Multi-Timescale-Based Partial Optimal Control of a Proton-Exchange Membrane Fuel Cell
Abstract
1. Introduction
- (i)
- (ii)
- Development of the control-oriented dynamic PEMFC transient model. The transient model is developed from basic physics principles, where double layer charge effect is taken into account as well as the input delays due to gas starvation during transients. The transient model is specifically developed and experimentally validated for Greenlight Innovation TP50 (www.greenlightinnovation.com) fuel cell attached to the Greenlight Innovation G60 testing station, currently operating at Villanova University research lab.
- Application of the multistage control technique to the developed transient model. This is a novel control technique recently developed by the authors, particularly suitable for multi-timescale systems.
2. Materials and Methods
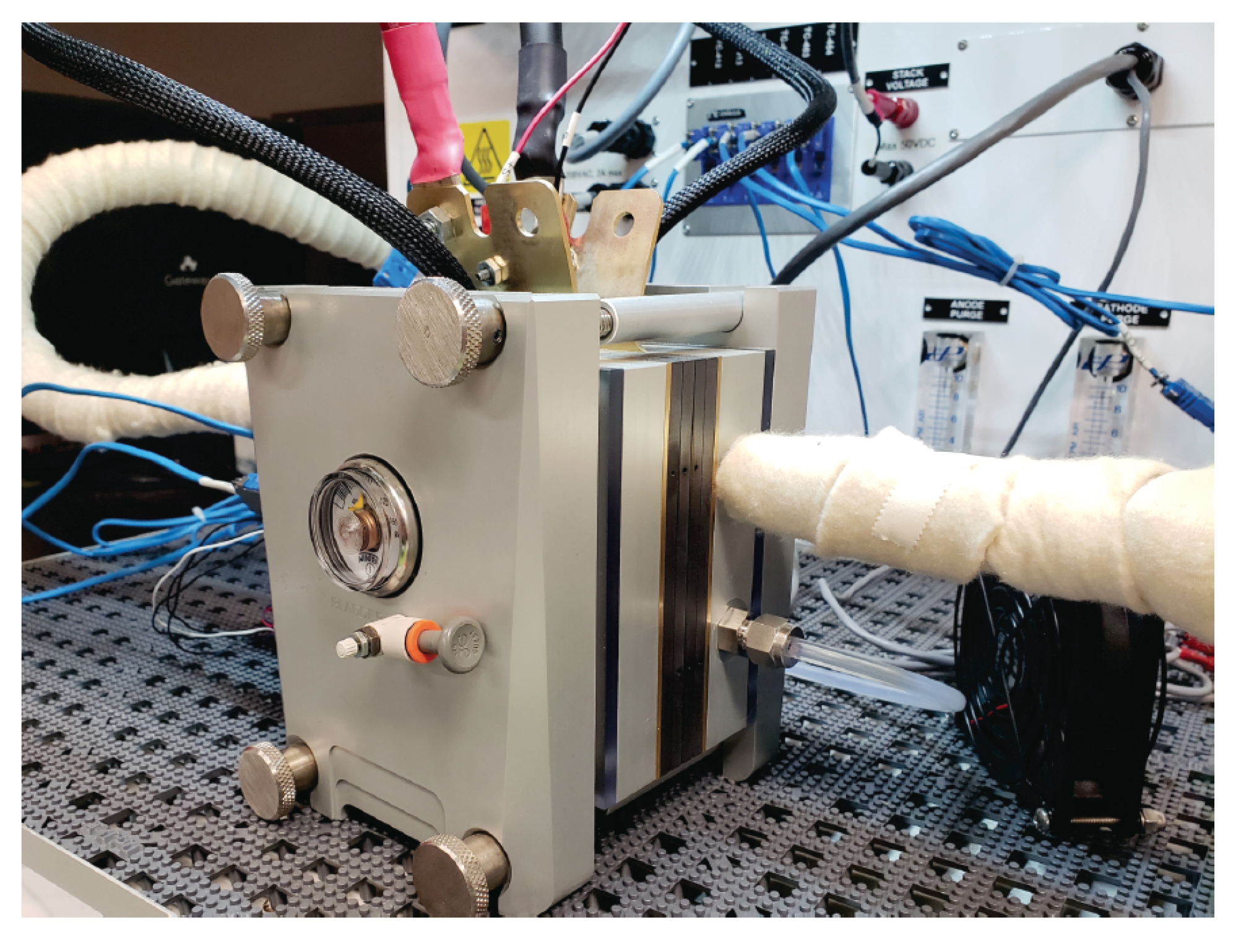
2.1. Experimental Setup
- Inlet pressure is maintained constant during transient periods.
- All gases present are assumed to be single phase gases.
- Flow velocity is the same on the cathode (CA) and anode (AN) sides at all points where the gas flow is considered laminar, despite the fact that on the anode side during low stoic and pure condition this assumption might be violated. Since this assumption is used only for pressure calculation, non-uniformity of the flow on the anode side can be neglected in the developed model.
- Oxidant, in this case air is consisted of only oxygen and nitrogen .
- Internal stack relative humidity never exceeds . In this model, liquid water content (known as flooding) is not considered.
- CA and AN volumes are calculated as single lumped volume.
- Stack temperature is constant.
2.2. Control-Oriented Transient PEMFC Model: Derivation
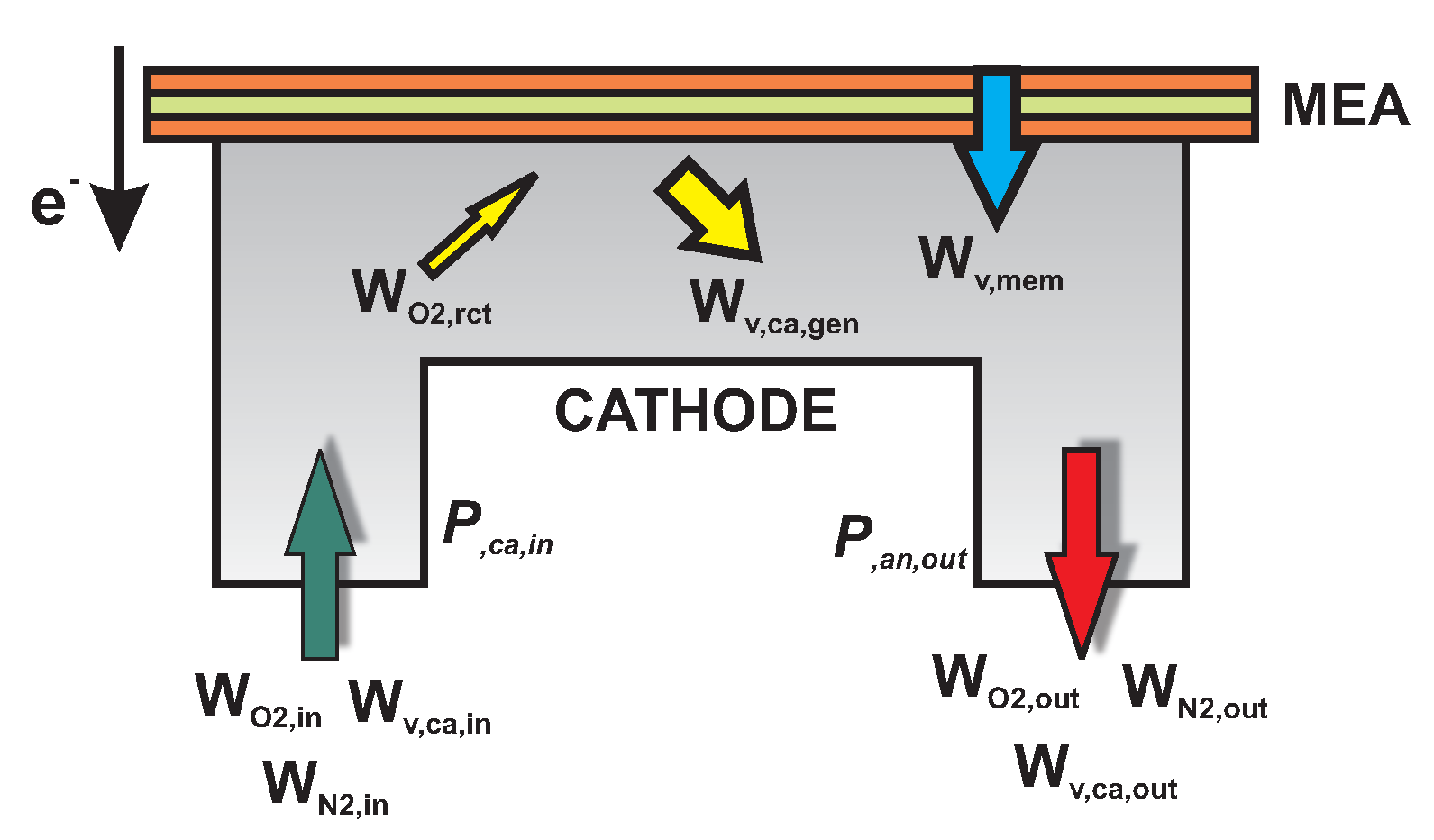
2.2.1. Cathode
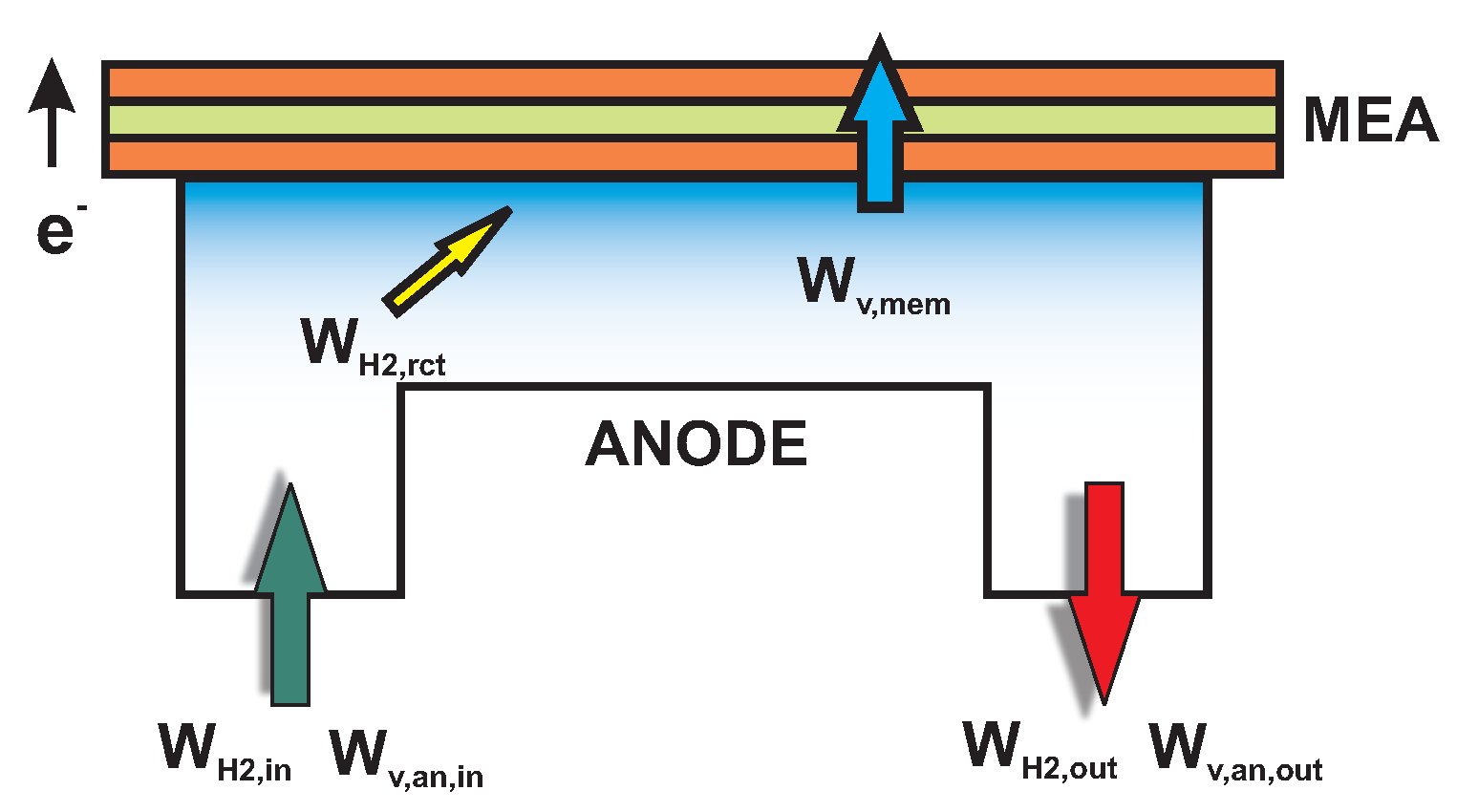
2.2.2. Anode
2.2.3. Outlet Pressures
- Friction losses. As flow is considered to be laminar at all times, this loss can be modeled by Darcy’s Law aswhere Darcy’s friction factor is given as , with Reynolds number and flow velocity , which depends on the inlet mass flow . In the previous expressions is fluid density, is fluid viscosity, is channel chord length, is hydraulic diameter of the rectangular flow channel, and stands for the channel cross sectional area.
- Channel bending losses. This pressure loss is a consequence of bends present in the serpentine channel pattern [47], dependable on flow velocitywhere are friction coefficients defined as (for a square duct) and (for bend angle) and (for laminar flow).
- Chemical reaction pressure losses dependable on flow velocity :
2.2.4. Stack Voltage
2.3. Multistage Control Technique
| Algorithm 1 Three stage technique implementation |
| input: Singularly perturbed matrices , , Equation (53) |
| output: Equivalent gain for feedback |
| solve for matrices (54); |
| solve for matrix (54); |
| set control gain law with very fast desired eigenvalues ; |
| solve for matrix (55); |
| set control gain law with fast desired eigenvalues ; |
| solve for matrix (55); |
| solve for matrix (55); |
| set control gain law with slow desired eigenvalues ; |
| set equivalent gain ; |
| → feedback control: ; |
- Matrix . If this cannot be satisfied then equation in (54) can be solved iteratively as a sequence of algebraic Sylvester like equationswith initial conditionCoefficient matrices have eigenvalues of different order and . Since there are no common eigenvalues a unique solution for exists [53]. Iteration algorithm for Sylvester like equation isConvergence rate of this algorithm is . After i iterations precision can be achieved [40].
- Matrices and . Those matrices are asymptotically stable, so they are always invertible [54]. Feedback controller gains and are chosen such that this is satisfied.
3. Results
3.1. Control-Oriented Transient PEMFC Model: Experimental Validation
3.2. Multistage Control Technique Application: Simulation Results and Analysis
Three Stage Control Technique
4. Discussion & Conclusions
4.1. Control-Oriented Transient PEMFC Model
- A complex physical system is represented by simple, but a realistic model, without overwhelming details. Following this modeling approach, scientist equipped with such a model can have full focus on designing a control strategies and controllers for the most dominant effects and study them theoretically and experimentally with less efforts.
- In this model setup stack current is treated as a measured disturbance. The stack current is the fastest physical variable and it can be used in various control designs, with accent on feedforward control approaches, which one possible future research direction.
- Since the inlet relative humidity is included in the model, it can be manipulated (controlled) to improve the water management in the stack, which is crucial for the correct operation of the fuel cell.
- The presented logic model can be easily expanded. Flexibility is important, because for example, if a researcher wants to focus on the analysis in the higher current densities operational range, one might want to consider adding extra states which represent liquid phase generated water.
- The system is in state space form, which enables us to perform controllability and observability analysis.
4.2. Multistage Control Technique Application
- Similarity transformations and preserve the controllability/observability property of the full system. If the original full-order system is controllable/observable then local systems will also be controllable/observable.
- The original full-order system is decoupled in several subsystems, each with the unique timescale. A composite feedback controller applied to the full-order system is constructed with different state feedback controllers: eigenvalue assignment, optimal, robust, etc.
- Computational costs are significantly reduced because all calculations are done with reduced order matrices where numerical ill-conditioning of higher order matrices is completely avoided. Instead, very high accuracy can be achieved by using iteration algorithms to solve nonlinear algebraic equations.
- Partial full-state feedback and partial output feedback (for subsystem for which only the output is available for feedback) can be implemented through this design. If certain subsystem does not need to be controlled, the gain for that subsystem is set to zero. The gains of the other subsystems are set accordingly and combined in the composite feedback matrix.
Author Contributions
Funding
Conflicts of Interest
Appendix A. Linearized State Space Matrices
Appendix A.1. Model Parameter Tables
| Parameter | Symbol | Value | Units |
|---|---|---|---|
| CA inlet pressure | 100 | kPag | |
| AN inlet pressure | 100 | kPag | |
| CA inlet temperature | 70 | C | |
| AN inlet temperature | 70 | C | |
| CA and AN endplate temperature | 80 | C |
| Symbol | Value |
|---|---|
| Symbol | Value |
|---|---|
| Constant | Symbol | Value | Units |
|---|---|---|---|
| Atmospheric pressure | 101325 | Pa | |
| Atmospheric temperature | 298.15 | K | |
| Air density | 1.23 | ||
| Universal gas constant | R | 8.314 | |
| Air gas constant | 286.9 | ||
| Oxygen gas constant | 259.8 | ||
| Nitrogen gas constant | 296.8 | ||
| Hydrogen gas constant | 4.124.3 | ||
| Water vapor gas constant | 461.5 | ||
| Air molar mass | |||
| Oxygen molar mass | |||
| Nitrogen molar mass | |||
| Hydrogen molar mass | |||
| Water vapor molar mass | |||
| Faraday’s constant | F | 96485 | |
| Number of cells in stack | n | 3 | - |
| Stack temperature | 353 | K | |
| CA volume | |||
| AN volume | |||
| CA orifice constant | |||
| AN orifice constant | |||
| Membrane dry density | |||
| Membrane dry weight | |||
| Membrane thickness | |||
| Oxygen mole fraction | - | ||
| CA channel chord length | m | ||
| AN channel chord length | m | ||
| Cross sectional area | m |
Appendix A.2. Lengthy Expressions in Three Stage Technique Derivation
References
- Alaswad, A.; Baroutaji, A.; Achour, H.; Carton, J.; Makky, A.A.; Olabi, A. Developments in fuel cell technologies in the transport sector. Int. J. Hydrog. Energy 2016, 41, 16499–16508. [Google Scholar] [CrossRef]
- Wilberforce, T.; Alaswad, A.; Palumbo, A.; Dassisti, M.; Olabi, A. Advances in stationary and portable fuel cell applications. Int. J. Hydrog. Energy 2016, 41, 16509–16522. [Google Scholar] [CrossRef]
- Huang, W.; Zhou, B.; Sobiesiak, A. Steady and unsteady modeling of single PEMFC with detailed thermoelectrochemical model. J. Electrochem. Soc. 2006, 153, A1945–A1954. [Google Scholar] [CrossRef]
- Shahsavari, S.; Desouza, A.; Bahrami, M.; Kjeang, E. Thermal analysis of air-cooled PEM fuel cells. Int. J. Hydrog. Energy 2012, 37, 18261–18271. [Google Scholar] [CrossRef]
- Afshari, E. Computational analysis of heat transfer in a PEM fuel cell with metal foam as a flow field. J. Therm. Anal. Calorim. 2019. [Google Scholar] [CrossRef]
- Ma, L.; Ingham, D.; Pourkashanian, M.; Carcadea, E. Review of the Computational Fluid Dynamics Modeling of Fuel Cells. ASME. J. Fuel Cell Sci. Technol. 2005, 2, 246–257. [Google Scholar] [CrossRef]
- Hu, G.; Fan, J. Transient computation fluid dynamics modeling of a single proton exchange membrane fuel cell with serpentine channel. J. Power Sour. 2007, 165, 171–184. [Google Scholar] [CrossRef]
- He, Y.; Chen, H.; Qu, B.; Zhang, T.; Pei, P.; Liang, C. Analysis of Proton Exchange Membrane Fuel Cell reactant gas dynamic response and distribution quality. Energy Proc. 2018, 152, 667–672. [Google Scholar] [CrossRef]
- Velisala, V.; Srinivasulu, G.N. Computational fluid dynamics study of 3-pass serpentine flow field configuration on proton exchange membrane fuel cell performance. Int. J. Ambient. Energy 2018, 1–6. [Google Scholar] [CrossRef]
- Kreuer, K.D.; Paddison, S.J.; Spohr, E.; Schuster, M. Transport in Proton Conductors for Fuel Cell Applications: Simulations, Elementary Reactions, and Phenomenology. Chem. Rev. 2004, 104, 4637–4678. [Google Scholar] [CrossRef]
- Wang, X.; Nguyen, T.V. Modeling the Effects of Capillary Property of Porous Media on the Performance of the Cathode of a PEMFC. J. Electrochem. Soc. 2008, 155, B1085–B1092. [Google Scholar] [CrossRef]
- Cho, J.; Neville, T.; Trogadas, P.; Bailey, J.; Shearing, P.; Brett, D.; Coppens, M.O. Capillaries for water management in polymer electrolyte membrane fuel cells. Int. J. Hydrog. Energy 2018, 43, 21949–21958. [Google Scholar] [CrossRef]
- Vargas, J.; Ordonez, J.; Bejan, A. Constructal PEM fuel cell stack design. Int. J. Heat Mass Transf. 2005, 48, 4410–4427. [Google Scholar] [CrossRef]
- Han, I.S.; Jeong, J.; Shin, H.K. PEM fuel-cell stack design for improved fuel utilization. Int. J. Hydrog. Energy 2013, 38, 11996–12006. [Google Scholar] [CrossRef]
- Baroutaji, A.; Carton, J.; Sajjia, M.; Olabi, A. Materials in PEM Fuel Cells. In Reference Module in Materials Science and Materials Engineering; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar] [CrossRef]
- Brunner, D.A.; Marcks, S.; Bajpai, M.; Prasad, A.K.; Advani, S.G. Design and characterization of an electronically controlled variable flow rate ejector for fuel cell applications. Int. J. Hydrog. Energy 2012, 37, 4457–4466. [Google Scholar] [CrossRef]
- Bavarian, M.; Soroush, M.; Kevrekidis, I.G.; Benziger, J.B. Mathematical modeling, steady-state and dynamic behavior, and control of fuel cells: A Review. Ind. Eng. Chem. Res. 2010, 49, 7922–7950. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, K.S.; Mishler, J.; Cho, S.C.; Adroher, X.C. A review of polymer electrolyte membrane fuel cells: Technology, applications, and needs on fundamental research. Appl. Energy 2011, 88, 981–1007. [Google Scholar] [CrossRef]
- Headley, A.; Yu, V.; Borduin, R.; Chen, D.; Li, W. Development and Experimental Validation of a Physics-Based PEM Fuel Cell Model for Cathode Humidity Control Design. IEEE/ASME Trans. Mechatron. 2016, 21, 1775–1782. [Google Scholar] [CrossRef]
- Zhu, M.; Xie, X.; Wu, K.; Najmi, A.U.H.; Jiao, K. Experimental investigation of the effect of membrane water content on PEM fuel cell cold start. Energy Proc. 2019, 158, 1724–1729. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, Z.; Liu, X.; Wu, W.T. Modeling of PEM fuel cell with thin MEA under low humidity operating condition. Appl. Energy 2019, 242, 1513–1527. [Google Scholar] [CrossRef]
- Hao, L.; Yu, H.; Hou, J.; Song, W.; Shao, Z.; Yi, B. Transient behavior of water generation in a proton exchange membrane fuel cell. J. Power Sour. 2008, 177, 404–411. [Google Scholar] [CrossRef]
- Jia, J.; Li, Q.; Wang, Y.; Cham, Y.T.; Han, M. Modeling and Dynamic Characteristic Simulation of a Proton Exchange Membrane Fuel Cell. IEEE Trans. Energy Convers. 2009, 24, 283–291. [Google Scholar] [CrossRef]
- Pei, P.; Yuan, X.; Gou, J.; Li, P. A new dynamic model for predicting transient phenomena in a PEM fuel cell system. Materials 2009, 2, 734–748. [Google Scholar] [CrossRef]
- Chen, H.; Jia, S.; Liu, B.; Zhang, T. Numerical Simulation of the Power Performance a Proton Exchange Membrane Fuel Cell Under Dynamic Loading Conditions. IEEE Access 2018, 6, 67992–68000. [Google Scholar] [CrossRef]
- Goshtasbi, A.; Chen, J.; Waldecker, J.R.; Hirano, S.; Ersal, T. On Parameterizing PEM Fuel Cell Models. In Proceedings of the American Control Conference, Philadelphia, PA, USA, 29 August 2019. [Google Scholar]
- de Bruijn, F.A.; Dam, V.A.T.; Janssen, G.J.M. Review: Durability and Degradation Issues of PEM Fuel Cell Components. Fuel Cells 2008, 8, 3–22. [Google Scholar] [CrossRef]
- Pei, P.; Chen, H. Main factors affecting the lifetime of Proton Exchange Membrane fuel cells in vehicle applications: A review. Appl. Energy 2014, 125, 60–75. [Google Scholar] [CrossRef]
- Qin, C.; Wang, J.; Yang, D.; Li, B.; Zhang, C. Proton Exchange Membrane Fuel Cell Reversal: A Review. Catalysts 2016, 6, 197. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, X.; Zhang, T.; Pei, P. The reactant starvation of the proton exchange membrane fuel cells for vehicular applications: A review. Energy Convers. Manag. 2019, 182, 282–298. [Google Scholar] [CrossRef]
- Dubau, L.; Castanheira, L.; Maillard, F.; Chatenet, M.; Lottin, O.; Maranzana, G.; Dillet, J.; Lamibrac, A.; Perrin, J.C.; Moukheiber, E.; et al. A review of PEM fuel cell durability: materials degradation, local heterogeneities of aging and possible mitigation strategies. Wiley Interdiscip. Rev. Energy Environ. 2014, 3, 540–560. [Google Scholar] [CrossRef]
- Liang, D.; Dou, M.; Hou, M.; Shen, Q.; Shao, Z.; Yi, B. Behavior of a unit proton exchange membrane fuel cell in a stack under fuel starvation. J. Power Sour. 2011, 196, 5595–5598. [Google Scholar] [CrossRef]
- Pei, P.; Yuan, X.; Chao, P.; Wang, X. Analysis on the PEM fuel cells after accelerated life experiment. Int. J. Hydrog. Energy 2010, 35, 3147–3151. [Google Scholar] [CrossRef]
- Daud, W.; Rosli, R.; Majlan, E.; Hamid, S.; Mohamed, R.; Husaini, T. PEM fuel cell system control: A review. Renew. Energy 2017, 113, 620–638. [Google Scholar] [CrossRef]
- Luna, J.; Usai, E.; Husar, A.; Serra, M. Enhancing the Efficiency and Lifetime of a Proton Exchange Membrane Fuel Cell Using Nonlinear Model-Predictive Control With Nonlinear Observation. IEEE Trans. Ind. Electron. 2017, 64, 6649–6659. [Google Scholar] [CrossRef]
- Milanovic, M.; Radisavljevic-Gajic, V. Optimal Linear-Quadratic Integral Feedback Controller Design with Disturbance Rejection form Proton Exchange Membrane Fuel Cell. In Proceedings of the Dynamic Systems and Control Conference, Atlanta, GA, USA, 3 October 2018. [Google Scholar]
- Zhou, D.; Gao, F.; Breaz, E.; Ravey, A.; Miraoui, A.; Zhang, K. Dynamic Phenomena Coupling Analysis and Modeling of Proton Exchange Membrane Fuel Cells. IEEE Trans. Energy Convers. 2016, 31, 1399–1412. [Google Scholar] [CrossRef]
- Radisavljevic-Gajic, V.; Milanovic, M. Three-Stage Feedback Controller Design With Applications to a Three Time-Scale System. In Proceedings of the Dynamic Systems and Control Conference, Minneapolis, MN, USA, 12 October 2016. [Google Scholar]
- Radisavljevic-Gajic, V.; Milanovic, M.; Clayton, G.M. Three-Stage Feedback Controller Design With Applications to Three Time-Scale Linear Control Systems. J. Dyn. Syst. Meas. Control. 2017, 139, 104502–104510. [Google Scholar] [CrossRef]
- Radisavljevic-Gajic, V.; Milanovic, M.; Rose, P. Multi-Stage and Multi-Time Scale Feedback Control of Linear Systems with Applications to Fuel Cells; Springer Nature: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]
- Benziger, J.; Chia, E.; Moxley, J.; Kevrekidis, I. The dynamic response of PEM fuel cells to changes in load. Chem. Eng. Sci. 2005, 60, 1743–1759. [Google Scholar] [CrossRef]
- Milanovic, M.; Rose, P.; Radisavljevic-Gajic, V.; Clayton, G.M. Five State Analytical Model of Proton Exchange Membrane Fuel Cell. In Proceedings of the Dynamic Systems and Control Conference, Tysons Corner, VA, USA, 11–13 October 2017. [Google Scholar]
- Wang, C.; Nehrir, M.H.; Shaw, S.R. Dynamic models and model validation for PEM fuel cells using electrical circuits. IEEE Trans. Energy Convers. 2005, 20, 442–451. [Google Scholar] [CrossRef]
- Purkushpan, J.T.; Stefanopoulou, A.G.; Peng, H. Control of Fuel Cell Power Systems, Principles, Modeling, Analysis and Feedback Design; Springer: London, UK, 2004. [Google Scholar]
- Springer, T.E.; Zawodzinski, T.A.; Gottesfeld, S. Polymer Electrolyte Fuel Cell Model. J. Electrochem. Soc. 1991, 138, 2334–2342. [Google Scholar] [CrossRef]
- Dutta, S.; Shimpalee, S.; Zee, J.V. Numerical prediction of mass-exchange between cathode and anode channels in a PEM fuel cell. Int. J. Heat Mass Transf. 2001, 44, 2029–2042. [Google Scholar] [CrossRef]
- Park, J.; Li, X. An experimental and numerical investigation on the cross flow through gas diffusion layer in a PEM fuel cell with a serpentine flow channel. J. Power Sour. 2007, 163, 853–863. [Google Scholar] [CrossRef]
- Larminie, J.; Dicks, A. Fuel Cell Systems Explained; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2003. [Google Scholar]
- O’Hayre, R.; Cha, S.W.; Colella, W.; Prinz, F.B. Fuel Cell Fundamentals; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Hinaje, M.; Raël, S.; Noiying, P.; Nguyen, D.A.; Davat, B. An Equivalent Electrical Circuit Model of Proton Exchange Membrane Fuel Cells Based on Mathematical Modelling. Energies 2012, 5, 2724–2744. [Google Scholar] [CrossRef]
- Kokotović, P.; Khalil, H.; O’Reilly, J. Singular Perturbation Methods in Control: Analysis and Design; Society for Industrial and Applied Mathematics: Philadelphia, PA, USA, 1999. [Google Scholar] [CrossRef]
- Naidu, D.S. Singular Perturbation Methodology in Control Systems; Peter Peregrinus Ltd.: London, UK, 1988. [Google Scholar]
- Chen, C.T. Linear System Theory and Design; Oxford University Press: Oxford, UK, 1999. [Google Scholar]
- Perov, A.I. Conditions for Invertibility and Hurwitz Stability. Funct. Anal. Its Appl. 2017, 51, 310–315. [Google Scholar] [CrossRef]
- Yoo, H.; Gajic, Z. New Designs of Linear Observers and Observer-Based Controllers for Singularly Perturbed Linear Systems. IEEE Trans. Autom. Control. 2018, 63, 3904–3911. [Google Scholar] [CrossRef]
- Munje, R.; Gu, D.; Desai, R.; Patre, B.; Zhang, W. Observer-Based Spatial Control of Advanced Heavy Water Reactor Using Time-Scale Decoupling. IEEE Trans. Nucl. Sci. 2018, 65, 2756–2766. [Google Scholar] [CrossRef]
- Radisavljevic-Gajic, V.; Rose, P. A new two-stage design of feedback controllers for a hydrogen gas reformer. Int. J. Hydrog. Energy 2014, 39, 11738–11748. [Google Scholar] [CrossRef]
- Radisavljević-Gajić, V.; Milanović, M.; Rose, P. Three-Stage Continuous-Time Feedback Controller Design. In Multi-Stage and Multi-Time Scale Feedback Control of Linear Systems with Applications to Fuel Cells; Springer: Cham, Switzerland, 2019; pp. 71–96. [Google Scholar] [CrossRef]
- Radisavljević-Gajić, V.; Milanović, M.; Rose, P. Four-Stage Continuous-Time Feedback Controller Design. In Multi-Stage and Multi-Time Scale Feedback Control of Linear Systems with Applications to Fuel Cells; Springer International Publishing: Cham, Switzerland, 2019; pp. 109–127. [Google Scholar] [CrossRef]
- Radisavljevic-Gajic, V.; Milanovic, M. Loss of Optimal Performance of the Finite-Horizon Continuous-Time Linear-Quadratic Controller Driven by a Reduced-Order Observer. J. Dyn. Syst. Meas. Control. 2018, 140, 061014. [Google Scholar] [CrossRef]










© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milanovic, M.; Radisavljevic-Gajic, V. Multi-Timescale-Based Partial Optimal Control of a Proton-Exchange Membrane Fuel Cell. Energies 2020, 13, 166. https://doi.org/10.3390/en13010166
Milanovic M, Radisavljevic-Gajic V. Multi-Timescale-Based Partial Optimal Control of a Proton-Exchange Membrane Fuel Cell. Energies. 2020; 13(1):166. https://doi.org/10.3390/en13010166
Chicago/Turabian StyleMilanovic, Milos, and Verica Radisavljevic-Gajic. 2020. "Multi-Timescale-Based Partial Optimal Control of a Proton-Exchange Membrane Fuel Cell" Energies 13, no. 1: 166. https://doi.org/10.3390/en13010166
APA StyleMilanovic, M., & Radisavljevic-Gajic, V. (2020). Multi-Timescale-Based Partial Optimal Control of a Proton-Exchange Membrane Fuel Cell. Energies, 13(1), 166. https://doi.org/10.3390/en13010166






