Winery Wastewater Treatment by Microalgae to Produce Low-Cost Biomass for Energy Production Purposes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microalgae Strains and Culture Conditions
2.2. Experimental Design
2.3. Biomass and Winery Wastewaters Characterization
2.4. Kinetic Parameters of Microalgae Growth
3. Results and Discussions
3.1. Winery Wastewater Characterization
3.2. Microalgal Biomass Growth Using Different Type and Concentration of Winery Wastewaters
3.3. Lipid Accumulation and Elemental Composition in Co-Culture Biomass
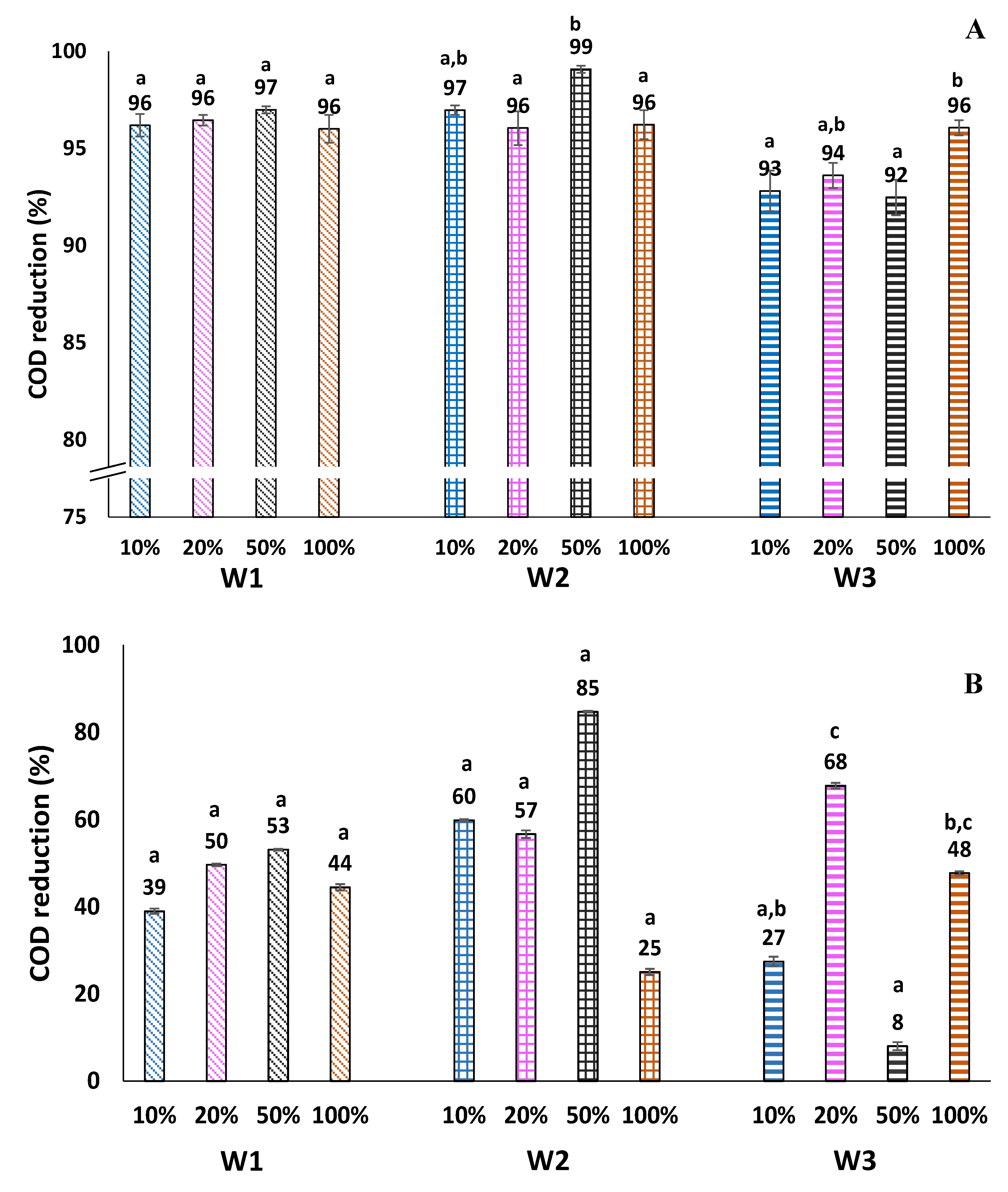
3.4. COD Removal from Winery Wastewater
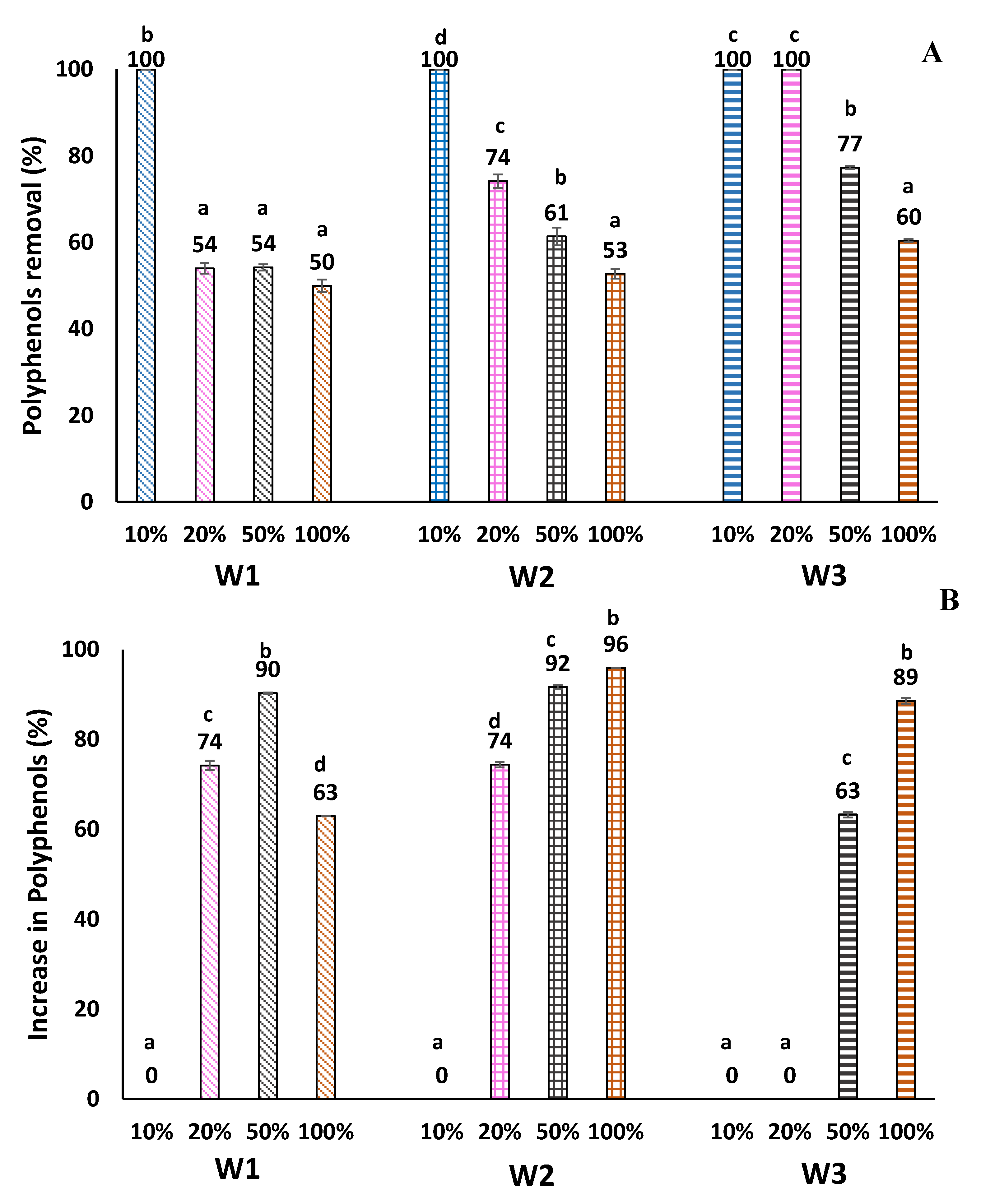
3.5. Polyphenols Removal
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lisý, M.; Lisá, H.; Jecha, D.; Baláš, M.; Križan, P. Characteristic properties of alternative biomass fuels. Energies 2020, 13, 1448. [Google Scholar] [CrossRef] [Green Version]
- Saad, M.G.; Dosoky, N.S.; Zoromba, M.S.; Shafik, H.M. Algal biofuels: Current status and key challenges. Energies 2019, 12, 1920. [Google Scholar] [CrossRef] [Green Version]
- Song, M.; Pei, H. The growth and lipid accumulation of Scenedesmus quadricauda during batch mixotrophic/heterotrophic cultivation using xylose as a carbon source. Bioresour. Technol. 2018, 263, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Suganya, T.; Varman, M.; Masjuki, H.H.; Renganathan, S. Macroalgae and microalgae as a potential source for commercial applications along with biofuels production: A biorefinery approach. Renew. Sustain. Energy Rev. 2016, 55, 909–941. [Google Scholar] [CrossRef]
- Da Silva Vaz, B.; Moreira, J.B.; de Morais, M.G.; Costa, J.A.V. Microalgae as a new source of bioactive compounds in food supplements. Curr. Opin. Food Sci. 2016, 7, 73–77. [Google Scholar] [CrossRef]
- Sathasivam, R.; Radhakrishnan, R.; Hashem, A.; Abd_Allah, E.F. Microalgae metabolites: A rich source for food and medicine. Saudi J. Biol. Sci. 2019, 26, 709–722. [Google Scholar] [CrossRef]
- Lam, M.K.; Lee, K.T. Microalgae biofuels: A critical review of issues, problems and the way forward. Biotechnol. Adv. 2012, 30, 673–690. [Google Scholar] [CrossRef]
- Hu, J.; Nagarajan, D.; Zhang, Q.; Chang, J.S.; Lee, D.J. Heterotrophic cultivation of microalgae for pigment production: A review. Biotechnol. Adv. 2018, 36, 54–67. [Google Scholar] [CrossRef]
- Zhou, W.; Wang, Z.; Xu, J.; Ma, L. Cultivation of microalgae Chlorella zofingiensis on municipal wastewater and biogas slurry towards bioenergy. J. Biosci. Bioeng. 2018, 126, 644–648. [Google Scholar] [CrossRef]
- Huy, M.; Kumar, G.; Kim, H.W.; Kim, S.H. Photoautotrophic cultivation of mixed microalgae consortia using various organic waste streams towards remediation and resource recovery. Bioresour. Technol. 2018, 247, 576–581. [Google Scholar] [CrossRef]
- Ioannou, L.A.; Puma, G.L.; Fatta-Kassinos, D. Treatment of winery wastewater by physicochemical, biological and advanced processes: A review. J. Hazard. Mater. 2015, 286, 343–368. [Google Scholar] [CrossRef] [PubMed]
- Gasol, C.M.; Rieradevall, J.; Ruggieri, L.; Cadena, E.; Martı, J.; Gabarrell, X.; Gea, T.; Sort, X.; Sa, A.; Valle, C. Recovery of organic wastes in the Spanish wine industry. Technical, economic and environmental analyses of the composting process. J. Clean. Prod. 2009, 17, 830–838. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.L.; Pratap, R. Advances in Biological Treatment of Industrial Waste Water and Their Recycling for a Sustainable Future; Springer: Berlin/Heidelberg, Germany, 2019; ISBN 978-981-13-1467-4. [Google Scholar]
- Mohana, S.; Acharya, B.K.; Madamwar, D. Distillery spent wash: Treatment technologies and potential applications. J. Hazard. Mater. 2009, 163, 12–25. [Google Scholar] [CrossRef] [PubMed]
- Federation, W.E. Standard Methods for the Examination of Water and Wastewater Part 1000 Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 1999. [Google Scholar]
- Ortiz Montoya, E.Y.; Casazza, A.A.; Aliakbarian, B.; Perego, P.; Converti, A.; De Carvalho, J.C.M. Production of Chlorella vulgaris as a source of essential fatty acids in a tubular photobioreactor continuously fed with air enriched with CO2 at different concentrations. Biotechnol. Prog. 2014, 30, 916–922. [Google Scholar] [CrossRef]
- Casazza, A.A.; Ferrari, P.F.; Aliakbarian, B.; Converti, A.; Perego, P. Effect of UV radiation or titanium dioxide on polyphenol and lipid contents of Arthrospira (Spirulina) platensis. Algal Res. 2015, 12, 308–315. [Google Scholar] [CrossRef]
- Brezoiu, A.; Matei, C.; Deaconu, M.; Stanciuc, A.; Trifan, A.; Gaspar-pintiliescu, A.; Berger, D. Polyphenols extract from grape pomace. Characterization and valorisation through encapsulation into mesoporous silica-type matrices. Food Chem. Toxicol. 2019, 133, 110787. [Google Scholar] [CrossRef]
- Heredia-arroyo, T.; Wei, W.; Ruan, R.; Hu, B. Mixotrophic cultivation of Chlorella vulgaris and its potential application for the oil accumulation from non-sugar materials. Biomass Bioenergy 2011, 5. [Google Scholar] [CrossRef]
- Andrade, M.R.; Costa, J.A.V. Mixotrophic cultivation of microalga Spirulina platensis using molasses as organic substrate. Aquaculture 2007, 264, 130–134. [Google Scholar] [CrossRef]
- Ganeshkumar, V.; Subashchandrabose, S.R.; Dharmarajan, R.; Venkateswarlu, K.; Naidu, R.; Megharaj, M. Use of mixed wastewaters from piggery and winery for nutrient removal and lipid production by Chlorella sp. MM3. Bioresour. Technol. 2018, 256, 254–258. [Google Scholar] [CrossRef]
- Sati, H.; Mitra, M.; Mishra, S.; Baredar, P. Microalgal lipid extraction strategies for biodiesel production: A review. Algal Res. 2019, 38, 101413. [Google Scholar] [CrossRef]
- Ferreira, G.F.; Ríos Pinto, L.F.; Maciel Filho, R.; Fregolente, L.V. A review on lipid production from microalgae: Association between cultivation using waste streams and fatty acid profiles. Renew. Sustain. Energy Rev. 2019, 109, 448–466. [Google Scholar] [CrossRef]
- Santana, H.; Cereijo, C.R.; Teles, V.C.; Nascimento, R.C.; Fernandes, M.S.; Brunale, P.; Campanha, R.C.; Soares, I.P.; Silva, F.C.P.; Sabaini, P.S.; et al. Microalgae cultivation in sugarcane vinasse: Selection, growth and biochemical characterization. Bioresour. Technol. 2017, 228, 133–140. [Google Scholar] [CrossRef]
- Kwak, H.S.; Kim, J.Y.H.; Woo, H.M.; Jin, E.S.; Min, B.K.; Sim, S.J. Synergistic effect of multiple stress conditions for improving microalgal lipid production. Algal Res. 2016, 19, 215–224. [Google Scholar] [CrossRef]
- Malandra, L.; Wolfaardt, G.; Zietsman, A.; Viljoen-Bloom, M. Microbiology of a biological contactor for winery wastewater treatment. Water Res. 2003, 37, 4125–4134. [Google Scholar] [CrossRef]
- Zhan, J.; Rong, J.; Wang, Q. Mixotrophic cultivation, a preferable microalgae cultivation mode for biomass/bioenergy production, and bioremediation, advances and prospect. Int. J. Hydrogen Energy 2016, 42, 8505–8517. [Google Scholar] [CrossRef]
- Torrijos, M.; Moletta, R. Winery wastewater depollution by sequencing batch reactor. Water Sci. Technol. 1997, 35, 249–257. [Google Scholar] [CrossRef]
- Lindner, A.V.; Pleissner, D. Utilization of phenolic compounds by microalgae. Algal Res. 2019, 42, 101602. [Google Scholar] [CrossRef]
- Cerniglia, C.E.; Gibson, D.T.; Van Baalen, C. Oxidation of naphthalene by cyanobacteria and microalgae. J. Gen. Microbiol. 1980, 116, 495–500. [Google Scholar] [CrossRef] [Green Version]
- Pinto, G.; Pollio, A.; Previtera, L.; Temussi, F. Biodegradation of phenols by microalgae. Biotechnol. Lett. 2002, 24, 2047–2051. [Google Scholar] [CrossRef]
- Papazi, A.; Ioannou, A.; Symeonidi, M.; Doulis, A.G.; Kotzabasis, K. Bioenergetic strategy of microalgae for the biodegradation of tyrosol and hydroxytyrosol. Z. Fur Nat. Sect. C J. Biosci. 2017, 72, 227–236. [Google Scholar] [CrossRef]
- Ferrarini, R.; Versari, A.; Galassi, S. A preliminary comparison between nanofiltration and reverse osmosis membranes for grape juice treatment. J. Food Process. Eng. 2001, 50, 113–116. [Google Scholar] [CrossRef]


| TS a (g/L) | TSS b (g/L) | pH | COD c (gO2/L) | PC d (mg GAE/L) | |
|---|---|---|---|---|---|
| W1 e | 13.15 ± 0.48 | 1.26 ± 0.02 | 3.42 | 116.30 ± 8.13 | 143.33 ± 0.13 |
| W2 f | 11.51 ± 0.24 | 0.39 ± 0.04 | 3.31 | 119.30 ± 1.06 | 139.72 ± 0.03 |
| W3 g | 4.69 ± 0.24 | 0.60 ± 0.05 | 3.82 | 36.90 ± 0.88 | 98.52 ± 0.23 |
| Concentration | 10% (v/v) | 20% (v/v) | 50% (v/v) | 100% (v/v) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wastewater | W1 | W2 | W3 | W1 | W2 | W3 | W1 | W2 | W3 | W1 | W2 | W3 | Control |
| Xfa (gDW/L) | 1.03 ± 0.37 | 1.85 ± 0.24 | 1.85 ± 0.15 | 0.67 ± 0.10 | 1.04 ± 0.41 | 2.07 ± 0.20 | 0.18 ± 0.10 | 0.84 ± 0.00 | 0.34 ± 0.00 | 0.40 ± 0.10 | 1.07 ± 0.24 | 0.03 ± 0.01 | 2.14 ± 0.06 |
| Xmaxb (gDW/L) | 1.03 ± 0.37 | 1.85 ± 0.024 | 1.85 ± 0.10 | 1.87 ± 0.32 | 2.63 ± 0.00 | 2.07 ± 0.20 | 1.66 ± 0.05 | 2.12 ± 0.01 | 0.72 ± 0.00 | 1.64 ± 0.01 | 2.07 ± 0.00 | 0.91 ± 0.10 | 2.04 ± 0.29 |
| µc (day−1) | 0.07 ± 0.02 | 0.12 ± 0.00 | 0.10 ± 0.00 | 0.01 ± 0.00 | 0.04 ± 0.03 | 0.08 ± 0.00 | 0.00 ± 0.00 | 0.08 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.05 ± 0.01 | 0.00 ± 0.00 | 0.09 ± 0.01 |
| µmaxd (day−1) | 0.07 ± 0.02 | 0.12 ± 0.00 | 0.10 ± 0.00 | 0.29 ± 0.01 | 0.39 ± 0.00 | 0.09 ± 0.00 | 0.48 ± 0.01 | 0.04 ± 0.02 | 0.03 ± 0.00 | 0.60 ± 0.00 | 0.17 ± 0.00 | 0.07 ± 0.01 | 0.10 ± 0.00 |
| νe (gDW/L day) | 0.07 ± 0.02 | 0.12 ± 0.016 | 0.11 ± 0.01 | 0.04 ± 0.00 | 0.49 ± 0.00 | 0.08 ± 0.01 | 0.00 ± 0.00 | 0.06 ± 0.00 | 0.01 ± 0.00 | 0.03 ± 0.00 | 0.07 ± 0.01 | 0.00 ± 0.00 | 0.14 ± 0.02 |
| νmaxf (gDW/L day) | 0.07 ± 0.02 | 0.12 ± 0.016 | 0.11 ± 0.01 | 0.47 ± 0.08 | 0.66 ± 0.03 | 0.15 ± 0.01 | 0.33 ± 0.01 | 0.42 ± 0.00 | 0.05 ± 0.00 | 0.82 ± 0.00 | 0.25± 0.01 | 0.11 ± 0.00 | 0.14 ± 0.01 |
| Concentration | Wastewater | CLa (gL/100 gDW) | νL b (gL/100 L·day) | νLmax c (g L/100 L·day) |
|---|---|---|---|---|
| 10% | W1 | 8.36 ± 1.70 | 0.29 ± 0.01 | 0.29 ± 0.01 |
| W2 | 12.61 ± 0.00 | 1.13 ± 0.01 | 1.13 ± 0.01 | |
| W3 | 12.31 ± 0.00 | 1.07 ± 0.02 | 1.07 ± 0.02 | |
| 20% | W1 | 10.03 ± 0.68 | 0.29 ± 0.00 | 3.43 ± 0.10 |
| W2 | 13.34 ± 3.00 | 0.11 ± 0.01 | 7.10 ± 0.22 | |
| W3 | 10.93 ± 0.02 | 1.14 ± 0.01 | 1.14 ± 0.01 | |
| 50% | W1 | 9.98 ± 0.04 | 0.00 ± 0.00 | 2.31 ± 0.09 |
| W2 | 19.66 ± 0.00 | 0.00 ± 0.00 | 6.37 ± 0.18 | |
| W3 | 21.95 ± 0.00 | 0.00 ± 0.00 | 2.41 ± 0.12 | |
| 100% | W1 | 7.37 ± 2.27 | 0.00 ± 0.00 | 2.80 ± 0.14 |
| W2 | 7.30 ± 0.00 | 0.00 ± 0.00 | 1.43 ± 0.13 | |
| W3 | 8.39 ± 2.02 | 0.00 ± 0.00 | 0.43 ± 0.07 | |
| Control | 10.54 ± 0.00 | 1.08 ± 0.09 | 1.08 ± 0.09 |
| Concentration | Wastewater | H | C | N | S |
|---|---|---|---|---|---|
| Control | 2.93 ± 0.43 b,c,d,e | 38.43 ± 1.08 a,b | 6.30 ± 0.37 a,b,c | 0.00 ± 0.37 a | |
| 10% (v/v) | W1 | 4.78 ± 0.43 d,e,f | 40.60 ± 1.08 a,b | 6.40 ± 0.37 a,b,c | 0.49 ± 0.49 a |
| W2 | 5.48 ± 0.56 e,f | 43.20 ± 1.12 a,b | 6.84 ± 0.17 b,c | 0.00 ± 0.00a | |
| W3 | 6.40 ± 0.39 f | 42.50 ± 3.64 a,b | 7.29 ± 0.22 a,b,c | 0.08 ± 0.09 a | |
| 20% (v/v) | W1 | 4.21 ± 0.53 d,e,f | 39.65 ± 3.74 a,b | 6.20 ± 0.3 a,b,c | 0.04 ± 0.05 a |
| W2 | 4.14 ± 0.25 c,d,e,f | 40.40 ± 1.42 a,b | 6.60 ± 0.33 a,b,c | 0.00 ± 0.00 a | |
| W3 | 3.32 ± 0.40b,c,d,e | 41.95 ± 1.02 a,b | 6.91 ± 0.68 b,c | 0.00 ± 0.00 a | |
| 50% (v/v) | W1 | 1.14 ± 0.07 a,b | 42.33 ± 1.12 a,b | 6.49 ± 0.14 a,b,c | 0.00 ± 0.00 a |
| W2 | 1.56 ± 0.24 a,b,c | 42.74 ± 1.12 a,b | 6.82 ± 0.25 b,c | 0.04 ± 0.00 a | |
| W3 | 2.84 ± 0.12 b,c,d,e | 36.58 ± 1.02 a | 5.38 ± 0.40 a | 0.00 ± 0.00 a | |
| 100% (v/v) | W1 | 1.04 ± 0.60 a,b | 45.05 ± 0.94 b | 5.79 ± 0.28 a,b | 0.00 ± 0.00 a |
| W2 | 2.62 ± 2.03 a,b,c,d | 39.67 ± 1.63 a,b | 6.24 ± 0.20 a,b,c | 0.00 ± 0.00 a | |
| W3 | 0.09 ± 0.13 a | 43.32 ± 3.25 a,b | 6.58 ± 0.68 a,b,c | 0.00 ± 0.00 a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spennati, E.; Casazza, A.A.; Converti, A. Winery Wastewater Treatment by Microalgae to Produce Low-Cost Biomass for Energy Production Purposes. Energies 2020, 13, 2490. https://doi.org/10.3390/en13102490
Spennati E, Casazza AA, Converti A. Winery Wastewater Treatment by Microalgae to Produce Low-Cost Biomass for Energy Production Purposes. Energies. 2020; 13(10):2490. https://doi.org/10.3390/en13102490
Chicago/Turabian StyleSpennati, Elena, Alessandro Alberto Casazza, and Attilio Converti. 2020. "Winery Wastewater Treatment by Microalgae to Produce Low-Cost Biomass for Energy Production Purposes" Energies 13, no. 10: 2490. https://doi.org/10.3390/en13102490







