539. [Google Scholar] [CrossRef]
- Pozo-Antonio, J.S.; Papanikolaou, A.; Philippidis, A.; Melessanaki, K.; Rivas, T.; Pouli, P. Cleaning of gypsum-rich black crusts on granite using a dual wavelength Q-Switched Nd:YAG laser. Constr. Build. Mater. 2019, 226, 721–733. [Google Scholar] [CrossRef]
- Striova, J.; Fontana, R.; Barucci, M.; Felici, A.; Marconi, E.; Pampaloni, E.; Raffaelli, M.; Riminesi, C. Optical devices provides unprecedented insights into the laser cleaning of calcium oxalate layers. Microchem. J. 2016, 124, 331–337. [Google Scholar] [CrossRef]
- Pouli, P.; Zafiropulos, V.; Balas, C.; Doganis, Y.; Galanos, A. Laser cleaning of inorganic encrustation on excavated objects: Evaluation of the cleaning result by means of multi-spectral imaging. J. Cult. Herit. 2003, 4, 338–342. [Google Scholar] [CrossRef]
- Shi, T.; Wang, C.; Mi, G.; Yana, F. A study of microstructure and mechanical properties of aluminum alloy using laser cleaning. J. Manuf. Process. 2019, 42, 60–66. [Google Scholar] [CrossRef]
- Chen, Y.; Yan, X.; Zhang, X.; Shi, X.; Ma, J. Surface-enhanced Raman spectroscopy quantitative analysis of polycyclic aromatic hydrocarbons based on supports vector machine algorithm. Chin. J. Lasers 2019, 46, 0311005-1–0311005-8. [Google Scholar] [CrossRef]
- Villa-Aleman, E.; Bridges, N.J.; Shehee, T.C.; Houk, A.L. Raman microspectroscopy of PuO2 particulate aggregates. J. Nucl. Mater. 2019, 515, 140–149. [Google Scholar] [CrossRef]









| C | Si | Mn | S | P | Fe |
|---|---|---|---|---|---|
| 0.14 | 0.13 | 0.44 | 0.031 | 0.015 | 0.244 |
| Corrosion Degree | Description | Image Example |
|---|---|---|
| A | The metal is covered by the oxide layer and almost no corrosion component can be found. |  |
| B | The corrosion has happened, parts of the oxide layer have fallen off, and some small corrosion blocks can be observed. |  |
| C | The oxide layer has fallen off and many big corrosion blocks can be observed. |  |
| D | The oxide layer has fallen off completely. The corrosion layer has covered the metal surface completely. |  |
| Intensity of Importance | Definition |
|---|---|
| 1 | Equally important |
| 3 | Weakly important |
| 5 | Essentially important |
| 7 | Very strongly important |
| 9 | Absolutely important |
| 2, 4, 6, 8 | Importance between the above odd numbers |
| Maximum Average Output Power (W) | Central Emission Wavelength (nm) | Emission Bandwidth (nm) | Pulsed Energy (mJ) |
| 190–210 | 1055–1075 | 5–10 | 10 |
| Pulse Repetition Rate (kHz) | Pulse Duration Time (ns) | Initial Output Beam Diameter (mm) | Beam Quality |
| 2–50 | 30–400 | 6–9 | 10–14 |
| Sensor Type | Sensor Size (μm) | Maximum Resolution | Focal Length (mm) | Frame Rate (Hz) | Working Wavelength |
|---|---|---|---|---|---|
| CMOS | 3.2 × 3.2 | 2048 × 1536 | 5.0 | 12 | Visible light waveband |
| Laser Type | Laser Wavelength (nm) | Spatial Resolution (μm) |
| Argon ion laser | 514.5 | X, Y coordinate directions~1 μm Z coordinate direction~ 2 μm |
| Spectral Range (cm−1) | Spectral Resolution (cm−1) | Spectral Repeatability (cm−1) |
| 100–4000 | 1 | ≤0.1 |
| Num | MT_Coa | M0G_Ent | MInt | M0G_Con | M0G_Prom | M0G_Hom |
|---|---|---|---|---|---|---|
| 1 | 2.12781 | 2.811652 | 106 | 0.593458 | 187.165814 | 0.774368 |
| 2 | 2.233168 | 2.583998 | 93 | 0.449418 | 678.798712 | 0.830039 |
| 3 | 2.270004 | 2.470134 | 80 | 0.387261 | 849.111131 | 0.843735 |
| 4 | 2.377367 | 2.649662 | 94 | 0.446554 | 880.062174 | 0.831126 |
| 5 | 2.236245 | 2.762098 | 99 | 0.426323 | 204.382459 | 0.810559 |
| 6 | 2.31929 | 2.758776 | 96 | 0.41641 | 212.443985 | 0.815336 |
| 7 | 2.227868 | 2.749216 | 100 | 0.415732 | 227.420047 | 0.815706 |
| 8 | 2.299521 | 2.740829 | 93 | 0.415828 | 247.127022 | 0.818207 |
| 9 | 2.336464 | 2.747704 | 101 | 0.380215 | 244.702209 | 0.827395 |
| 10 | 2.365239 | 2.72145 | 101 | 0.354979 | 243.449295 | 0.837478 |
| Criteria | Texture Information | Intensity Information | Patch Information |
|---|---|---|---|
| Texture information | 1 | 9 | 9 |
| Intensity information | 1/9 | 1 | 1/2 |
| Patch information | 1/9 | 2 | 1 |
| Alternatives | MT_Coa | MG_Ent | Alternatives | MInt | MG_Con | Alternatives | MG_Prom | MG_Hom |
|---|---|---|---|---|---|---|---|---|
| MT_Coa | 1 | 7 | MInt | 1 | 1/4 | MG_Prom | 1 | 3 |
| MG_Ent | 1/7 | 1 | MG_Con | 4 | 1 | MG_Hom | 1/3 | 1 |
| Name | MT_Coa | MG_Ent | MInt | MG_Con | MG_Prom | MG_Hom |
|---|---|---|---|---|---|---|
| Value | 0.7124 | 0.1018 | 0.0144 | 0.0574 | 0.0855 | 0.0285 |
| α-Fe2O3 | Fe3O4 | γ-Fe2O3 |
| 220, 293, 404, 495, 612 | 432, 513, 1322 | 517, 655, 1580 |
| γ-FeOOH | α-FeOOH | FeO |
| 216, 269, 380, 525, 1307 | 396, 483 | 595 |
| Corrosion Degree B | Corrosion Degree C | ||||
|---|---|---|---|---|---|
| Laser Energy Density (J/cm2) | Corrosion Component | Raman Shift (peak area) | Laser Energy Density (J/cm2) | Corrosion Component | Raman Shift (peak area) |
| 5.662 | α-FeOOH | 490.3 (11,518.88), 392.8 (2425.73) | 5.662 | α-FeOOH | 466.9 (12,997.08) |
| γ-FeOOH | 216.9 (7401.40), 520.1 (4482.71), 1301.1 (98.98) | γ-FeOOH | 212.2 (10,467.41) | ||
| α-Fe2O3 | 282.8 (8309.26) | α-Fe2O3 | 497.5 (38,790.21), 272.5 (13,488.55) | ||
| γ-Fe2O3 | 640.8 (5231.48) | γ-Fe2O3 | - | ||
| Fe3O4 | 1322.0 (3007.09) | Fe3O4 | 430.8 (1125.86) | ||
| FeO | - | FeO | - | ||
| 7.077 | α-FeOOH | - | 7.077 | α-FeOOH | 394.3 (1701.64) |
| γ-FeOOH | 208.0 (2185.95) | γ-FeOOH | 212.0 (3762.90) | ||
| α-Fe2O3 | 274.4 (2979.47) | α-Fe2O3 | 281.6 (5765.28) | ||
| γ-Fe2O3 | 476.7 (26211.11) | γ-Fe2O3 | - | ||
| Fe3O4 | 450.2 (2996.49) | Fe3O4 | 454.7 (4919.38), 505.938 (5360.09), 1322.0 (1641.25) | ||
| FeO | - | FeO | - | ||
| 8.493 | α-FeOOH | 392.8 (2639.24), 476.3 (1692.18) | 8.493 | α-FeOOH | - |
| γ-FeOOH | 217.0 (5715.80), 1310.2 (3222.05) | γ-FeOOH | 238.7 (1553.6) | ||
| α-Fe2O3 | 282.1 (9718.34), 502.326 (2209.62), 596.0 (1303.29) | α-Fe2O3 | 484.3 (32,360.16) | ||
| γ-Fe2O3 | 1580.0 (3343.43) | γ-Fe2O3 | 653.4 (2749.88) | ||
| Fe3O4 | 442.2 (2342.28) | Fe3O4 | - | ||
| FeO | - | FeO | - | ||
| 9.908 | α-FeOOH | 392.7 (2675.52), 470.7 (4229.27) | 9.908 | α-FeOOH | 475.7 (543.99) |
| γ-FeOOH | 213.5 (16,940.84), 1308.2 (6210.08) | γ-FeOOH | 217.7 (1848.46), 395.845 (544.35), 1304.3 (107.90) | ||
| α-Fe2O3 | 281.1 (9671.83) | α-Fe2O3 | 282.2 (1903.03) | ||
| γ-Fe2O3 | - | γ-Fe2O3 | - | ||
| Fe3O4 | 439.2 (854.79) | Fe3O4 | 511.8 (394.78) | ||
| FeO | - | FeO | 595.5 (148.63) | ||
| 11.323 | α-FeOOH | 473.7 (2564.50) | 11.323 | α-FeOOH | 475.5 (1191.03) |
| γ-FeOOH | 212.8 (7475.18), 271.9 (6789.98), 383.3 (2340.0) | γ-FeOOH | 213.1 (7357.98), 1294.3 (2154.61) | ||
| α-Fe2O3 | 287.0 (2735.15), 401.6 (329.61) | α-Fe2O3 | 276.9 (8337.21), 489.8 (3448.43) | ||
| γ-Fe2O3 | - | γ-Fe2O3 | - | ||
| Fe3O4 | 508.5 (1025.06), 1322.7 (869.99) | Fe3O4 | 516.8 (846.0) | ||
| FeO | - | FeO | 601.4 (247.48) | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Xue, Y.; Li, J.; Wu, W.; Lan, J. Investigation of Laser Power Output and Its Effect on Raman Spectrum for Marine Metal Corrosion Cleaning. Energies 2020, 13, 12. https://doi.org/10.3390/en13010012
Liu H, Xue Y, Li J, Wu W, Lan J. Investigation of Laser Power Output and Its Effect on Raman Spectrum for Marine Metal Corrosion Cleaning. Energies. 2020; 13(1):12. https://doi.org/10.3390/en13010012
Chicago/Turabian StyleLiu, Haoting, Yafei Xue, Jiacheng Li, Weijie Wu, and Jinhui Lan. 2020. "Investigation of Laser Power Output and Its Effect on Raman Spectrum for Marine Metal Corrosion Cleaning" Energies 13, no. 1: 12. https://doi.org/10.3390/en13010012





