1. Introduction
Adsorption heat transformation (AHT) technology [
1,
2,
3] is a promising way to utilize renewable and waste heat [
4,
5], which should replace fossil fuels in the immediate future. Closed AHT cycles operate by exchanging heat with the ambience. In essence, the heat of condensation/evaporation is rejected/absorbed to/from ambient thermal baths, such as air, soil, water reservoirs (sea, river, geothermal water, etc.). Therefore, the operating conditions of AHT cycles, first of all, the evaporator and condenser temperatures, are dictated by climatic conditions of the territory where AHT is used. The main AHT applications are cooling and air-conditioning [
1,
2], which are in demand in countries with warm or even hot climates. Water is basically used as a working fluid (adsorptive). It is ecologically sound and has a large evaporation enthalpy (Δ
He = 44.2 kJ/mol = 2.45 kJ/g at 20 °C [
6]). This allows a high first law efficiency (or coefficient of performance, COP) to be obtained [
3,
7] as well as promotes a large specific useful heat
Qus per cycle. The principal disadvantages of water as a working fluid are:
- (1)
A high freezing point of 0 °C. Ice formation in the evaporator/condenser at Tam < 0 °C is deemed to restrict the water application in AHT by the outdoor temperature above 0 °C;
- (2)
Relatively low vapour pressure (
Table 1) that can decelerate mass transport, which is determined by vapour pressure gradients in adsorbent bed or/and grain [
8]. An intelligent compromise between reasonable values of COP and specific cooling power (SCP) has to be reached.
If the ambient temperature
Tam < 0 °C, methanol [
10,
11] and ammonia [
12,
13] are suggested as working fluids as they have a much lower melting temperature and higher vapour pressure (
Table 1). The latter is advantageous for gaining high SCP at the expense of lower COP and
Qus due to a smaller evaporation heat of both methanol and ammonia. All these considerations are especially important for a novel AHT cycle “Heat from Cold” (HeCol), which has recently been proposed for upgrading the ambient heat during the wintertime in cold countries [
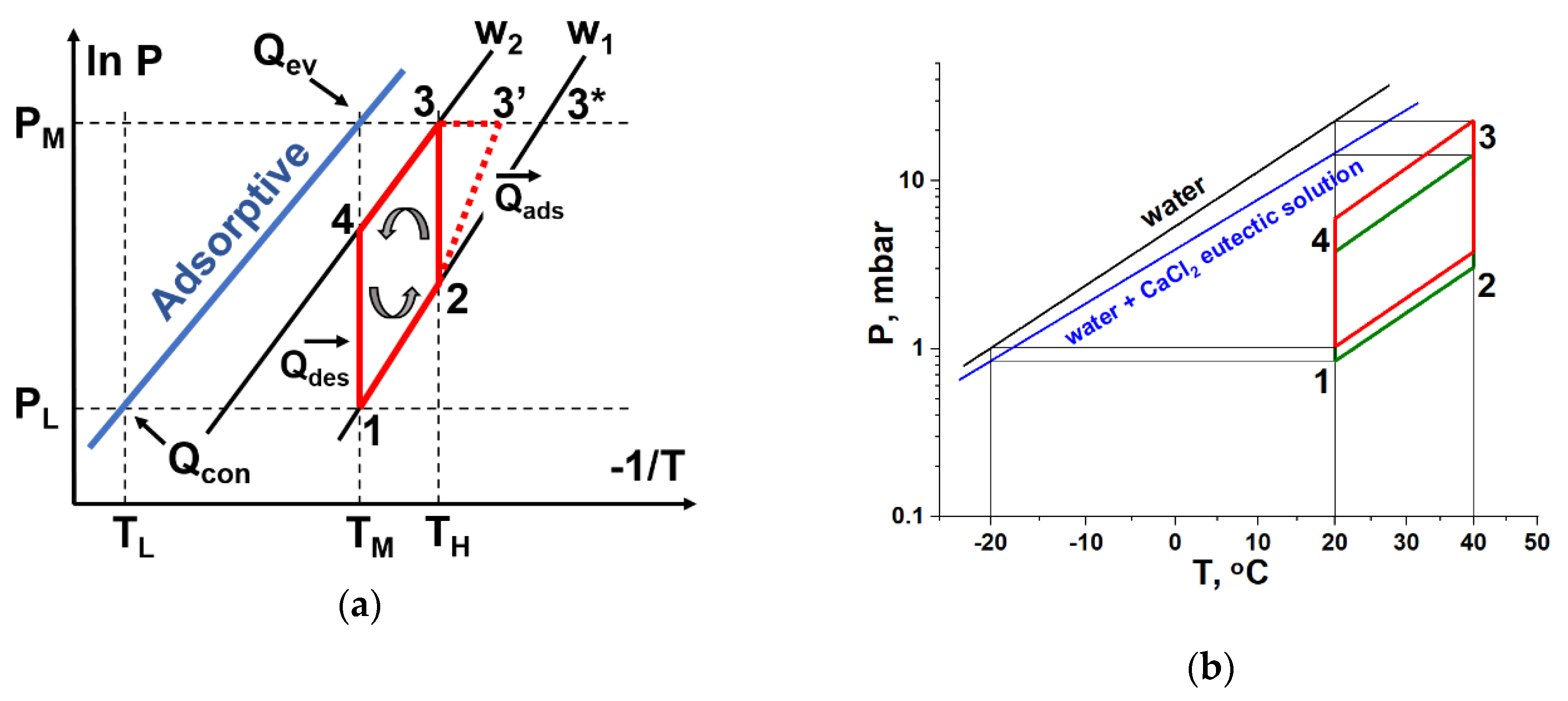
14]. A typical 3T HeCol cycle consists of two isosters and two isotherms (
Figure 1). The regeneration (or desorption) phase occurs due to a drop of vapour pressure over adsorbent at its low and constant temperature
TM (stage 4–1 in
Figure 1). The adsorption (or useful heat generation) phase is initiated by pressure jump at constant (2–3, isothermal cycle) or raising (2–3', non-isothermal cycle) temperature. The HeCol condenser is maintained at the ambient temperature
TL; the temperature of evaporator and adsorber is equal to the temperature of another natural thermal bath, namely a non-freezing water basin, such as a river, lake, sea, and underground water with
TM = (0–20 °C).
For the first theoretical [
15] and experimental [
16,
17] studies of the HeCol cycle, methanol was used as an adsorptive because of its low freezing temperature. These studies demonstrated that the proposed HeCol cycle is feasible and the generated useful heat can be suitable for heating low-energy buildings in countries with a cold climate (northern parts of Canada, Europe, Russia, the USA, etc.), as well as for the Arctic zone. However, the specific useful heat was found to be quite modest (300–400 J/g-adsorbent), which could be mostly due to the low evaporation heat of methanol, which is half of that for water (
Table 1). It leads to the enhanced size of HeCol units, which, in its turn, increases inert thermal masses [
18] of a HeCol unit and can further reduce the COP and the useful heat. Therefore, it is worthy of performing a thermodynamic evaluation of the HeCol useful heat, if water would be used as adsorptive, and compare it with that with methanol [
15,
17]. In this hypothetical analysis (
Section 2), we neglect ice formation in the condenser. This analysis shows that it is very attractive to use water vapour as adsorptive in the HeCol cycle; therefore, special measures should be made to avoid ice formation in the condenser. For instance, it was proposed in [
19] to mix pure water with ethylene glycol as an anti-freezing agent. The glycol remains in the evaporator and, hence, water is used as a refrigerant.
In this communication, we propose to use in the condenser and evaporator of the HeCol unit an aqueous salt solution instead of pure water. It is well-known that the freezing point of a solvent (water) is decreased upon the addition of a non-volatile solute (salt) [
20]. The depression of the freezing temperature depends on the salt nature and its concentration in solution. The lowest possible melting temperature corresponds to the eutectic mixing ratio of the salt and water. The eutectic temperature for different "salt–water" mixtures can vary in a wide range (
Table 2) to cover possible winter conditions. Further decline of the melting temperature can be reached by water [
21] or solution [
22] confinement to small pores of a host matrix. Since the salt is non-volatile, only water vapour is in the gas phase over the aqueous salt solution and serves as adsorptive.
The proposed substitution of pure water with the salt solution leads to lower vapour pressure over the salt solution (
Table 3) caused by interaction of water molecules with anions and cations of the salt dissociated in solution. The substitution can affect the cycle boundary pressures, the uptake variation, and a driving force for adsorption and desorption. These issues are considered theoretically in
Section 3. As for HeCol cycle based on water as adsorptive, sorption dynamics is of crucial importance, we have also performed an experimental measurement of water uptake/release curves under the reduced vapour pressure typical for a eutectic (CaCl
2 – H
2O) solution in the evaporator/condenser. The dynamic tests in
Section 4 have aimed to evaluate whether the ad/desorption rate under these severe conditions is acceptable for HeCol cycles.
2. Effect of Methanol Substitution with Water
Here, we evaluate how the substitution of methanol with water can affect the performances of the typical HeCol cycle in terms of the specific useful heat
Qus [J/g_adsorbent] generated for a consumer. It should be noted that the specific useful heat
Qus is equal for both isothermal and non-isothermal cycles. This heat is equal to the adsorption heat
Qads released at stage 2-3 minus the sensible heat
Qsen consumed at stage 1-2 related to the adsorbent mass
ma
where Δ
Hads is the specific adsorption heat [J/g_adsorbate], ∆
w is the specific adsorbate mass exchanged in the cycle [g_adsorbate/g_adsorbent],
C and
M are the overall specific heat capacity and the mass of inert components,
TH and
TM are the temperatures of adsorption and regeneration (
Figure 1a). In Equation (1), the specific heat of vapour in the gas phase is neglected as its mass is very small. The overall specific heat capacity
C and mass
M of inert components concern the adsorbent, adsorbate, and metal heat exchanger that can be called as an "adsorbent – heat exchanger" unit (AdHEx). As seen from Equation (1), there is a threshold exchange ∆
w* = [
C∙
M∙(
TH –
TM)] / (Δ
Hads∙
ma), at which the adsorption heat just compensates sensible heating of the AdHEx unit.
The specific useful heat is compared for water and methanol as adsorptive for the HeCol unit tested in [
17]. It consists of a plate-tube finned heat exchanger loaded with a composite sorbent LiCl/(silica gel), which is promising for sorbing both water [
26] and methanol [
27]. For this composite, Δ
Hads = (2.86 ± 0.25) kJ/g for water [
26] and (1.30 ± 0.08) kJ/g for methanol [
27]. A fined flat-tube HEx (Yamaha Aerox) made of aluminum with the dimensions 190 x 200 x 30 mm
3 (
MAl = 0.50 kg and
ma = 0.25 kg) [
17] is considered. Equation (1) can be re-written as:
where
ωLiCl = 0.21 is the mass fraction of the salt in the composite,
w1 is the initial adsorbate content (0.1 g/g and 0.01 g/g for water and methanol, respectively);
TH = 313 K and
TM = 293 K (
Figure 1b). The specific heat capacity
C of inert components is presented in
Table 4.
The useful heat of the HeCol cycle is generated only if ∆
w > ∆
w* = 0.023 and 0.044 g/g for water and methanol (
Figure 2). The heat linearly increases at larger adsorbate exchange ∆
w and would be much larger if water is used as adsorptive instead of methanol. Therefore, the use of salt solutions instead of pure water is deemed to lead to significantly increasing the HeCol useful heat.
3. Effect of Aqueous Salt Solution on the HeCol Cycle
Here, we compare how the substitution of pure water with a eutectic (CaCl
2 – H
2O) solution affects the cycle boundary pressures and its useful heat
Qus. The analysis is performed for the typical HeCol cycle with
TL = −20 °C,
TM = 20 °C, and
TH = 40 °C (
Figure 1b). The composites (21 wt.%) LiCl/ (silica gel) and (33 wt.%) CaCl
2/ (silica gel), which are advanced sorbents of both methanol and water vapour [
30], are considered here.
As the vapour–liquid (solid) equilibrium line for the solution lies below the line for water (ice), the proposed HeCol cycle is also shifted to lower pressure (
Figure 1b and
Table 5). It can be disadvantageous for ad/desorption dynamics; this issue is discussed in
Section 4. The equilibrium uptake reduces so that the specific exchange Δ
w in the cycle changes from Δ
w = 0.38 g/g to 0.32 g/g. The released adsorption heat
Qads = Δ
Hads∙
ma∙∆
w is consequently decreased. For the LiCl/silica, this reduction is acceptable as the specific useful heat
Qus = Δ
Hads∙
ma∙(∆
w – ∆
w*) = 850 J/(g_H
2O) is only slightly lower than that for pure water (1020 J/g). Thus, it remains much larger as compared with methanol as adsorptive (490 J/g), at least, for the HeCol prototype tested in [
16,
17]. Although for the CaCl
2/silica composite the uptake changes are significantly smaller (
Table 5), a similar relation between the useful heats is found: 710 J/g > 480 J/g > 350 J/g.
Thus, from the thermodynamic point of view, the usage of eutectic (CaCl
2 – H
2O) solution in both evaporator and condenser could essentially increase the specific useful heat generated in the HeCol cycle as compared with methanol. However, the water boundary pressures are more than ten times lower than those for methanol (
Table 5), and dynamic analysis is strictly necessary to realize whether the application of the salt solution is feasible. In this case, it seems difficult to make a relevant theoretical evaluation, which properly accounts for various heat and mass transfer resistances in this system. For this reason, we have performed an experimental dynamic study of water sorption and desorption under the reduced water vapour pressures.
5. Summary
A new cycle "Heat from Cold" (HeCol) has recently been proposed for upgrading the ambient heat in cold countries [
14]. The useful heat of this cycle essentially increases at lower temperature of the ambient air [
15], which is far below 0 °C. For this reason, methanol was used as a working fluid for the first theoretical and experimental investigations of the HeCol cycle. These studies confirmed that the cycle is feasible; however, its specific useful heat is restricted by 300–400 J/g_adsorbent [
17] due to the relatively low evaporation heat of methanol. This paper has aimed to analyze whether water, having much larger evaporation heat, can be applied as a working fluid for HeCol cycle.
First, a thermodynamic evaluation showed that the HeCol useful heat would be much larger if water is used as adsorptive instead of methanol (
Figure 2). This heat is generated only if the uptake change in the cycle is larger than a threshold value ∆
w*, at which the adsorption heat just compensates the sensible heating of the AdHEx unit. For the particular HeCol unit tested in [
16,
17], this value is estimated for water and methanol as ∆
w* = 0.023 and 0.044 g/g.
To use water as adsorptive, it is necessary to avoid ice formation in the condenser. Here, we propose to use in the condenser and evaporator of a HeCol unit an aqueous salt solution instead of pure water. For instance, the freezing point of a eutectic CaCl
2 solution is as low as −55.5 °C and the solution remains liquid above this temperature. The salt addition results in lower vapour pressure over the salt solution (
Table 3) so that the cycle boundary pressures (
Figure 1b and
Table 7) and the uptake variation (
Table 5) reduce appropriately. Despite this decrease, the specific useful heat still remains much larger as compared with methanol as adsorptive; e.g., 870 J/g versus 520 J/g for the HeCol prototype tested in [
16,
17]. Thus, the proposed substitution is very advantageous from the thermodynamic point of view.
The dynamic tests were performed to evaluate whether the adsorption and desorption rates under the reduced vapour pressure are acceptable for the HeCol cycle. The dynamics are studied by a volumetric LPJ set-up described in [
33] for three selected adsorbents under larger and smaller pressure drops Δ
P (
Table 7). The adsorption dynamics is quite fast and ensures the initial useful power as large as 11.3 ± 0.4 kW/kg (
Table 8). The driving force for water desorption is smaller than for adsorption; therefore, it is slower, and the initial power varies from 2.1 to 3.4 kW/g. However, the experimental desorption conversion can be smaller than the equilibrium one due to the large desorption heat (for the composite "CaCl
2 in mesoporous silica") and/or subtle driving force for desorption (for the silica Siogel). The composites "salt in porous matrix" [
30] are promising for the HeCol cycle from the thermodynamic point of view; however, an intelligent compromise between the useful heat and specific power has to be reached.
It is worth noting that a eutectic aqueous solution of CaCl2 provides a very low freezing point of −55.5 °C that can cover almost all possible winter conditions. For most of the cold countries, a higher freezing temperature is sufficient, e.g., −33.5 or −21.2 °C for eutectic aqueous solutions of MgCl2 or NaCl. In this case, the reduction of the water vapour pressure is smaller and leads to the enlargement of both uptake change Δw and driving force ΔP for desorption; hence, higher specific useful heat and power can be expected. Thus, a choice of the proper salt can be an effective tool to manage these basic outputs of the HeCol cycle.
In a broader sense, this approach can be extended to other AHT cycles working at the evaporator or condenser temperature below 0 °C. It is notable if a large adsorption heat is crucially important, as for adsorptive heat storage and, first of all, for its long-term (seasonal) version. For this application, the useful heat actually supplied to a consumer is dramatically lower than the theoretical value [
37]. This is due to the fact that the sensible heat of the storage unit is completely lost and has to be recovered.