Phytoremediation—From Environment Cleaning to Energy Generation—Current Status and Future Perspectives
Abstract
1. Introduction
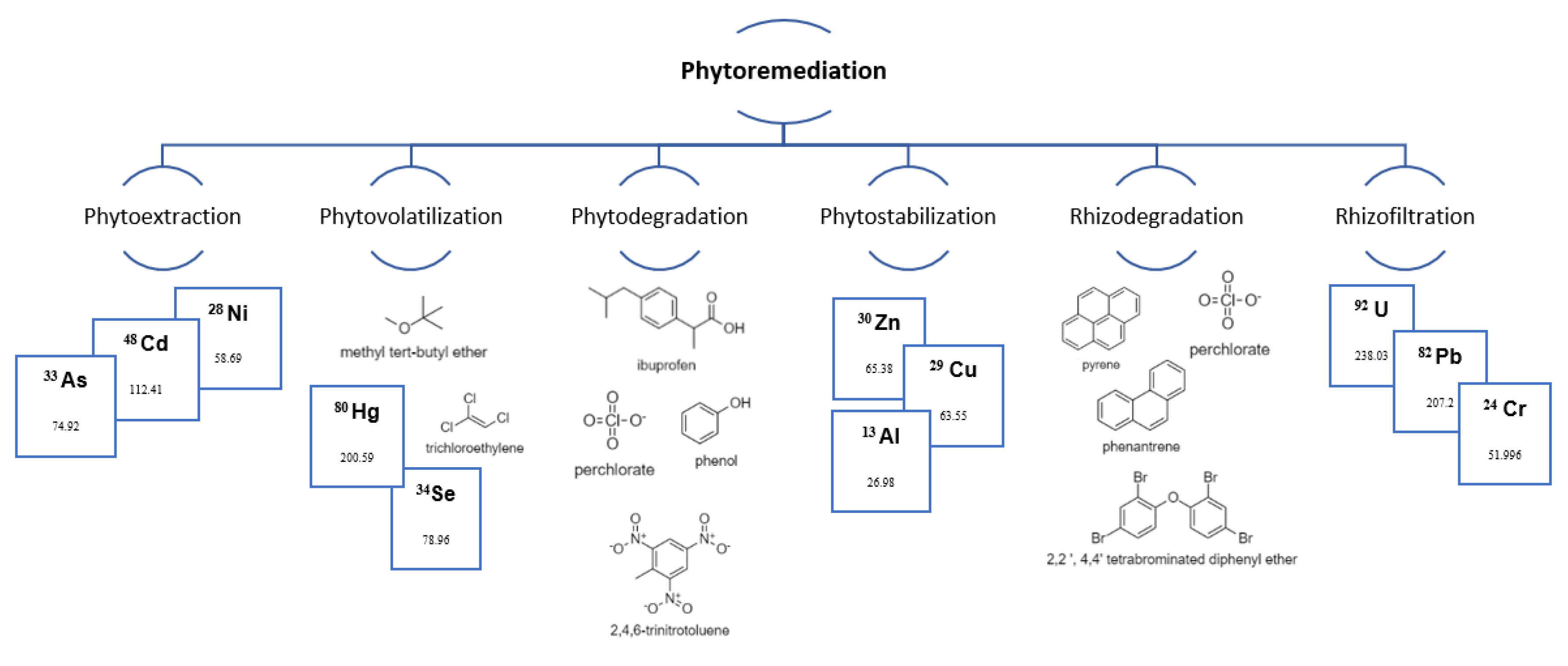
2. Mechanisms of Phytoremediation
2.1. Phytoextraction
2.2. Phytovolatilization
2.3. Phytodegradation
2.4. Phytostabilization
2.5. Rhizodegradation
2.6. Rhizofiltration
3. Parameters Affecting Phytoremediation Process
3.1. Soil pH
3.2. Inorganic Fertilizers
3.3. Organic Amendments
3.4. Contaminant Concentration
3.5. Mobility, Bioavailability and Chelating Agents
3.6. Plant Growth, Biomass Production and Accumulation Capacity
3.7. Microbial Activity
4. Phytoremediation—Benefits and Limitations
5. Energy Generation from Harvested Plants
6. Summary
Author Contributions
Funding
Conflicts of Interest
References
- Cachada, A.; Rocha-Santos, T.; Duarte, A.C. Soil and pollution: An introduction to the main issues. In Soil Pollution; Duarte, A.C., Cachada, A., Rocha-Santos, T., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 1–28. [Google Scholar]
- Sun, B.; Zhang, L.; Yang, L.; Zhang, F.; Norse, D. Agricultural non-point source pollution in China: Causes and mitigation measures. AMBIO 2012, 41, 370–379. [Google Scholar] [CrossRef]
- Nadal, M.; Schuhmacher, M.; Domingo, J.L. Metal pollution of soils and vegetation in an area with petrochemical industry. Sci. Total Environ. 2004, 321, 59–69. [Google Scholar] [CrossRef]
- Colvile, R.N.; Hutchinson, E.J.; Mindell, J.S.; Warren, R.F. The transport sector as a source of air pollution. Atmos. Environ. 2001, 35, 1537–1565. [Google Scholar] [CrossRef]
- Saier, M.H.; Trevors, J.T. Phytoremediation. Water Air Soil Pollut. 2010, 205, 61–63. [Google Scholar] [CrossRef]
- Kang, J.W. Removing environmental organic pollutants with bioremediation and phytoremediation. Biotechnol. Lett. 2014, 36, 1129–1139. [Google Scholar] [CrossRef]
- Vishnoi, S.R.; Srivastava, P.N. Phytoremediation—Green for environmental clean. In Taal 2007, Proceedings of the 12th World Lake Conference, Jodhpur, India, 28 October–2 November 2007; Sengupta, M., Dalwani, R., Eds.; Jai Narain Vyas University: Jodhpur, India, 2008; pp. 1016–1021. [Google Scholar]
- Cunningham, S.D.; Anderson, T.A.; Schwab, A.P.; Hsu, F.C. Phytoremediation of soils contaminated with organic pollutants. Adv. Agron. 1996, 56, 56–114. [Google Scholar]
- Etim, E.E. Phytoremediation and its mechanisms: A review. Int. J. Energy Environ. 2012, 2, 120–136. [Google Scholar]
- Wani, S.H.; Sanghera, G.S.; Athokpam, H. Phytoremediation: Curing soil problems with crops Phytoremediation: Curing soil problems with crops. Afr. J. Agric. Res. 2012, 7, 3991–4002. [Google Scholar]
- Chen, Y.; Shen, Z.; Li, X. The use of vetiver grass (Vetiveria zizanioides) in the phytoremediation of soils contaminated with heavy metals. Appl. Geochem. 2004, 19, 1553–1565. [Google Scholar] [CrossRef]
- Singh, S.; Eapen, S.; Thorat, V.; Kaushik, C.P.; Raj, K.; Souza, S.F.D. Phytoremediation of 137 cesium and 90 strontium from solutions and low-level nuclear waste by Vetiveria zizanoides. Ecotoxicol. Environ. Saf. 2008, 69, 306–311. [Google Scholar] [CrossRef]
- Kruger, E.L.; Anhalt, J.C.; Sorenson, D. Atrazine degradation in pesticide-contaminated soils: Phytoremediation potential. In Phytoremediation of Soil and Water Contaminants; Kruger, E.L., Anderson, T.A., Coats, J.R., Eds.; American Chemical Society: Washington, DC, USA, 1997; pp. 54–64. [Google Scholar]
- Huang, X.; El-Alawi, Y.; Penrose, D.M.; Glick, B.R.; Greenberg, B.M. A multi-process phytoremediation system for removal of polycyclic aromatic hydrocarbons from contaminated soils. Environ. Pollut. 2004, 130, 465–476. [Google Scholar] [CrossRef]
- Gahlawat, S.; Gauba, P. Phytoremediation of aspirin and tetracycline by Brassica juncea. Int. J. Phytoremediation 2016, 18, 929–935. [Google Scholar] [CrossRef]
- Ishikawa, S.; Noriharu, A.E.; Murakami, M. Soil science and plant nutrition is Brassica juncea a suitable plant for phytoremediation of cadmium in soils with moderately low cadmium contamination?—Possibility of using other plant species for Cd- phytoextraction. Soil Sci. Plant Nutr. 2010, 52, 32–42. [Google Scholar] [CrossRef]
- Tlustos, P.; Fischerova, Z.; Szakova, J.; Sichorova, K. A comparison of phytoremediation capability of selected plant species for given trace elements. Environ. Pollut. 2006, 144, 93–100. [Google Scholar]
- Ang, W.; Ui, C.; Ong, D. Phytoremediation of polluted waters potentials and prospects of wetland plants. Acta Biotechnol. 2002, 22, 199–208. [Google Scholar]
- Yoon, J.; Cao, X.; Zhou, Q.; Ma, L.Q. Accumulation of Pb, Cu, and Zn in native plants growing on a contaminated Florida site. Sci. Total Environ. 2006, 368, 456–464. [Google Scholar] [CrossRef]
- Moosavi, S.G.; Seghatoleslami, M.J. Phytoremediation: A review. Adv. Agric. Biol. 2013, 1, 5–11. [Google Scholar]
- Sarma, H. Metal hyperaccumulation in plants: A review focusing on phytoremediation technology. J. Environ. Sci. Technol. 2011, 4, 118–138. [Google Scholar] [CrossRef]
- Ghosh, M.; Singh, S.P. A review on phytoremediation of heavy metals and utilization of it’s by products. J. Energy Environ. 2005, 6, 214–231. [Google Scholar]
- Cunningham, S.D.; Berti, W.R. Remediation of contaminated soils with green plants: An overview. In Vitro Cell. Dev. Biol. 1993, 29, 207–212. [Google Scholar] [CrossRef]
- Brennan, M.A.; Shelley, M.L. A model of the uptake, translocation, and accumulation of lead (Pb) by maize for the purpose of phytoextraction. Ecol. Eng. 1999, 12, 271–297. [Google Scholar] [CrossRef]
- Yang, Y.; Shen, Q. Phytoremediation of cadmium-contaminated wetland soil with Typha latifolia L. and the underlying mechanisms involved in the heavy-metal uptake and removal. Environ. Sci Pollut. Res. Int. 2020, 27, 4905–4916. [Google Scholar] [CrossRef] [PubMed]
- Holubik, O.; Vanek, A.; Mihaljevic, M.; Vejvodova, K. Higher Tl bioaccessibility in white mustard (hyper-accumulator) grown under the soil than hydroponic conditions: A key factor for the phytoextraction use. J. Environ. Manag. 2020, 255, 109880. [Google Scholar] [CrossRef] [PubMed]
- Hamzah, M.; Kamran, M.; Zhou, Y. Appraising growth, oxidative stress and copper phytoextraction potential of flax (Linum usitatissimum L.) grown in soil differentially spiked with copper. J. Environ. Manag. 2020, 257, 109994. [Google Scholar]
- Zhang, Y.; Liu, G. Uptake, accumulation and phytoextraction efficiency of cesium in Gypsophila paniculata. Int. J. Phytoremediation 2019. [Google Scholar] [CrossRef]
- Marathe, S.; Ravichandran, N. Potential of sunflower to extract heavy metals from leachate. Int. J. Geosci. 2019, 10, 1115–1127. [Google Scholar] [CrossRef]
- Marchiol, L.; Assolari, S.; Sacco, P.; Zerbi, G. Phytoextraction of heavy metals by canola (Brassica napus) and radish (Raphanus sativus) grown on multicontaminated soil. Environ. Pollut. 2004, 132, 21–27. [Google Scholar] [CrossRef]
- Gupta, A.K.; Sinha, S. Phytoextraction capacity of the Chenopodium album L. grown on soil amended with tannery sludge. Bioresour. Technol. 2007, 98, 442–446. [Google Scholar] [CrossRef]
- Keeling, S.M.; Stewart, R.B.; Anderson, C.W.N.; Robinson, B.H. Nickel and cobalt phytoextraction by the hyperaccumulator Berkheya coddii: Implications for polymetallic phytomining and phytoremediation. Int. J. Phytoremediation 2003, 5, 235–244. [Google Scholar] [CrossRef]
- Brooks, R.R.; Wither, E.D. Nickel accumulation by Rinorea bengalensis (Wall.) O.K. J. Geochem. Explor. 1977, 7, 295–300. [Google Scholar] [CrossRef]
- Goncalaves, M.T.; Goncalaves, S.C.; Portugal, A.; Silva, S.; Sousa, J.P.; Freitas, H. Effects of nickel hyperaccumulation in Alyssum pintodasilvae on model arthropods representatives of two trophic levels. Plant Soil 2007, 293, 177–188. [Google Scholar] [CrossRef]
- Broadhurst, C.L.; Chaney, R.L.; Angle, J.S.; Erbe, E.F.; Maugel, T.K. Nickel localization and response to increasing Ni soil levels in leaves of the Ni hyperaccumulator Alyssum murale. Plant Soil 2004, 265, 225–242. [Google Scholar] [CrossRef]
- Tongbin, C.; Chaoyang, W.; Zechun, H.; Qifei, H.; Quanguo, L.; Zilian, F. Arsenic hyperaccumulator Pteris vittata, L. and its arsenic accumulation. Chin. Sci. Bull. 2002, 47, 902–905. [Google Scholar]
- Sun, Y.; Zhou, Q.; Diao, C. Effects of cadmium and arsenic on growth and metal accumulation of Cd-hyperaccumulator Solanum nigrum L. Bioresour. Technol. 2007, 99, 1103–1110. [Google Scholar] [CrossRef]
- Zhao, F.J.; Lombi, E.; Breedon, T.; Grath, S.P.M. Zinc hyperaccumulation and cellular distribution in Arabidopsis halleri. Plant Cell Environ. 2000, 23, 507–514. [Google Scholar] [CrossRef]
- Vázquez, M.D.; Barceló, J.; Poschenrieder, C.; Mádico, J.; Hatton, P.; Baker, A.J.M.; Cope, G.H. Localization of zinc and cadmium in Thlaspi caerulescens (Brassicaceae), a Metallophyte that can Hyperaccumulate both metals. J. Plant Physiol. 1992, 140, 350–355. [Google Scholar] [CrossRef]
- Arnold, C.W.; Parfitt, D.G.; Kaltreider, M. Phytovolatilization of oxygenated gasoline-impacted groundwater at an underground storage tank site via conifers. Int. J. Phytoremediation 2007, 9, 53–69. [Google Scholar] [CrossRef]
- Edwards, M.R.A.; Hetu, M.F.; Columbus, M.; Silva, A.; Lefebvre, D.D. The effect of ethylene glycol on the phytovolatilization of 1,4—Dioxane. Int. J. Phytoremediation 2011, 13, 37–41. [Google Scholar] [CrossRef]
- Chen, Z.; Kuschk, P.; Reiche, N.; Borsdorf, H.; Kästner, M.; Köser, H. Comparative evaluation of pilot scale horizontal subsurface-flow constructed wetlands and plant root mats for treating groundwater contaminated with benzene and MTBE. J. Hazard. Mater. 2012, 209, 510–515. [Google Scholar] [CrossRef]
- Nagata, T.; Morita, H.; Akizawa, T.; Pan-Tou, H. Development of a transgenic tobacco plant for phytoremediation of methylmercury pollution. Appl. Microbiol. Biotechnol. 2010, 87, 781–786. [Google Scholar] [CrossRef]
- Rahman, R.A.A.; Abou-Shanab, R.A.; Moawad, H. Mercury detoxification using genetic engineered. Glob. Nest. J. 2008, 10, 432–438. [Google Scholar]
- Sakakibara, M.; Watanabe, A.; Inoue, M.; Sano, S.; Kaise, T. Phytoextraction and phytovolatilization of arsenic from as-contaminated soils by Pteris vittata. Proc. Annu. Int. Conf. Soils Sediments Water Energy 2010, 12, 26. [Google Scholar]
- Tagmount, A.; Berken, A.; Terry, N. An essential role of S-Adenosyl-L-Methionine: L-Methionine S-Methyltransferase in selenium volatilization by plants. Methylation of selenomethionine to selenium-methyl-L-Selenium-Methionine, the precursor of volatile selenium. J. Plant. Physiol. 2002, 130, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Limmer, M.A.; Burken, J.G. Phytovolatilization of organic contaminants. Environ. Sci. Technol. 2016, 50, 6632–6643. [Google Scholar] [CrossRef] [PubMed]
- Peter, L.; Clausen, W.; Broholm, M.M.; Gosewinkel, U.; Trapp, S. Test of aerobic TCE degradation by willows (Salix viminalis) and willows inoculated with TCE-cometabolizing strains of Burkholderia cepacia. Environ. Sci. Pollut. Res. 2017, 24, 18320–18331. [Google Scholar]
- Orchard, B.J.; Doucette, W.J.; Chard, J.; Bugbee, B. Uptake of trichloroethylene by hybrid poplar trees grown hydroponically in flow-through plant growth chambers. Environ. Toxicol. Chem. 2000, 19, 895–903. [Google Scholar] [CrossRef]
- Yu, X.; Gu, J. Uptake, metabolism, and toxicity of methyl tert -butyl ether (MTBE) in weeping willows. J. Hazard. Mater. 2006, 137, 1417–1423. [Google Scholar] [CrossRef]
- Ferro, A.M.; Kennedy, J.; LaRue, J.C. Phytoremediation of 1,4-dioxane-containing recovered groundwater. Int. J. Phytoremediation 2013, 15, 911–923. [Google Scholar] [CrossRef]
- De Souza, M.P.; Pickering, I.J.; Walla, M.; Terry, N. Selenium assimilation and volatilization from selenocyanate-treated Indian mustard and muskgrass. J. Plant Physiol. 2002, 128, 625–633. [Google Scholar] [CrossRef]
- Shrestha, B.; Lipe, S.; Johnson, K.A.; Zhang, T.Q.; Retzlaff, W.; Lin, Z. Soil hydraulic manipulation and organic amendment for the enhancement of selenium volatilization in a soil-pickleweed system. Plant Soil 2006, 288, 189–196. [Google Scholar] [CrossRef]
- Heaton, A.C.P.; Rugh, C.L.; Wang, N.; Meagher, R.B. Phytoremediation of mercury- and methylmercury- polluted soils using genetically engineered plants. J. Soil Contam. 1998, 7, 497–509. [Google Scholar] [CrossRef]
- Newman, L.A.; Wang, X.; Muiznieks, I.A.; Ekuan, G.; Ruszaj, M.; Cortellucci, R.; Domroes, D.; Karscig, G.; Newman, T.; Crampton, R.S.; et al. Remediation of trichloroethylene in an artificial aquifer with trees: A controlled field study. Environ. Sci. Technol. 1999, 33, 2257–2265. [Google Scholar] [CrossRef]
- Doucette, W.; Klein, H.; Chard, J.; Dupont, R.; Plaehn, W.; Bugbee, B. Volatilization of trichloroethylene from trees and soil: Measurement and scaling approaches. Environ. Sci. Technol. 2013, 47, 5813–5820. [Google Scholar] [CrossRef]
- Narayanan, M.; Davis, L.C.; Erickson, L.E. Fate of volatile chlorinated organic compounds in a laboratory chamber with alfalfa plants. Environ. Sci. Technol. 1995, 29, 2437–2444. [Google Scholar] [CrossRef] [PubMed]
- James, C.A.; Xn, G.; Doty, S.L.; Muiznieks, I.; Newman, L.; Strand, S.E. A mass balance study of the phytoremediation of perchloroethylene-contaminated groundwater. Environ. Pollut. 2009, 157, 2564–2569. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Burken, J.G. TCE diffusion to the atmosphere in phytoremediation applications. Environ. Sci. Technol. 2003, 37, 2534–2539. [Google Scholar] [CrossRef] [PubMed]
- Balarak, D.; Yousefi, Z.; Zazouli, M.A. Phytodegradation potential of phenol from aqueous solution by Azolla filiculoides. J. Bioremed. Biodegredation 2014. [Google Scholar] [CrossRef]
- Mirck, J.; Isebrands, J.G.; Verwijst, T.; Ledin, S. Development of short-rotation willow coppice systems for environmental purposes in Sweden. Biomass Bioenergy 2005, 28, 219–228. [Google Scholar] [CrossRef]
- Kagalkar, A.N.; Jadhav, M.U.; Bapat, V.A.; Govindwar, S.P. Phytodegradation of the triphenylmethane dye malachite green mediated by cell suspension cultures of Blumea malcolmii Hook. Bioresour. Technol. 2011, 102, 10312–10318. [Google Scholar] [CrossRef] [PubMed]
- Hasan, S.; Jyoti, P.; Singh, N. Mycorrhizae and phytochelators as remedy in heavy metal contaminated land remediation. Int. Res. J. Environ. Sci. 2013, 2, 74–78. [Google Scholar]
- Schwitzguébel, J.P.; Meyer, J.B.; Kidd, P. Pesticides removal using plants: Phytodegradation. In Phytoremediation Rhizoremediation; Mackova, M., Dowling, D., Macek, T., Eds.; Springer: Dordrecht, The Netherlands, 2006; pp. 179–198. [Google Scholar]
- Susarla, S.; Bacchus, S.T.; Wolfe, N.L.; McCutcheon, S.C. Phytotransformation of perchlorate and identification of metabolic products in Myriophyllum aquaticum. Int. J. Phytoremediation 1999, 1, 97–107. [Google Scholar] [CrossRef]
- Vanderford, M.; Shanks, J.V.; Hughes, J.B. Phytotransformation of trinitrotoluene (TNT) and distribution of metabolic products in Myriophyllum aquaticum. Biotechnol. Lett. 1997, 19, 277–280. [Google Scholar] [CrossRef]
- Yoon, J.M.; Oliver, D.J.; Shanks, J.V. Phytotransformation of 2,4-dinitrotoluene in Arabidopsis thaliana: Toxicity, fate, and gene expression studies in vitro. Biotechnol. Prog. 2006, 22, 1524–1531. [Google Scholar] [CrossRef] [PubMed]
- Gujarathi, N.P.; Haney, B.J.; Linden, J.C. Phytoremediation potential of Myriophyllum aquaticum and Pistia stratiotes to modify antibiotic growth promoters, tetracycline, and oxytetracycline, in Aqueous wastewater systems. Int. J. Phytoremediation 2005, 7, 99–112. [Google Scholar] [CrossRef]
- Thi, T.; Hoang, T.; Thi, L. A preliminary study on phytoremediation of antibiotic. Int. J. Phytoremediation 2013, 15, 65–76. [Google Scholar]
- Gałwa-Widera, M. Plant-based technologies for removal of pharmaceuticals and personal care products. In Pharmaceuticals and Personal Care Products: Waste Management and Treatment Technology; Prasad, M.T.W., Vithanage, M., Kapley, A., Eds.; Butterworth–Heinemann Elsevier Inc.: Oxford, UK, 2019. [Google Scholar]
- He, Y.; Langenhoff, A.A.M.; Sutton, N.B.; Rijnaarts, H.H.M.; Blokland, M.H.; Chen, F.; Huber, C.; Schröder, P. Metabolism of ibuprofen by Phragmites australis: Uptake and phytodegradation. Environ. Sci. Technol. 2017, 51, 4576–4584. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.; Zhu, G.; Liu, Y.; Wu, B.; Jern, W. Phytoextraction, phytotransformation and rhizodegradation of ibuprofen associated with Typha angustifolia in a horizontal subsurface flow constructed wetland. Water Res. 2016, 102, 294–304. [Google Scholar] [CrossRef]
- Singh, V.; Pandey, B.; Suthar, S. Ecotoxicology and environmental safety phytotoxicity and degradation of antibiotic o floxacin in duckweed (Spirodela polyrhiza) system. Ecotoxicol. Environ. Saf. 2019, 179, 88–95. [Google Scholar] [CrossRef]
- Datta, R.; Das, P.; Smith, S.; Punamiya, P.; Ramanathan, D.M.; Reddy, R.; Sarkar, D. Phytoremediation potential of vetiver grass (Chrysopogon Zizanioides (L.)) for tetracycline. Int. J. Phytoremediation 2013, 15, 343–351. [Google Scholar] [CrossRef]
- Topal, M.; Senel, G.U.; Obek, E.; Topal, E.I.A. Removal of tetracycline and the degradation products by Lemna gibba L. exposed to secondary effluents. Environ. Prog. Sustain. Energy. 2015, 34, 1311–1325. [Google Scholar] [CrossRef]
- Li, M.; Cheng, Y.; Ding, T.; Wang, H.; Wang, W.; Li, J.; Ye, Q. Phytotransformation and metabolic pathways of 14C-carbamazepine in carrot and celery. J. Agric. Food Chem. 2020, 68, 3362–3371. [Google Scholar] [CrossRef] [PubMed]
- Ryšlavá, H.; Pomeislová, A.; Pšondrová, S.; Hýsková, V.; Smrček, S. Phytoremediation of carbamazepine and its metabolite 10,11-epoxycarbamazepine by C3 and C4 plants. Environ. Sci. Pollut. Res. Int. 2015, 22, 20271–20282. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Ma, X. Phytoremediation of ethion by water hyacinth (Eichhornia crassipes) from water. Bioresour. Technol. 2006, 97, 1050–1054. [Google Scholar] [CrossRef] [PubMed]
- Rani, R.; Padole, P.; Juwarkar, A.; Chakrabarti, T. Phytotransformation of phorate by Brassica juncea (Indian Mustard). Water Air Soil Pollut. 2012, 223, 1383–1392. [Google Scholar] [CrossRef]
- Rizzi, L.; Petruzzelli, G.; Poggio, G.; Guidi, G.V. Soil physical changes and plant availability of Zn and Pb in a treatability test of phytostabilization. Chemosphere 2004, 57, 1039–1046. [Google Scholar] [CrossRef]
- Van Nevel, L.; Mertens, J.; Staelens, J.; Schrijver, A.D.; Tack, F.; Neve, S.D.; Meers, E.; Verheyen, K. Elevated Cd and Zn uptake by aspen limits the phytostabilization potential compared to five other tree species. Ecol. Eng. 2011, 37, 1072–1080. [Google Scholar] [CrossRef]
- Hattab, N.; Motelica-Heino, M.; Bourrat, X.; Mench, M. Mobility and phytoavailability of Cu, Cr, Zn, and As in a contaminated soil at a wood preservation site after 4 years of aided phytostabilization. Environ. Sci. Pollut. Res. Int. 2014, 21, 10307–10319. [Google Scholar] [CrossRef][Green Version]
- Guo, P.; Wang, T.; Liu, Y.; Xia, Y. Phytostabilization potential of evening primrose (Oenothera glazioviana) for copper-contaminated sites. Environ. Sci. Pollut. Res. Int. 2016, 21, 631–640. [Google Scholar] [CrossRef]
- Farahat, E.A. Trace metal accumulation by Ranunculus sceleratus: Implications for phytostabilization. Environ. Sci. Pollut. Res. Int. 2018, 25, 4214–4222. [Google Scholar] [CrossRef]
- Alkorta, I.; Becerril, J.M.; Garbisu, C. Phytostabilization of metal contaminated soils. Rev. Environ. Health 2010, 25, 135–146. [Google Scholar] [CrossRef]
- Arienzo, M.; Adamo, P.; Cozzolino, V. The potential of Lolium perenne for revegetation of contaminated soil from a metallurgical site. Sci. Total Environ. 2004, 319, 13–25. [Google Scholar] [CrossRef]
- Dhir, B.; Sharmila, P.; Saradhi, P.P. Potential of aquatic macrophytes for removing contaminants from the environment. Crit. Rev. Environ. Sci. Technol. 2009, 39, 754–783. [Google Scholar] [CrossRef]
- Ruttens, A.; Mench, M.; Colpaert, J.V.; Boisson, J.; Carleer, R.; Vangronsveld, J. Phytostabilization of a metal contaminated sandy soil. I: Influence of compost and/or inorganic metal immobilizing soil amendments on phytotoxicity and plant availability of metals. Environ. Pollut. 2006, 144, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Santibáñez, C.; Verdugo, C.; Ginocchio, R. Phytostabilization of copper mine tailings with biosolids: Implications for metal uptake and productivity of Lolium perenne. Sci. Total Environ. 2008, 395, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Nouri, J.; Lorestani, B.; Yousefi, N.; Khorasani, N.; Hasani, A.H.; Seif, F.; Cheraghi, M. Phytoremediation potential of native plants grown in the vicinity of Ahangaran lead—Zinc mine (Hamedan, Iran). Environ. Earth Sci. 2011, 62, 639–644. [Google Scholar] [CrossRef]
- Wong, M.Y.M. Ecological restoration of mine degraded soils, with emphasis on metal contaminated soils. Chemosphere 2003, 50, 775–780. [Google Scholar] [CrossRef]
- Pavel, P.; Puschenreiter, M.; Wenzel, W.W.; Diacu, E.; Barbu, C.H. Aided phytostabilization using Miscanthus sinensis × giganteus on heavy metal-contaminated soils. Sci. Total Environ. 2014, 479, 125–131. [Google Scholar] [CrossRef]
- Lee, S.; Ji, W.; Lee, W.; Koo, N.; Koh, I.H.; Kim, M.; Park, J. Influence of amendments and aided phytostabilization on metal availability and mobility in Pb/Zn mine tailings. J. Environ. Manag. 2014, 139, 15–21. [Google Scholar] [CrossRef]
- Pérez-Esteban, J.; Escolástico, C.; Moliner, A.; Masaguer, A.; Ruiz-Fernández, J. Phytostabilization of metals in mine soils using Brassica juncea in combination with organic amendments. Plant Soil 2014, 377, 97–109. [Google Scholar] [CrossRef]
- Prapagdee, S.; Piyatiratitivorakul, S.; Petsom, A.; Tawinteung, N. Application of biochar for enhancing cadmium and zinc phytostabilization in Vigna radiata L. cultivation. Water Air Soil Pollut. 2014, 225, 1–13. [Google Scholar] [CrossRef]
- Radziemska, M.; Gusiatin, Z.M.; Bilgin, A. Potential of using immobilizing agents in aided phytostabilization on simulated contamination of soil with lead. Ecol. Eng. 2017, 102, 490–500. [Google Scholar] [CrossRef]
- Meeinkuirt, W.; Kruatrachue, M.; Pichtel, J.T.; Phusantisampan, T.; Saengwilai, P. Influence of organic amendments on phytostabilization of Cd-contaminated soil by Eucalyptus camaldulensis. Sci. Asia 2016, 42, 83–91. [Google Scholar] [CrossRef]
- Phusantisampan, T.; Meeinkuirt, W.; Saengwilai, P. Phytostabilization potential of two ecotypes of Vetiveria zizanioides in cadmium-contaminated soils: Greenhouse and field experiments. Environ. Sci. Pollut. Res. 2016, 23, 20027–20038. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Roos, P.; Zhu, Y.; Jakobsen, I. Arbuscular mycorrhizas contribute to phytostabilization of uranium in uranium mining tailings. J. Environ. Radioact. 2008, 99, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Zhou, Z.; Gao, Y.; Yuan, X.; Ai, Y.; Zhang, J.; Zuo, W.; Taylor, A.A.; Nan, S.; Li, F. The influences of Arbuscular mycorrhizal fungus on phytostabilization of lead/zinc tailings using of four plant species. Int. J. Phytoremediation 2017, 19, 739–745. [Google Scholar] [CrossRef]
- Ouaryi, A.; Boularbah, A.; Sanguin, H.; Hafidi, M.; Baudoin, E.; Ouahmane, L.; Roux, C.L.; Galiana, A.; Prin, Y.; Duponnois, R. High potential of symbiotic interactions between native mycorrhizal fungi and the exotic tree Eucalyptus camaldulensis for phytostabilization of metal-contaminated arid soils. Int. J. Phytoremediation 2016, 18, 41–47. [Google Scholar] [CrossRef]
- Masciandaro, G.; Macci, C.; Peruzzi, E.; Ceccanti, B.; Doni, S. Organic matter—Microorganism—Plant in soil bioremediation: A synergic approach. Rev. Environ. Sci. Biotechnol. 2013, 12, 399–419. [Google Scholar] [CrossRef]
- Gkorezis, P.; Daghio, M.; Franzetti, A.; Hamme, J.D.V.; Sillen, W.; Vangronsveld, J. The interaction between plants and bacteria in the remediation of petroleum hydrocarbons: An environmental perspective. Front. Microbiol. 2016, 7, 1–27. [Google Scholar] [CrossRef]
- Abdullah, S.R.S.; Al-Baldawi, I.A.; Almansoory, A.F.; Purwanti, I.F.; Al-Sbani, N.H.; Sharuddin, S.S.N. Plant-assisted remediation of hydrocarbons in water and soil: Application, mechanisms, challenges and opportunities. Chemosphere 2020, 247, 125932. [Google Scholar] [CrossRef]
- Kaimi, E.; Mukaidani, T.; Tamaki, M. Effect of rhizodegradation in diesel-contaminated soil under different soil conditions. Plant Prod. Sci. 2007, 10, 105–111. [Google Scholar] [CrossRef]
- Maqbool, F.; Wang, Z.; Xu, Y.; Zhao, J.; Gao, D.; Zhao, Y.; Bhatti, Z.A.; Xing, B. Rhizodegradation of petroleum hydrocarbons by Sesbania cannabina in bioaugmented soil with free and immobilized consortium. J. Hazard. Mater. 2012, 237, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Allamin, I.A.; Halmi, M.I.E.; Yasid, N.A.; Ahmad, S.A.; Abdullah, S.R.S.; Shukor, Y. Rhizodegradation of petroleum oily sludge-contaminated soil using Cajanus cajan increases the diversity of soil microbial community. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ramos, D.T.; Maranho, L.T.; Godoi, A.F.L.; Filho, M.A.S.C.; Lacerda, L.G.; Vasconcelos, E.C. Petroleum hydrocarbons rhizodegradation by Sebastiania commersoniana (Baill.) L. B. SM. & Downs. Water Air Soil Pollut. 2009, 9, 293–302. [Google Scholar]
- Lu, H.; Zhang, Y.; Liu, B.; Liu, J.; Ye, J.; Yan, C. Rhizodegradation gradients of phenanthrene and pyrene in sediment of mangrove (Kandelia candel (L.) Druce). J. Hazard. Mater. 2011, 196, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Wang, H.; Lu, H.; Jiang, S.; Dai, M.; Liu, J.; Yan, C. Rhizodegradation potential and tolerance of Avicennia marina (Forsk.) Vierh in phenanthrene and pyrene contaminated sediments. Mar. Pollut. Bull. 2016, 110, 112–118. [Google Scholar] [CrossRef]
- Yifru, D.D.; Nzengung, V.A. Use of dissolved organic carbon to biostimulate rapid rhizodegradation of perchlorate in soil. J. Bioremed. Biodegrad. 2007. [Google Scholar] [CrossRef]
- Mwegoham, W.; Mbuya, O.S.; Jain, A.; Ugochukwu, N.H.; Abazinge, M. Use of chicken manure extract for biostimulation and enhancement of perchlorate rhizodegradation in soil and water media. Bioremediat. J. 2007, 11, 61–70. [Google Scholar] [CrossRef]
- Lee, M.; Yang, M. Rhizofiltration using sunflower (Helianthus annuus L.) and bean (Phaseolus vulgaris L. var. vulgaris) to remediate uranium contaminated groundwater. J. Hazard. Mater. 2010, 173, 589–596. [Google Scholar] [CrossRef]
- Verma, P.; George, K.V.; Singh, H.V.; Singh, S.; Juwarkar, A.A.; Singh, R.N. Modeling rhizofiltration: Heavy-metal uptake by plant roots. Environ. Model. Assess. 2006, 11, 387–394. [Google Scholar] [CrossRef]
- Tomé, F.V.; Rodríguez, P.B.; Lozano, J.C. Elimination of natural uranium and 226 Ra from contaminated waters by rhizofiltration using Helianthus annuus L. Sci. Total Environ. 2008, 393, 351–357. [Google Scholar] [CrossRef]
- Eapen, S.; Suseelan, K.N.; Tivarekar, S.; Kotwal, S.A.; Mitra, R. Potential for rhizofiltration of uranium using hairy root cultures of Brassica juncea and Chenopodium amaranticolor. Environ. Res. 2003, 91, 127–133. [Google Scholar] [CrossRef]
- Mikheev, A.N.; Lapan, O.V.; Madzhd, S.M. Experimental foundations of a new method for rhizofiltration treatment of aqueous ecosystems from 137 Cs. J. Water Chem. Technol. 2017, 39, 245–249. [Google Scholar] [CrossRef]
- Yang, M.; Jawitz, J.W.; Lee, M. Uranium and cesium accumulation in bean (Phaseolus vulgaris L. var. vulgaris) and its potential for uranium rhizofiltration. J. Environ. Radioact. 2015, 140, 42–49. [Google Scholar] [CrossRef]
- Kamel, H.A.; Eskander, S.B.; Aly, M.A.S. Physiological response of Epipremnum aureum for cobalt-60 and cesium-137 translocation and rhizofiltration. Int. J. Phytoremediation 2007, 9, 403–417. [Google Scholar] [CrossRef] [PubMed]
- Veselý, T.; Tlustoš, P.; Száková, J. The use of water lettuce (Pistia stratiotes L.) for rhizofiltration of a highly polluted solution by cadmium and lead. Int. J. Phytoremediation 2011, 13, 859–872. [Google Scholar]
- Muhammad, D.; Chen, F.; Zhao, J.; Zhang, G.; Wu, F. Comparison of EDTA- and citric acid- enhanced phytoextraction of heavy metals in artificially metal contaminated soil by Typha angustifoli. Int. J. Phytoremediation 2009, 11, 558–574. [Google Scholar] [CrossRef]
- Tandy, S.; Schulin, R.; Nowack, B. Uptake of metals during chelant-assisted phytoextraction with EDDS related to the solubilized metal concentration. Environ. Sci. Technol. 2006, 40, 2753–2758. [Google Scholar] [CrossRef]
- Fokkema, M.J.; Song, J.; Luo, Y.M.; Japenga, J.; Zhao, F.J. Feasibility of phytoextraction to remediate cadmium and zinc contaminated soils. Environ. Pollut 2008, 156, 905–914. [Google Scholar]
- Puschenreiter, M.; Stöger, G.; Lombi, E.; Horak, O.; Wenzel, W.W. Phytoextraction of heavy metal contaminated soils with Thlaspi goesingense and Amaranthus hybridus: Rhizosphere manipulation using EDTA and ammonium sulfate. J. Soil Sci. Plant Nutr. 2001, 164, 615–621. [Google Scholar] [CrossRef]
- Quartacci, M.F.; Argilla, A.; Baker, A.J.M. Phytoextraction of metals from a multiply contaminated soil by Indian mustard. Chemosphere 2006, 63, 918–925. [Google Scholar] [CrossRef]
- Wenzel, W.W.; Unterbrunner, R.; Sommer, P.; Sacco, P. Chelate-assisted phytoextraction using canola (Brassica napus L.) in outdoors pot and lysimeter experiments. Plant Soil 2003, 249, 83–96. [Google Scholar] [CrossRef]
- Meers, E.; Hopgood, M.; Lesage, E.; Vervaeke, P.; Tack, F.M.G.; Verloo, M.G. Enhanced phytoextraction: In search of EDTA alternatives enhanced phytoextraction: In search of EDTA alternatives. Int. J. Phytoremediation 2004, 6, 95–109. [Google Scholar] [CrossRef]
- Luo, C.; Shen, Z.; Li, X.; Baker, A.J.M. Enhanced phytoextraction of Pb and other metals from artificially contaminated soils through the combined application of EDTA and EDDS. Chemosphere 2006, 63, 1773–1784. [Google Scholar] [CrossRef]
- Bani, A.; Echevarria, G.; Sulçe, S.; Louis, J.; Alfred, M. In-situ phytoextraction of Ni by a native population of Alyssum murale on an ultramafic site (Albania). Plant Soil 2007, 293, 79–89. [Google Scholar] [CrossRef]
- Li, Y.M.; Chaney, R.L.; Brewer, E.P.; Angle, J.S.; Nelkin, J. Phytoextraction of nickel and cobalt by hyperaccumulator alyssum species grown on nickel-contaminated soils. Environ. Sci. Technol. 2003, 37, 1463–1468. [Google Scholar] [CrossRef]
- El-Mahrouk, E.M.; Eisa, E.A.E.; Ali, H.M.; Hegazy, M.A.E.; Abd El-Gayed, M.E.S. Populus nigra as a phytoremediator for Cd, Cu, and Pb in contaminated soil. BioResources 2020, 15, 869–893. [Google Scholar]
- Komarek, M.; Tlustos, P.; Száková, J.; Chrastný, V.; Ettler, V. The use of maize and poplar in chelant-enhanced phytoextraction of lead from contaminated agricultural soils. Chemosphere 2007, 67, 640–651. [Google Scholar] [CrossRef]
- Wilde, E.W.; Brigmon, R.L.; Dunn, D.L.; Heitkamp, M.A.; Dagnan, D.C. Phytoextraction of lead from firing range soil by Vetiver grass. Chemosphere 2005, 61, 1451–1457. [Google Scholar] [CrossRef] [PubMed]
- Freitas, E.; Williams, C.; Souza, A.; Bruno, F. Citric acid-assisted phytoextraction of lead: A field experiment. Chemosphere 2013, 92, 213–217. [Google Scholar] [CrossRef]
- Zhuang, P.; Yang, Q.W.; Wang, H.B.; Shu, W.S. Phytoextraction of heavy metals by eight plant species in the field. Water Air Soil Pollut. 2007, 184, 235–242. [Google Scholar] [CrossRef]
- Wu, Q.T.; Wei, Z.B.; Ouyang, Y. Phytoextraction of metal-contaminated soil by Sedum alfredii H: Effects of chelator and Co-planting. Water Air Soil Pollut. 2007, 180, 131–139. [Google Scholar] [CrossRef]
- Sun, Y.; Zhou, Q.; An, J.; Liu, W.; Liu, R. Chelator-enhanced phytoextraction of heavy metals from contaminated soil irrigated by industrial wastewater with the hyperaccumulator plant (Sedum alfredii Hance). Geoderma 2009, 150, 106–112. [Google Scholar] [CrossRef]
- Zhuang, P.; Ye, Z.H.; Lan, C.Y.; Xie, Z.W.; Shu, W.S. Chemically assisted phytoextraction of heavy metal contaminated soils using three plant species. Plant Soil 2005, 276, 153–162. [Google Scholar] [CrossRef]
- Duquène, L.; Vandenhove, H.; Tack, F.; Meers, E.; Baeten, J.; Wannijn, J. Enhanced phytoextraction of uranium and selected heavy metals by Indian mustard and ryegrass using biodegradable soil amendments. Sci. Total Environ. 2008, 407, 1496–1505. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Feng, X.; Anderson, C.W.N.; Qiu, G.; Ping, L.; Bao, Z. Ammonium thiosulphate enhanced phytoextraction from mercury contaminated soil—Results from a greenhouse study. J. Hazard. Mater. 2011, 186, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Al-Baldawi, I.A.; Abdullah, I.A.; Anuar, S.R.S.; Hassan, N.; Abu, H. Phytotransformation of methylene blue from water using aquatic plant (Azolla pinnata). Environ. Technol. Innov. 2018, 11, 15–22. [Google Scholar] [CrossRef]
- Gong, Y.; Chen, J.; Pu, R. The enhanced removal and phytodegradation of sodium dodecyl sulfate (SDS) in wastewater using controllable water hyacinth. Int. J. Phytoremediation 2019. [Google Scholar] [CrossRef]
- Dolphen, R.; Thiravetyan, P. Phytodegradation of ethanolamines by Cyperus alternifolius: Effect of molecular size phytodegradation of ethanolamines by Cyperus alternifolius: Effect of molecular size. Int. J. Phytoremediation 2015, 17, 686–692. [Google Scholar] [CrossRef]
- De Farias, V.; Maranho, L.T.; de Vasconcelos, E.C.; Carvalho Filho, M.A.D.; Lacerda, L.G.; Azevedo, J.A.M.; Pandey, A.; Soccol, C.R. Phytodegradation potential of Erythrina crista-galli L., Fabaceae, in petroleum-contaminated soil. Appl. Biochem. Biotechnol. 2009, 157, 10–22. [Google Scholar] [CrossRef]
- Vazguez, S.; Agha, R.; Granado, A.; Sarro, M.; Esteban, E.; Penalosa, J.; Carpena, R. Use of white lupin plant for phytostabilization. Water Air Soil Pollut. 2006, 177, 349–365. [Google Scholar] [CrossRef]
- Ehsan, M.; Santamaría-Delgado, K.; Vázquez-Alarcón, A.; Alderete-Chavez, A.; Cruz-Landero, N.; Jaén-Contreras, D.; Augustine Molumeli, P. Phytostabilization of cadmium contaminated soils by Lupinus uncinatus Schldl. Span. J. Agric. Res. 2009, 7, 390–397. [Google Scholar] [CrossRef]
- Fatnassi, I.C.; Chiboub, M.; Saadani, O.; Jebara, M.; Jebara, S.H. Phytostabilization of moderate copper contaminated soils using co-inoculation of Vicia faba with plant growth promoting bacteria. J. Basic Microbiol. 2015, 55, 303–311. [Google Scholar] [CrossRef]
- Meeinkuirt, W.; Kruatrachue, M.; Tanhan, P.; Chaiyarat, R.; Pokethitiyook, P. Phytostabilization potential of Pb mine tailings by two grass species, Thysanolaena maxima and Vetiveria zizanioides. Water Air Soil Pollut. 2013, 224, 1–12. [Google Scholar] [CrossRef]
- Ramana, S.; Biswas, A.K.; Ajay; Singh, A.B.; Ahirwar, N.K.; Rao, A.S. Potential of rose for phytostabilization of chromium contaminated soils. J. Plant Physiol. 2013, 18, 381–383. [Google Scholar] [CrossRef]
- Wu, Q.; Wang, S.; Thangavel, P.; Li, Q.; Zheng, H.; Bai, J.; Qiu, R. Phytostabilization potential of Jatropha curcas L. in polymetallic acid mine tailings. Int. J. Phytoremediation 2011, 13, 788–804. [Google Scholar] [CrossRef]
- Sylvain, B.; Mikael, M.-H.; Florie, M.; Joussein, E.; Soubrand-Colin, M.; Sylvain, B.; Domenico, M. Phytostabilization of As, Sb and Pb by two willow species (S. viminalis and S. purpurea) on former mine technosols. Catena 2016, 136, 44–53. [Google Scholar] [CrossRef]
- Yfru, D.D.; Nzengung, V.A. Organic carbon biostimulates rapid rhizodegradation of perchlorate. Environ. Toxicol. Chem. 2008, 27, 2419–2426. [Google Scholar] [CrossRef]
- Xiang, L.; Song, Y.; Bian, Y.; Liu, G.; Herzberger, A.; Gu, C.; Jiang, X.; Wang, F. Manure amendment reduced plant uptake and enhanced rhizodegradation of 2, 2’, 4, 4’-tetrabrominated diphenyl ether in soil. Biol. Fertil. Soils 2018, 54, 807–817. [Google Scholar] [CrossRef]
- Yadav, B.K.; Siebel, M.A.; Van Bruggen, J.J.A. Rhizofiltration of a heavy metal (Lead) containing wastewater using the wetland plant Carex pendula. Clean Soil Air Water 2011, 39, 467–474. [Google Scholar] [CrossRef]
- Abubakar, M.M.; Ahmad, M.M.; Getso, B.U. Rhizofiltration of heavy metals from eutrophic water using Pistia stratiotes in a controlled environment. J. Environ. Sci. Toxicol. Food Technol. 2014, 8, 1–3. [Google Scholar] [CrossRef]
- Veselý, T.; Tlustoš, P.; Száková, J. Organic acid enhanced soil risk element (Cd, Pb and Zn) leaching and secondary bioconcentration in water lettuce (Pistia stratiotes, L.) in the rhizofiltration process. Int. J. Phytoremediation 2012, 14, 335–349. [Google Scholar]
- Salido, A.L.; Hasty, K.L.; Lim, J.M.; Butcher, D.J. Phytoremediation of arsenic and lead in contaminated soil using Chinese brake ferns (Pteris vittata) and Indian mustard (Brassica juncea). Int. J. Phytoremediation 2003, 5, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, S.D.; Shann, J.R.; Crowley, D.E.; Anderson, T.A. Phytoremediation of contaminated water and soil. In Phytoremediation of Soil and Water Contaminants; Kruger, E.L., Anderson, T.A., Coats, J.L., Eds.; American Chemical Society: Washington, DC, USA, 1997. [Google Scholar]
- Chuan, M.C.; Shu, G.Y.; Liu, J.C. Solubility of heavy metals in a contaminated soil: Effects of redox potential and pH. Water Air Soil Pollut. 1996, 90, 543–556. [Google Scholar] [CrossRef]
- Barrow, N.J.; Whelan, B.R. Comparing the effects of pH on the sorption of metals by soil and by goethite, and on uptake by plants. Eur. J. Soil Sci. 1998, 49, 683–692. [Google Scholar] [CrossRef]
- Bradl, H.B. Adsorption of heavy metal ions on soils and soils constituents. J. Colloid Interface Sci. 2004, 277, 1–18. [Google Scholar] [CrossRef]
- Willscher, S.; Jablonski, L.; Fona, Z.; Rahmi, R.; Wittig, J. Phytoremediation experiments with Helianthus tuberosus under different pH and heavy metal soil concentrations. Hydrometallurgy 2017, 168, 153–158. [Google Scholar] [CrossRef]
- Bagga, D.K.; Peterson, S. Phytoremediation of arsenic- contaminated soil as affected by the chelating agent CDTA and different levels of soil pH. Remediat. J. 2001, 12, 77–85. [Google Scholar] [CrossRef]
- Brown, S.L.; Chaney, R.L.; Angle, J.S.; Baker, A.J.M. Phytoremediation potential of thlaspi caerulescens and bladder campion for Zinc- and cadmium-contaminated soil. J. Environ. Qual. 1994, 23, 1151–1157. [Google Scholar] [CrossRef]
- Chen, Y.X.; Lin, Q.; Luo, Y.M.; He, Y.F.; Zhen, S.J.; Yu, Y.L.; Tian, G.M.; Wong, M.H. The role of citric acid on the phytoremediation of heavy metal contaminated soil. Chemosphere 2003, 50, 807–811. [Google Scholar] [CrossRef]
- Hattori, H.; Kuniyasu, K.; Chiba, K.; Chino, M. Soil science and plant nutrition effect of chloride application and low soil pH on cadmium uptake from soil by plants. Soil Sci. Plant. Nutr. 2006, 52, 89–94. [Google Scholar] [CrossRef]
- Saleh, H.M. Water hyacinth for phytoremediation of radioactive waste simulate contaminated with cesium and cobalt radionuclides. Nucl. Eng. Des. 2012, 242, 425–432. [Google Scholar] [CrossRef]
- Singh, D.; Gupta, R.; Tiwari, A. Potential of duckweed (Lemna minor) for removal of lead from wastewater by phytoremediation. J. Pharm. Res. 2012, 5, 1578–1582. [Google Scholar]
- Corradini, E.; de Moura, M.R.; Mattoso, L.H.C. A preliminary study of the incorparation of NPK fertilizer into chitosan nanoparticles. Express Polym. Lett. 2010, 4, 509–515. [Google Scholar] [CrossRef]
- Liao, X.Y.; Chen, T.B.; Xiao, X.Y.; Xie, H.; Yan, X.L.; Zhai, L.M.; Wu, B. Selecting appropriate forms of nitrogen fertilizer to enhance soil arsenic removal by Pteris vittata: A new approach in phytoremediation. Int. J. Phytoremediation 2007, 9, 269–280. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, R.; Xia, S.; Wang, L.; Liu, C.; Zhang, R.; Fan, Z.; Chen, F.; Liu, Y. Interactions between N, P, and K fertilizers affect the environment and the yield and quality of satsumas. Glob. Ecol. Conserv. 2019, 19, e00663. [Google Scholar] [CrossRef]
- Wu, L.; Li, H.; Luo, Y.M.; Christie, P. Nutrients can enhance phytoremediation of copper-polluted soil by Indian mustard. Environ. Geochem. Health 2004, 26, 331–335. [Google Scholar] [CrossRef]
- Schwartz, C.; Echevarria, G.; Morel, J.L. Phytoextraction of cadmium with Thlaspi caerulescens. Plant Soil 2003, 249, 27–35. [Google Scholar] [CrossRef]
- Jacobs, A.; Noret, N.; Van Baekel, A.; Liénard, A.; Colinet, G.; Drouet, T. Influence of edaphic conditions and nitrogen fertilizers on cadmium and zinc phytoextraction efficiency of Noccaea caerulescens. Sci. Total Environ. 2019, 665, 649–659. [Google Scholar] [CrossRef]
- Di Luca, G.A.; Hadad, H.R.; Mufarrege, M.M.; Maine, M.A.; Sánchez, G.C. Improvement of Cr phytoremediation by Pistia stratiotes in presence of nutrients. Int. J. Phytoremediation 2014, 16, 167–178. [Google Scholar] [CrossRef]
- Merkl, N.; Schultze-Kraft, R.; Arias, M. Influence of fertilizer levels on phytoremediation of crude oil- contaminated soils with the tropical pasture grass Brachiaria brizanth (Hochst. ex A. Rich.) Stapf. Int. J. Phytoremediation 2005, 7, 217–230. [Google Scholar] [CrossRef]
- Cartmill, A.D.; Cartmill, D.L.; Alarcón, A. Controlled release fertilizer increased phytoremediation of petroleum-contaminated sandy soil. Int. J. Phytoremediation 2014, 16, 285–301. [Google Scholar] [CrossRef] [PubMed]
- Jayaweera, M.W.; Kasturiarachchi, J.C.; Kularatne, R.K.A.; Wijeyekoon, S.L.J. Contribution of water hyacinth (Eichhornia crassipes (Mart.) Solms) grown under different nutrient conditions to Fe-removal mechanisms in constructed wetlands. J. Environ. Manag. 2008, 87, 450–460. [Google Scholar] [CrossRef] [PubMed]
- Ji, P.; Sun, T.; Song, Y.; Ackland, M.L.; Liu, Y. Strategies for enhancing the phytoremediation of cadmium-contaminated agricultural soils by Solanum nigrum L. Environ. Pollut. 2011, 159, 762–768. [Google Scholar] [CrossRef] [PubMed]
- Simon, E. Heavy metals in soils, vegetation development and heavy metal tolerance in plant populations from metalliferous areas. New Phytol. 1978, 81, 175–188. [Google Scholar] [CrossRef]
- Lu, A.; Zhang, S.; Shan, X. Time effect on the fractionation of heavy metals in soils. Geoderma 2005, 125, 225–234. [Google Scholar] [CrossRef]
- Goyal, S.; Mishra, M.M.; Hooda, I.S.; Singh, R. Organic matter-microbial biomass relationships in field experiments under tropical conditions: Effects of inorganic fertilization and organic amendments. Soil Biol. Biochem. 1992, 24, 1081–1084. [Google Scholar] [CrossRef]
- Fontaine, S.; Mariotti, A.; Abbadie, L. The priming effect of organic matter: A question of microbial competition? Soil Biol. Biochem. 2003, 35, 837–843. [Google Scholar] [CrossRef]
- Wai Mun, H.; Lai Hoe, A.; Don Koo, L. Assessment of Pb uptake, translocation and immobilization in kenaf (Hibiscus cannabinus L.) for phytoremediation of sand tailings. J. Environ. Sci. 2008, 20, 1341–1347. [Google Scholar]
- Pillai, S.S.; Girija, N.; Williams, G.P.; Koshy, M. Impact of organic manure on the phytoremediation potential of Vetiveria zizanioides in chromium-contaminated soil. Chem. Ecol. 2013, 29, 270–279. [Google Scholar] [CrossRef]
- Cheng, K.Y.; Lai, K.M.; Wong, J.W.C. Chemosphere effects of pig manure compost and nonionic-surfactant tween 80 on phenanthrene and pyrene removal from soil vegetated with Agropyron elongatum. Chemosphere 2008, 73, 791–797. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, J.; Zhu, Z. Pig manure vermicompost (PMVC) can improve phytoremediation of Cd and PAHs co-contaminated soil by Sedum alfredii. J. Soil Sediments 2012, 12, 1089–1099. [Google Scholar] [CrossRef]
- Wei, S.; Li, Y.; Zhou, Q.; Srivastava, M.; Chiu, S.; Zhan, J.; Wu, Z.; Sun, T. Effect of fertilizer amendments on phytoremediation of Cd-contaminated soil by a newly discovered hyperaccumulator Solanum nigrum L. J. Hazard. Matter. 2010, 176, 269–273. [Google Scholar] [CrossRef]
- Wei, S.; Wang, S.; Zhou, Q.; Zhan, J.; Ma, L.; Wu, Z.; Sun, T.; Prasad, M.N.V. Potential of Taraxacum mongolicum Hand-Mazz for accelerating phytoextraction of cadmium in combination with eco-friendly amendments. J. Hazard. Mater. 2010, 181, 480–484. [Google Scholar] [CrossRef]
- Wei, S.; Zhu, J.; Zhou, Q.; Zhan, J. Fertilizer amendment for improving the phytoextraction of cadmium by a hyperaccumulator Rorippa globosa (Turcz.) Thell. J. Soil Sediments 2011, 11, 915–922. [Google Scholar] [CrossRef]
- Nwaichi, E.O.; Onyeike, E.N.; Wegwu, M.O. Comparison of chicken manure and urea fertilizers as potential soil amendments for enhanced phytoextraction of heavy metals. Bioremediat. J. 2010, 14, 180–188. [Google Scholar] [CrossRef]
- Houben, D.; Evrard, L.; Sonnet, P. Beneficial effects of biochar application to contaminated soils on the bioavailability of Cd, Pb and Zn and the biomass production of rapeseed (Brassica napus L.). Biomass Bioenergy 2013, 57, 196–204. [Google Scholar] [CrossRef]
- Han, T.; Zhao, Z.; Bartlam, M.; Wang, Y. Combination of biochar amendment and phytoremediation for hydrocarbon removal in petroleum-contaminated soil. Environ. Sci. Pollut. Res. 2016, 23, 21219–21228. [Google Scholar] [CrossRef] [PubMed]
- Saum, L.; Jiménez, M.B.; Crowley, D. Influence of biochar and compost on phytoremediation of oil-contaminated soil. Int. J. Phytoremediation 2018, 20, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Evangelou, M.W.H.; Daghan, H.; Schaeffer, A. The influence of humic acids on the phytoextraction of cadmium from soil. Chemosphere 2004, 57, 207–213. [Google Scholar] [CrossRef]
- Angin, I.; Turan, M.; Ketterings, Q.M.; Cakici, A. Humic acid addition enhances B and Pb phytoextraction by vetiver grass (Vetiveria zizanioides (L.) Nash). Water Air Soil Pollut. 2008, 188, 335–343. [Google Scholar] [CrossRef]
- Valdrighi, M.M.; Pera, A.; Agnolucci, M.; Frassinetti, S.; Lunardi, D.; Vallini, G. Effects of compost-derived humic acids on vegetable biomass production and microbial growth within a plant (Cichorium intybus)-soil system: A comparative study. Agric. Ecosyst. Environ. 1996, 58, 133–144. [Google Scholar] [CrossRef]
- Vargas, C.; Pérez-Esteban, J.; Escolástico, C.; Masaguer, A.; Moliner, A. Phytoremediation of Cu and Zn by vetiver grass in mine soils amended with humic acids. Environ. Sci. Pollut. Res. 2016, 23, 13521–13530. [Google Scholar] [CrossRef] [PubMed]
- Ke, L.; Wong, T.W.Y.; Wong, A.H.Y.; Wong, Y.S.; Tam, N.F.Y. Negative effects of humic acid addition on phytoremediation of pyrene-contaminated sediments by mangrove seedlings. Chemosphere 2003, 52, 1581–1591. [Google Scholar] [CrossRef]
- Matraszek, R.; Hawrylak-Nowak, B. Current issues growth and mineral composition of nickel-stressed plants under conditions of supplementation with excessive amounts of calcium and iron. J. Toxicol. Environ. Health 2010, 73, 1260–1273. [Google Scholar] [CrossRef] [PubMed]
- Sundaramoorthy, P.; Chidambaram, A.; Ganesh, K.S.; Unnikannan, P.; Baskaran, L. Comptes rendus biologies chromium stress in paddy: (i) Nutrient status of paddy under chromium stress; (ii) Phytoremediation of chromium by aquatic and terrestrial weeds. Comptes Rendus Biol. 2010, 333, 597–607. [Google Scholar] [CrossRef]
- Gomes, M.P.; Marques, T.C.L.L.S.M.; Carneiro, M.M.L.C.; Soares, Â.M. Anatomical characteristics and nutrient uptake and distribution associated with the Cd-phytoremediation capacity of Eucalyptus camaldulenses Dehnh. J. Soil Sci. Plant Nutr. 2012, 12, 481–495. [Google Scholar] [CrossRef]
- Dheeba, B.; Sampathkumar, P.A. Comparative study on the phytoextraction of five common plants against chromium toxicity. Orient. J. Chem. 2012, 28, 867–879. [Google Scholar] [CrossRef]
- Sekhar, K.C.; Kamala, C.T.; Chary, N.S.; Balaram, V.; Garcia, G. Potential of Hemidesmus indicus for phytoextraction of lead from industrially contaminated soils. Chemosphere 2005, 58, 507–514. [Google Scholar] [CrossRef]
- Williams, A.; Amarasiriwardena, D.; Xing, B. Comparison of natural organic acids and synthetic chelates at enhancing phytoextraction of metals from a multi-metal contaminated soil. Environ. Pollut. 2006, 140, 114–123. [Google Scholar]
- Evangelou, M.W.H.; Ebel, M.; Schaeffer, A. Evaluation of the effect of small organic acids on phytoextraction of Cu and Pb from soil with tobacco Nicotiana tabacum. Chemosphere 2006, 63, 996–1004. [Google Scholar] [CrossRef]
- Liu, D.; Islam, E.; Li, T.; Yang, X. Comparison of synthetic chelators and low molecular weight organic acids in enhancing phytoextraction of heavy metals by two ecotypes of Sedum alfredii Hance. J. Hazard. Matter. 2008, 153, 114–122. [Google Scholar] [CrossRef]
- Evangelou, M.W.H.; Ebel, M.; Hommes, G.; Schaeffer, A. Biodegradation: The reason for the inefficiency of small organic acids in chelant-assisted phytoextraction. Water Air Soil Pollut. 2008, 195, 177–188. [Google Scholar] [CrossRef]
- Wu, Q.; Deng, J.; Long, X.; Morel, J.; Schwartz, C. Selection of appropriate organic additives for enhancing Zn and Cd phytoextraction by hyperaccumulators. J. Environ. Sci. 2006, 18, 2–7. [Google Scholar] [CrossRef]
- Hsiao, K.; Kao, P.; Hseu, Z. Effects of chelators on chromium and nickel uptake by Brassica juncea on serpentine-mine tailings for phytoextraction. J. Hazard. Matter. 2007, 148, 366–376. [Google Scholar] [CrossRef]
- Evangelou, M.W.H.; Ebel, M.; Schaeffer, A. Chelate assisted phytoextraction of heavy metals from soil. Effect, mechanism, toxicity, and fate of chelating agents. Chemosphere 2007, 68, 989–1003. [Google Scholar] [CrossRef]
- Kos, B.; Lestan, D. Chelator induced phytoextraction and in situ soil washing of Cu. Environ. Pollut. 2004, 132, 333–339. [Google Scholar] [CrossRef]
- Quartacci, M.F.; Baker, A.J.M. Nitrilotriacetate- and citric acid-assisted phytoextraction of cadmium by Indian mustard (Brassica juncea (L.) Czernj, Brassicaceae). Chemosphere 2005, 59, 1249–1255. [Google Scholar] [CrossRef]
- Luo, C.; Shen, Z.; Lou, L.; Li, X. EDDS and EDTA-enhanced phytoextraction of metals from artificially contaminated soil and residual effects of chelant compounds. Environ. Pollut. 2006, 144, 862–871. [Google Scholar] [CrossRef]
- Neugschwandtner, R.W.; Tlusto, P.; Komárek, M. Phytoextraction of Pb and Cd from a contaminated agricultural soil using different EDTA application regimes: Laboratory versus field scale measures of efficiency. Geoderma 2008, 144, 446–454. [Google Scholar] [CrossRef]
- Bucheli-Witschel, M.; Egli, T. Environmental fate and microbial degradation of aminopolycarboxylic acids. FEMS Microbiol. Rev. 2001, 25, 69–106. [Google Scholar] [CrossRef]
- Keller, C.; Hammer, D.; Keller, C.; Hammer, D.; Kayser, A.; Richner, W.; Brodbeck, M.; Sennhauser, M. Root development and heavy metal phytoextraction efficiency: Comparison of different plant species in the field. Plant Soil 2003, 249, 67–81. [Google Scholar] [CrossRef]
- Tolra, R.; Pongrac, P.; Poschenrieder, C.; Vogel-Mikus, K.; Regvar, M.; Barceló, J. Distinctive effects of cadmium on glucosinolate profiles in Cd hyperaccumulator Thlaspi praecox and non-hyperaccumulator Thlaspi arvense. Plant Soil 2006, 288, 333–341. [Google Scholar] [CrossRef]
- Shen, Z.G.; Zhao, F.J.; McGrath, S.P. Uptake and transport of zinc in the hyperaccumulator Thiaspi caerulescens and the non-hyperaccumulator Thiaspi ochroleucum. Plant Cell Environ. 1997, 20, 898–906. [Google Scholar] [CrossRef]
- Lambrechts, T.; Lequeue, G.; Lobet, G.; Godin, B.; Bielders, C.L.; Lutts, S. Comparative analysis of Cd and Zn impacts on root distribution and morphology of Lolium perenne and Trifolium repens: Implications for phytostabilization. Plant Soil 2014, 376, 229–244. [Google Scholar] [CrossRef]
- Simon, L. Stabilization of metals in acidic mine spoil with amendments and red fescue (Festuca rubra L.) growth. Environ. Geochem. Health 2005, 27, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Kacprzak, M.; Grobelak, A.; Grosser, A.; Napora, A. The potential of biosolid application for the phytostabilisation of metals. Desalin. Water Treat. 2014, 52, 3955–3964. [Google Scholar] [CrossRef]
- Jadia, C.D.; Fulekar, M.H. Phytotoxicity and remediation of heavy metals by fibrous root grass (sorghum). J. Appl. Biosci. 2008, 10, 491–499. [Google Scholar]
- Gunarathne, V.; Mayakaduwa, S.; Ashiq, A.; Weerakoon, S.; Biswas, J.; Vithanage, M. Transgenic plants: Benefits, applications, and potential risks in phytoremediation. In Transgenic Plant Technology for Remediation of Toxic Metals and Metalloids; Prasad, M.N.V., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 89–102. [Google Scholar]
- Liu, D.; An, Z.; Mao, Z.; Ma, L.; Lu, Z. Enhanced heavy metal tolerance and accumulation by transgenic sugar beets expressing Streptococcus thermophilus StGCS-GS in the Presence of Cd, Zn and Cu alone or in combination. PLoS ONE 2015, 10, e0128824. [Google Scholar] [CrossRef]
- He, J.; Li, H.; Ma, C.; Zhang, Y.; Polle, A.; Rennenberg, H.; Cheng, X.; Luo, Z. Overexpression of bacterial γ-glutamylcysteine synthetasemediates changes in cadmium influx, allocation anddetoxification in poplar. New Phytol. 2015, 205, 240–254. [Google Scholar] [CrossRef]
- Sharma, R.; Yeh, K. The dual benefit of a dominant mutation in Arabidopsis IRON DEFICIENCY TOLERANT1 for iron biofortification and heavy metal phytoremediation. Plant Biotechnol. J. 2020, 18, 1200–1210. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.; Liu, B.; Liu, J.; He, J.; Lv, L.; Wang, H.; Xie, X.; Tao, Q.; Chen, Q. Phytoremediation of acetochlor residue by transgenic Arabidopsis expressing the acetochlor N-dealkylase from Sphingomonas wittichii DC-6. Sci. Total Environ. 2020, 723, 138687. [Google Scholar] [CrossRef]
- Zhang, L.; Rylott, E.L.; Bruce, N.C.; Strand, S.E. Genetic modifcation of western wheatgrass (Pascopyrum smithii) for the phytoremediation of RDX and TNT. Planta 2019, 249, 1007–1115. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Cang, L.; Wang, Q.; Zhou, D.; Cheng, J.; Xu, H. Roles of abiotic losses, microbes, plant roots, and root exudates on phytoremediation of PAHs in a barren soil. J. Hazard. Matter. 2010, 176, 919–925. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.G. Role of soil microbes in the rhizospheres of plants growing on trace metal contaminated soils in phytoremediation. J. Trace Elem. Med. Biol. 2005, 18, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.; Moon, H.S.; Nam, K.; Kim, J.Y.; Kim, T.S. Application of phosphate-solubilizing bacteria for enhancing bioavailability and phytoextraction of cadmium (Cd) from polluted soil. Chemosphere 2012, 88, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Ahemad, M. Phosphate-solubilizing bacteria-assisted phytoremediation of metalliferous soils: A review. 3 Biotech 2015, 5, 111–121. [Google Scholar] [CrossRef]
- Ahemad, M. Enhancing phytoremediation of chromium-stressed soils through plant-growth-promoting bacteria. J. Genet. Eng. Biotechnol. 2015, 13, 51–58. [Google Scholar] [CrossRef]
- Chang, P.; Gerhardt, K.E.; Huang, X.; Yu, X.; Glick, B.R.; Gerwing, P.D.; Greenberg, B.M. Plant growth-promoting bacteria facilitate the growth of barley and oats in salt-impacted soil: Implications for phytoremediation of saline soils. Int. J. Phytoremediation 2014, 16, 1133–1147. [Google Scholar] [CrossRef]
- Ma, Y.; Rajkumar, M.; Zhang, C.; Freitas, H. Inoculation of Brassica oxyrrhina with plant growth promoting bacteria for the improvement of heavy metal phytoremediation under drought conditions. J. Hazard. Mater. 2016, 320, 36–44. [Google Scholar] [CrossRef]
- Marques, A.P.; Moreira, H.; Franco, A.R.; Rangel, A.O.; Castro, P.M. Inoculating Helianthus annuus (sunflower) grown in zinc and cadmium contaminated soils with plant growth promoting bacteria—Effects on phytoremediation strategies. Chemosphere 2013, 392, 74–83. [Google Scholar] [CrossRef]
- Rajkumar, M.; Freitas, H. Effects of inoculation of plant-growth promoting bacteria on Ni uptake by Indian mustard. Bioresour. Technol. 2008, 99, 3491–3498. [Google Scholar] [CrossRef]
- Bhaduri, A.M.; Fulekar, M.H. Assessment of arbuscular mycorrhizal fungi on the phytoremediation potential of Ipomoea aquatica on cadmium uptake. 3 Biotech 2012, 2, 193–198. [Google Scholar] [CrossRef]
- Gunathilakae, N.; Yapa, N.; Hettiarachchi, R. Effect of arbuscular mycorrhizal fungi on the cadmium phytoremediation potential of Eichhornia crassipes (Mart.) Solms. Groundw. Sustain. Dev. 2018, 7, 477–482. [Google Scholar] [CrossRef]
- Farraji, H.; Zaman, N.Q.; Tajuddin, R.M.; Faraji, H. Advantages and disadvantages of phytoremediation: A concise review. Int. J. Environ. Tech. Sci. 2016, 2, 69–75. [Google Scholar]
- Wan, X.; Lei, M.; Chen, T. Cost—Benefit calculation of phytoremediation technology for heavy-metal-contaminated soil. Sci. Total Environ. 2015, 563, 796–802. [Google Scholar] [CrossRef]
- Chen, C.; Chiou, I. Remediation of heavy metal-contaminated farm soil using management framework. Environ. Eng. Sci. 2008, 25, 11–32. [Google Scholar] [CrossRef]
- Purakayastha, T.J.; Chhonkar, P.K. Phytoremediation of heavy metal contaminated soils. In Soil Heavy Metals; Sherameti, J., Varma, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; Volume 19, pp. 389–429. [Google Scholar]
- Robinson, B.H.; Anderson, C.W.N.; Dickinson, N.M. Phytoextraction: Where’s the action? J. Geochem. Explor. 2015, 151, 34–40. [Google Scholar] [CrossRef]
- Mohanty, M. Post-harvest management of phytoremediation technology. J. Environ. Anal. Toxicol. 2016. [Google Scholar] [CrossRef]
- Simonnot, M.; Vaughan, J.; Laubie, B. Processing of bio-ore to products. In Agromining: Farming for Metals; van der Ent, A., Baker, A.J.M., Reeves, R.D., Chaney, R.L., Anderson, C.W.N., Meech, J.A., Erskine, P.D., Simonnot, M.-O., Vaughan, J., et al., Eds.; Springer International Publishing AG: Cham, Switzerland, 2018; pp. 39–51. [Google Scholar]
- Vaughan, J.; Riggio, J.; Chen, J.; Peng, H.; Harris, H.; van der Ent, A. Characterisation and hydrometallurgical processing of nickel from tropical agromined bio-ore. Hydrometllurgy 2017, 169, 346–355. [Google Scholar] [CrossRef]
- Han, Z.; Guo, Z.; Zhang, Y.; Xiao, X.; Xu, Z.; Sun, Y. Adsorption-pyrolysis technology for recovering heavy metals in solution using contaminated biomass phytoremediation. Resour. Conserv. Recycl. 2018, 129, 20–26. [Google Scholar] [CrossRef]
- Zhao, Z.; Yan, H. Assessment of the biomass power generation industry in China. Renew. Energy 2012, 37, 53–60. [Google Scholar] [CrossRef]
- Sürmen, Y. The necessity of biomass energy for the Turkish economy. Energy Sources 2003, 25, 83–92. [Google Scholar] [CrossRef]
- Uddin, M.N.; Techato, K.; Taweekun, J.; Rahman, M.M.; Rasul, M.G.; Mahlia, T.M.I.; Ashrafur, S.M. An Overview of recent developments in biomass pyrolysis technologies. Energies 2018, 11, 3115. [Google Scholar] [CrossRef]
- Balat, M.; Ayar, G. Biomass energy in the world, use of biomass and potential trends. Energy Sources 2005, 27, 931–940. [Google Scholar] [CrossRef]
- McKendry, P. Energy production from biomass (part 1): Overview of biomass. Bioresour. Technol. 2002, 83, 37–46. [Google Scholar] [CrossRef]
- He, J.; Strezov, V.; Kumar, R.; Weldekidan, H.; Jahan, S.; Dastjerdi, B.H.; Zhou, X.; Kan, T. Pyrolysis of heavy metal contaminated Avicennia marina biomass from phytoremediation: Characterisation of biomass and pyrolysis products. J. Clean Prod. 2019, 234, 1235–1245. [Google Scholar] [CrossRef]
- Chandra, V.; Bajpai, O.; Singh, N. Energy crops in sustainable phytoremediation. Renew. Sustain. Energy Rev. 2016, 54, 58–73. [Google Scholar]
- Tripathi, V.; Edrisi, S.A.; Abhilash, P.C. Towards the coupling of phytoremediation with bioenergy production. Renew. Sust. Energ. Rev. 2016, 57, 1386–1389. [Google Scholar] [CrossRef]
- Witters, N.; Mendelshon, R.O.; Van Slycken, S.; Weyens, N.; Schreurs, E.; Meers, E.; Tack, F.; Carleer, R.; Vangronsveld, J. Phytoremediation, a sustainable remediation technology? Conclusions from a case study. I: Energy production and carbon dioxide abatement. Biomass Bioenerg 2012, 39, 454–469. [Google Scholar] [CrossRef]
- Hunce, S.Y.; Clemente, R.; Bernal, M.P. Energy production potential of phytoremediation plant biomass: Helianthus annuus and Silybum marianum. Ind. Crop. Prod. 2019, 135, 206–216. [Google Scholar] [CrossRef]
- Meers, E.; Van Slycken, S.; Adriaensen, K.; Ruttens, A.; Vangronsveld, J.; Du Laing, G.; Witters, N.; Thewys, T.; Tack, F.M. The use of bio-energy crops (Zea mays) for ‘phytoattenuation’ of heavy metals on moderately contaminated soils: A field experiment. Chemosphere 2010, 78, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Balsamo, R.A.; Kelly, W.J.; Satrio, J.A.; Ruiz-Felix, M.N.; Fetterman, M.; Wynn, R.; Hagel, K. Utilization of grasses for potential biofuel production and phytoremediation of heavy metal contaminated soils. Int. J. Phytoremediation 2015, 17, 448–455. [Google Scholar] [CrossRef]
- Porte, A.F.; de Souza, R.; Kaercher, J.A.; Klamt, R.A.; Schmatz, W.L.; Da Silva, W.L.T.; Severo Filho, W.A. Sunflower biodiesel production and application in family farms in Brazil. Fuel 2010, 12, 3718–3724. [Google Scholar] [CrossRef]
- Hesami, S.M.; Zilouei, H.; Karimi, K.; Asadinezhad, A. Enhanced biogas production from sunflower stalks using hydrothermal and organosolv pretreatment. Ind. Crop. Prod. 2015, 76, 449–455. [Google Scholar] [CrossRef]
- Houou, R.; Kindomihou, V. Impact of nitrogen fertilization on the oil, protein, starch, and ethanol yield of corn (Zea mays L.) grown for biofuel production. J. Life Sci. 2011, 5, 1013–1021. [Google Scholar]
- Amon, T.; Amon, B.; Kryvoruchko, V.; Zollitsch, W.; Mayer, K.; Gruber, L. Biogas production of maize and dairy cattle manure—Influence of biomass composition on the methane yield. Agric. Ecosyst. Environ. 2007, 118, 173–182. [Google Scholar] [CrossRef]
- Wilkes, M.A.; Takei, I.; Caldwell, R.A.; Trethowan, R.M. The effect of genotype and environment on biodiesel quality prepared from Indian mustard (Brassica juncea) grown in Australia. Ind. Crop. Prod. 2013, 28, 124–132. [Google Scholar] [CrossRef]
- Lee, W.; Kuan, W. Miscanthus as cellulosic biomass for bioethanol production. Biotechnol. J. 2015, 10, 840–854. [Google Scholar] [CrossRef]
- Kiesel, A.; Lewandowski, I. Miscanthus as biogas substrate—Cutting tolerance and potential for anaerobic digestion. Gcb Bioenergy 2017, 7, 153–167. [Google Scholar] [CrossRef]
- Kim, H.; Song, H.; Jeong, M.; Seo, Y.L.; Yang, J.K.; Yoo, S.B.; Choi, M.S. Bioethanol production by enzymatic saccharification of Salix viminalis var. Gigantea Biomass. For. Sci. Technol. 2012, 10, 67–72. [Google Scholar]
- Horn, S.J.; Estevez, M.M.; Nielsen, H.K.; Linjordet, R.; Eijsink, V.G. Biogas production and saccharification of Salix pretreated at different steam explosion conditions. Bioresour. Technol. 2011, 102, 7932–7936. [Google Scholar] [CrossRef]

| Mechanism | Plant | Research Location | Medium | Experimental Details | Contaminant and Initial Concentration | Conclusions | Reference |
|---|---|---|---|---|---|---|---|
| Phytoextraction | Typha Latifolia | China | wetland soil | Greenhouse pot experiment (pots—20 × 20 × 20 cm, day/night temperature of 25/18 °C, relative humidity from 70 to 80%) Exposition to contaminants: 90 days | Cd (0.1 and 30 mg/kg soil) |
| [25] |
| Typha Angustifolia | China | soil (1/2 compost, 1/4 vermiculite and 1/4 sand) | Greenhouse pot experiment (pots—20 cm high, capacity 5 L). Addition of chelator: 2.5, 5, and 10 mM EDTA or citric acid Exposition to contaminants: 20 days + 25 days after chelator application | Cd (50 mg/kg soil) Cu (10 mg/kg soil) Pb (20 mg/kg soil) Cr (10 mg/kg soil) |
| [121] | |
| Helianthus annuus | Switzerland | loamy topsoil from an agricultural field | Greenhouse pot experiment (16 h (21 °C)/8 h (16 °C) day/night cycle, light intensity at leaf height of 10,900 lux) Addition of chelator: 10 mM EDDS Exposition to contaminants: 4 weeks + 5 days after chelator application | Cu (360 mg/kg soil) Zn (530 mg/kg soil) |
| [122] | |
| Thlaspi caerulescens | Netherlands | soil contaminated due to atmospheric deposition of metal-bearing dust from a smelter | Greenhouse pot experiment (cone-shaped pots—18 cm in height with a top diameter of 18 cm and a bottom diameter of 14 cm, relative humidity from 30 to 60%, 16 h (20 °C)/8h (18 °C) day/night cycle, artificial light intensity of 400 W/m2) Exposition to contaminants: 113 days | Cd (from 0.51 = 0.03 to 9.6 ± 0.6 mg/kg soil) Zn (from 33.3 ± 1.4 to 776 ± 43 mg/kg soil) |
| [123] | |
| Thlaspi goesingense | Austria | soil collected from the vicinity of a former Pb-Zn smelter (sand, silt, clay) | Greenhouse pot experiment Addition of chelator: EDTA and (NH4)2SO4Exposition to contaminants: 143 days + 7 days after chelator application | Cd (6.3 and 19.7 mg/kg soil) Cu (174 and 232 mg/kg soil) Ni (23.9 and 126 mg/kg soil) Pb (12300 mg/kg soil) Zn (714 and 2710 mg/kg soil) |
| [124] | |
| Brassica juncea | Italy | soil from farming area contaminated by high metal polluted irrigation water | Greenhouse pot experiment (pots—22 cm diameter plastic, 16 h (25 °C)/8h (20 °C)day/night cycle, relative humidity of 75%, 400 µmol m−2s−1 photon flux density) Addition of chelator: 5 mmol/kg NTA or citric acid Exposition to contaminants: 43 days + 7 days after chelator application | Cd (40 mg/kg soil) Cr (186 mg/kg soil) Cu (313 mg/kg soil) Pb (1331 mg/kg soil) Zn (3326 mg/kg soil) |
| [125] | |
| Brassica napus | Austria | soil from former industrial site | Outdoors pot experiment Addition of chelator: 0.21, 0.41, 0.83, and 1.65 g/kg EDTA Exposition to contaminants: 60 days (application of chelator at 35th day and 50th day) Field-lysimeter experiment (spacing of 15 × 10 cm between individual plants) Addition of chelator: 0.25, 0.50, 1.0 and 2.01 g/kg EDTA Exposition to contaminants: about 6 months | Cu (225 mg/kg soil) Pb (77.1 mg/kg soil) Zn (344 mg/kg soil) |
| [126] | |
| Zea mays | Belgium | dredged sediments from the river (clay, silt, sand) | Greenhouse pot experiment (12 h/12 h day/night cycle, temperature from 18.5 to 22.5 °C, relative humidity from 60 to 70%.) Addition of chelator: 2mmol/kg EDTA, citric acid, and ammonium acetate Exposition to contaminants: 6 weeks (application of chelator 1 day before sowing or at 10th day after sowing) | Cu (145 ± 11 mg/kg soil) Zn (874 ± 61 mg/kg soil) Cd (9 ± 0.2 mg/kg soil) Pb (181 ± 6 mg/kg soil) Ni (58 ± 6 mg/kg soil) |
| [127] | |
| China | soil from disused agricultural field | Greenhouse pot experiment (pots—12 cm diameter and 12 cm height, temperature from 18 to 23 °C) Addition of chelator: EDTA or/and EDDS (variable ratios) Exposition to contaminants: 2 weeks + 2 weeks after chelator application | Pb (2500 mg/kg soil) Cu (500 mg/kg soil) Zn (1000 mg/kg soil) Cd (15 mg/kg soil) |
| [128] | ||
| Alyssum murale | Albania | soil from ultramafic area | Field experiment (area divided into six 36-m2 plots) Exposition to contaminants: 3 months | Ni (3500 mg/kg soil) |
| [129] | |
| Canada | agricultural soils collected near a historic Ni refinery | Greenhouse pot experiment (pots—18 cm diameter, 16 h (28 °C)/8 h (20 °C)day/night cycle) Exposition to contaminants: 120 days | Ni (1720 and 2570 mg/kg soil) Co (24 and 37 mg/kg soil) |
| [130] | ||
| Populus nigra | Egypt | clay soil | Greenhouse pot experiment (16 h/8h day/night cycle, temperatures of 25 ± 2 °C, relative humidity from 40 to 50%, 300 µmol m−2s−1 photon flux density) Exposition to contaminants: 30 months | Cd (from 3.9 to 15.6 mg/kg soil) Cu (from 3.6 to 63.6 mg/kg soil) Pb (from 19.11 to 173.3 mg/kg soil) |
| [131] | |
| Populus nigra x Populus maximowiczii | Czech Republic | soil from mining and smelting area | Pot experiment in controlled outdoor vegetation hall Addition of chelator: 3, 6 and 9 mmol/kg EDTA or EDDS Exposition to contaminants: 100 days + 30 days after chelator addition | Pb (200 ± 2 and 1360 ± 10 mg/kg soil) |
| [132] | |
| Vetiveria zizanoides | USA | soil from firing range | Greenhouse pot experiment (pots—capacity 13.2 L) Addition of chelator: 5 mmol/kg EDTA Exposition to contaminants: 4 months (addition of chelator 1 week before harvesting) | Pb (300–4500 mg/kg soil) |
| [133] | |
| Brazil | soil from an area of slag deposition | Field experiment (different spacing between rows (0.80,0.65 and 0.50 m).) Addition of chelator: 40 mmol/kg citric acid Exposition to contaminants: 69 days (addition of chelator 1 week before harvesting) | Pb (1850 mg/kg soil) |
| [134] | ||
| Rumex crispus Rumex K-1 | China | soil form farmland near the Pb/Zn mine | Field experiment (experimental area was split into eight blocks and 32 plots (each plot 3 m × 2 m)) Addition of chelator: 6 mmol/kg EDTA Exposition to contaminants: about 100 days (addition of chelator 1 week before harvesting) | Pb (960 ± 54 mg/kg) Zn (1050 ± 89 mg/kg) Cd (7.2 ± 0.92 mg/kg) |
| [135] | |
| Sedum alfredii | China | paddy soil form Pb/Zn mining site | Field experiment (21 field plots of 1.2 m × 6.8 m) Addition of chelator: MGWL, citric acid and EDTA at a mole ratio of 1:10:2 Exposition to contaminants: about 2 months (addition of chelator 21 days before harvesting) | Pb (268 mg/kg soil) Zn (121 mg/kg soil) Cd (0.99 mg/kg soil) |
| [136] | |
| China | soil irrigated with industrial wastewater | Pot experiment (pots—15 cm height and 20 cm diameter) Addition of chelator: 5 and 8 mmol/kg EDTA or citric acid Exposition to contaminants: 30 days | Cd (3.0 ± 0.4 mg/kg) Cu (45.5 ± 1.8 mg/kg) Zn (168.8 ± 16.4 mg/kg) Pb (57.9 ± 1.4 mg/kg) |
| [137] | ||
| Viola baoshanensis | China | soil from farmland near the Pb/Zn mine | Field experiment (area of 0.8 ha and consisting of six consecutively rectangle paddy plots, each plant was planted with the space of 20 cm × 20 cm) Addition of chelator: 6 mmol/kg EDTA and 10 mmol/kg (NH4)2SO4 and NH4NO3 Exposition to contaminants: about 140 days (addition of EDTA 1 week before harvesting and (NH4)2SO4 and NH4NO3 weeks before) | Pb (975 ± 84 mg/kg) Zn (924 ± 94 mg/kg) Cd (9.8 ± 0.9 mg/kg) |
| [138] | |
| Brassica juncea | Belgium | soil from vicinity of a former radium production site soil naturally contaminated by uraniferous shale vein | Greenhouse pot experiment (12 h (16 °C)/12 h (11 °C) day/night cycle) Addition of chelator: 5 mmol/kg EDDS, NTA, citric acid, oxalic acid or ammonium citrate Exposition to contaminants: 4 weeks + 2 weeks after addition of chelator | Cd (1 and 2 mg/kg soil) Zn (704 and 151 mg/kg soil) Cu (372 and 430 mg/kg soil) U (14 and 41 mg/kg soil) Pb (254 and 35 mg/kg soil) Cr (467 and 209 mg/kg soil) |
| [139] | |
| Chenopodium album | India | soil with tannery sludge (10 wt% and 25 wt%) | Greenhouse pot experiment (pots—12 inches in diameter) Exposition to contaminants: 60 days | Fe (12.6 ± 0.3 and 16.7 ± 0.2 mg/kg soil) Mn (12.3 ± 0.2 and 15.1 ± 0.3 mg/kg soil) Zn (7.1 ± 1.2 and 13.6 ± 0.4 mg/kg soil) Cr (3.6 ± 0.1 and 5.6 ± 0.1 mg/kg soil) Cu (2.7 ± 0.1 and 3.5 ± 0.0 mg/kg soil) |
| [31] | |
| Chenopodium glaucum | China | soil from mercury mining area | Greenhouse pot experiment (temperature from 25 to 30 °C, relative humidity from 40 to 60%) Addition of chelator: 0.05, 0.1 and 0.2 µM ammonium thiosulphate Exposition to contaminants: 55 days + 5 days after addition of chelator | Hg (151.13 ± 5.0 mg/kg soil) |
| [140] | |
| Phytovolatilization | Pteris Vittata | Japan | soil from deposit site of neutralized acid mine drainage | Greenhouse pot experiment (temperature varied from 25 (night) to 45°C (day) in summer and 10 (night) and 25 °C (day) in winter) Exposition to contaminants: 18 months | As (6540 ± 380 mg/kg soil) |
| [45] |
| Brassica juncea | USA | upland and wetland soil | Greenhouse pot experiment (temperature of 25 °C, relative humidity of 40%) Exposition to contaminants: 10 days | Selenium compounds (20 and 200 µM) |
| [52] | |
| Salicornia bigelovii | USA | soil mixed with sand | Greenhouse pot experiment (pots—9 cm or 25 cm in diameter, 14 h/10 h day/night cycle) Addition of amendment: 10 g/pot finely-ground pickleweed shoot tissues Exposition to contaminants: 4 months | Se (2.5 and 10 mg/kg soil) |
| [53] | |
| Poplar | USA | soil irrigated by groundwater | Field experiment Exposition to contaminants: 5 months | Trichloroethylene (from 1.4 μg/L to 190 μg/L) |
| [55] | |
| Arabidopsis thaliana | USA | aqueous solution | Hydroponic experiment (ball canning jar—capacity 1000 mL) Plants engineered with a modified bacterial mercuric reductase gene, merA and merB Exposition to contaminants: 7 days | Hg (II) (1 and 5 mg/L) |
| [54] | |
| Salix babylonica | China | aqueous solution | Hydroponic experiment in a climate control chamber (temperature of 25.0 ± 1 °C under continuous artificial light) Exposition to contaminants: 168 h | Methyl tert-butyl ether (10, 25, 50, 100, 200, and 400 mg/L) |
| [50] | |
| Phytodegradation | Myriophyllum aquaticum | USA | aqueous solution | Outdoor hydroponic experiment (Erlenmeyer flasks—capacity 250 mL, under natural light) Exposition to contaminants: 10 days | Trinitrotoluene (from 80 to 100 mg/L) |
| [66] |
| USA | aqueous solution and sand treatment | Experiment in beaker (beaker—600 mL, temperature of 20 ± 1 °C, continuous lights (150 W, 120 V) at 25 cm above the plant) Exposition to contaminants: 10 days | Perchlorate (0.2, 2.0, and 20 mg/L) |
| [65] | ||
| Brassica juncea | India | aqueous solution | Greenhouse hydroponic experiment (Erlenmeyer flasks—capacity 250 mL, temperature 25 ± 2 °C, 12 h/12 h day/night cycle) Exposition to contaminants: 5 days | Insecticide phorate (5 mg/L) |
| [79] | |
| Azolla pinnata | Iraq | dye-contaminated water | Hydroponic experiment (temperature from 20 to 23 °C, under lamp light at approximation of 10,000–25,000 lux) Exposition to contaminants: 5 days | Methylene blue (5, 15, 25 mg/L) |
| [141] | |
| Azolla filiculoides | Iran | aqueous solution | Hydroponic experiment (pots - 15 cm height and 10 cm width, natural light and ambient temperature of 30 °C) Exposition to contaminants: 14 days | Phenol (5,10,25,50 mg/L) |
| [60] | |
| Phragmites australis | Netherlands | Perlite | Greenhouse pot experiment (12 h (22 °C)/12 h (17 °C)day/night cycle, relative humidity of 60% (day) and 70% (night)) Exposition to contaminants: 21 days | Ibuprofen (60 μg/L) |
| [71] | |
| Chromolaena odorata | China | aqueous solution with wastewater | Hydroponic experiment (tanks—48 × 36 × 20 cm, temperature of 20 °C) Addition 0.4 mg/kg Chromolaena odorata L. extract Exposition to contaminants: 15 days | SDS (10 and 20 mg/L) |
| [142] | |
| Eichhornia Crassipes | China | aqueous solution | Greenhouse hydroponic experiment (containers—7 × 12.5 cm, capacity—500 mL or 30 × 25 cm, temperature of 25 ± 1 °C, 14h/10h day/night cycle, light intensity 1400 lux) Exposition to contaminants: 240h or 3 weeks | Phosphorus pesticide ethion (0.01, 0.1 and 1 mg/L) |
| [78] | |
| Cyperus Alternifolius | Thailand | synthetic wastewater | Greenhouse hydroponic experiment (temperature of 30 ± 2 °C, 12 h/12 h day/night cycle, relative humidity of 70 ± 5%) Exposition to contaminants: 12 days | Ethanolamines (MEA, DEA, TEA) (1400 mg/L) |
| [143] | |
| Erythrina crista-galli | Brazil | petroleum-contaminated soil | Greenhouse pot experiment (pots—22- cm height and 24- cm diameter, temperature from 25 °C to 30 °C, relative humidity from 85% to 90%,) Exposition to contaminants: 120 days | Hydrocarbons (25, 50, 75 g/kg soil) |
| [144] | |
| Phytostabilization | Lupinus Albus | Spain | soils affected by acid pyrite sludge | Greenhouse pot experiment (pots—capacity 5.5 L, temperature from 25 to 35 °C, relative humidity from 55 to 85%, photosynthetic photon flux density 1300 μmol m−2 s−1) Addition of chelator: 0,5 mM NTA Exposition to contaminants: 3 weeks Field experiment (rows: 35 m long and 50 cm apart, 20 g seeds/m2) Exposition to contaminants: 6 months | As (about 60 nmol/g soil) Cd (about 10 nmol/g soil) Cd (5.5 kg/ha soil) As (92.5 kg/ha soil) |
| [145] |
| Lupinus uncinatus | Mexico | soil from crop field | Greenhouse pot experiment (pots—15 cm in diameter, day and night temperature from 25 to 29 °C and from 8 to 11 °C) Exposition to contaminants: 18 weeks | Cd (9, 18, and 27 mg/kg soil) |
| [146] | |
| Vicia faba | Tunisia | vineyard soil | Greenhouse pot experiment (pots—15 cm diameter) Bacteria inoculation Exposition to contaminants: 65 days | Cu (63.5 mg/kg soil) |
| [147] | |
| Brassica juncea | Spain | soil with mine tailings | Greenhouse pot experiment (pots—capacity 0.7 L, maximum temperature from 25 to 33 °C, minimum temperature from 6 to 9 °C Addition of amendments: pine bark compost and manure compost Exposition to contaminants: 110 days | Zn (from 10.6 ± 0.1 to 18.3 ± 0.6 mg/kg soil) Cu (from 12 ± 0.2 to 118 ± 16 mg/kg soil) |
| [94] | |
| Vetiveria zizanioides | Thailand | mine tailings | Greenhouse pot experiment Addition of amendments: organic fertilizer, Osmocote® and cow manure Exposition to contaminants: 3 months Field experiment (plots 4 × 4 each) Addition of amendments: organic fertilizer and Osmocote® Exposition to contaminants: above 1 year | Pb (>10000 mg/kg soil) |
| [148] | |
| Thailand | soil contaminated by creek irrigation with creek which passes through drainage from active Zn mines | Greenhouse pot experiment (pots—8-in. diameter and 7 in. height, temperature from 26 to 30 °C, relative humidity from 60 to 80%, 4000–46,000 lux light intensity, 12 h/12 h day/night cycle) Addition of amendments: 10 wt% of cow manure, pig manure, bat manure, or organic pelleted fertilizer Exposition to contaminants: 3 months | Cd (from 33.8 to 35.7 mg/kg soil) |
| [98] | ||
| Vigna radiata | Thailand | soil from an agricultural area | Greenhouse pot experiment (pots—capacity 16 L, 19 cm in top diameter, 15 cm in bottom diameter, 30 cm in height) Addition of amendments: 5, 10, 15 wt% of biochar Exposition to contaminants: 8 weeks | Cd (58 mg/kg soil) Zn (1220 mg/kg soil) |
| [95] | |
| Lolium perenne | Chile | mine tailings | Greenhouse pot experiment (pots—12.5 cm in diameter and 12 cm height, temperature of 23 °C) Addition of amendments: 6 and 12 wt.% of biosolid Exposition to contaminants: 6 months | Cd (<0.02 mg/kg soil) Cu (485 mg/kg soil) Zn (41 mg/kg soil) Mo (109 mg/kg soil) |
| [89] | |
| Lolium italicum | Italy | soil from mining area | Mesocosm experiment Addition of amendments: 10 and 30 wt.% of compost Exposition to contaminants: 60 days | Zn (437 and 4622 mg/kg soil) Pb (374 and 17,739 mg/kg soil) |
| [80] | |
| Miscanthus sinensis | Republic of Korea | mine tailings | Greenhouse pot experiment (pots—20 cm diameter and 25 cm height, temperature from 15 to 25 °C, relative humidity from 60 to 70%) Addition of amendments: 2 wt% of bone mill, furnace slag, bottom ash, or red mud Exposition to contaminants: 90 days | Cu (388.31 mg/kg soil) Zn (2210 mg/kg soil) Pb (3889 mg/kg soil) Cd (32.46 mg/kg soil) |
| [93] | |
| Miscanthus sinensis × giganteus | Romania | soil polluted by smelter activity | Field experiment Addition of amendments: 1 wt% of red mud Exposition to contaminants: 1 year | Zn (322 to 780 mg/kg soil) Cd (4.7 to 10.3 mg/kg soil) Pb (154 to 607 mg/kg soil) |
| [92] | |
| Rose | India | soil from agricultural field | Pot experiment (pots—capacity 4.5 kg) Exposition to contaminants: 60 days | Cr (25, 50, 100, and 200 mg/kg soil) |
| [149] | |
| Jatropha curcas | China | soil from mine site | Greenhouse pot experiment (pots—capacity 2 kg, temperature from 18 to 26 °C, under natural light) Addition of amendments: 0.1, 0.25, 0.5, and 1.0% of limestone Exposition to contaminants: 6 months | Al (10756 to 32,970 mg/kg soil) Zn (287 to 651 mg/kg soil) Cu (324 to 722 mg/kg soil) Pb (266 to 2466 mg/kg soil) Cd (1 to 19.85 mg/kg soil) |
| [150] | |
| Salix viminalis Salix purpurea | France | soil from settling basin of a former gold mine | Mesocosm experiment (temperature of 20 ± 2 °C, 16 h/8 h day/night cycle, photosynthetic photon flux density 1000 μmol·m−2·s−1) Exposition to contaminants: 45 days | As (24.6 ± 14.5 to 1593 ± 280.3 mg/kg soil) Sb (0.18 ± 0.004 to 83.04 ± 2.53 mg/kg soil) Pb (3.6 ± 0.8 to 221 ± 27.9 mg/kg soil) |
| [151] | |
| Rhizodegradation | Kandelia candel | China | sediment of mangrove wetland | Greenhouse rhizobox experiment (rhizobox—150 mm × 300 mm × 200 mm, temperature from 26 to 32 °C, natural illumination, relative humidity of 85%) Exposition to contaminants: 60 days | Phenanthrene (10 mg/kg soil) Pyrene (10 mg/kg soil) |
| [109] |
| Lolium multiflorum | Japan | soil contaminated with diesel | Greenhouse pot experiment (pots—capacity 0.3 L, temperature of 25 °C, 10 h/14 h day/night cycle relative humidity of 70%, photosynthetic photon flux density of 150 μ mol m-2 s-1) Exposition to contaminants: 152 days | TPH (7977 ± 146 mg/kg soil) |
| [105] | |
| Salix nigra | USA | hydroponic solution and contaminated groundwater | Greenhouse hydroponic experiment (Erlenmeyer flasks- capacity 2 L) Addition: 500 mg/L of DOC derived from acetate, ethanol, organic mushroom compost, or chicken litter extract Exposition to contaminants: up to 70 days | Perchlorate (22 to 25 mg/L and 40 mg/L) |
| [152] | |
| Salix babylonica | USA | hydroponic solution and humic or sandy loam soil | Greenhouse bioreactor experiment (plastic buckets—2 gallons or Erlenmeyer flasks—capacity 2 L) Addition: 150 or 300 mg/L of DOC derived from chicken manure Exposition to contaminants: up to 42 days | Perchlorate (65.85 mg/L to 119.89 mg/L) |
| [112] | |
| Sebastiania Commersoniana | Brazil | petroleum-contaminated soil | Greenhouse pot experiment (pots—22 cm × 24 cm, temperature from 25 to 30°C and relative humidity from 85 to 90%) Exposition to contaminants: 424 days | TPH (25, 50, 75 g/kg soil) |
| [108] | |
| Avicennia marina | China | sediments from mangrove wetland | Greenhouse pot experiment (pots—20 cm in diameter for top, 14 cm in diameter for bottom, and 20 cm in height, 12 h/12 h day/night cycle, relative humidity of 85%, temperature from 22 to 26 °C). Exposition to contaminants: 120 days | Phenanthrene and pyrene (5, 10, and 50 mg/kg soil) |
| [110] | |
| Daucus carota | China | loamy soil | Greenhouse pot experiment (temperature from 25 to 35 °C in the daytime and from 15 to 25 °C in the nighttime, relative humidity of 50 and 75%) Addition: 1, 2, 4 wt% of composted pig manure Exposition to contaminants: 90 days | 2, 2′, 4, 4′-tetrabrominated diphenyl ether (BDE) (0.4 mg/kg soil) |
| [153] | |
| Rhizofiltration | Brassica juncea | India | aqueous solution | Hydroponic experiment (Erlenmayer flask—capacity 250 mL, temperature of 25 °C) Exposition to contaminants: 10–12 days | U (25–5000 µM) |
| [116] |
| Helianthus annuus | Republic of Korea | Groundwater | Greenhouse hydroponic experiment (glass jars—12 cm × 12 cm × 8 cm, temperature of 25 °C, relative humidity of 80%, 16 h/8 h day/night) Exposition to contaminants: 72 hours | U (30–646 µg/L) |
| [113] | |
| Carex pendula | The Netherlands | synthetic wastewater | Greenhouse hydroponic experiment (pots—capacity 11.4 L, temperature from 18 to 27 °C, 16 h/8 h day/night cycle, light intensity (48–56 mE/m2/s), relative humidity of 50%) Exposition to contaminants: 2 weeks | Pb (1, 5, 10 mg/L) |
| [154] | |
| Pistia Stratiotes | Nigeria | aqueous solution | Hydroponic experiment (aquarium—one meter by half a meter, kept in semi-shaded area) Exposition to contaminants: 21 days | Cr (1800– 2400 mg/L) Pb (1800– 2400 mg/L) Ni (1800–2400 mg/L) |
| [155] | |
| Czech Republic | soil contaminated due to the atmospheric deposition of potentially risk elements from the Pb smelter | Greenhouse pot experiment (pots—capacity 2 L) Addition: 10 mM acetic, tartaric, citric, and oxalic acid Exposition to contaminants: 21 days | Cd (5.68 ± 0.1 mg/kg soil) Pb (822 ± 14 mg/kg soil) Zn (267 ± 36 mg/kg soil) |
| [156] | ||
| Czech Republic | aqueous solution | Greenhouse hydroponic experiment (glass pots—capacity 250 mL, temperature of 25°C (day) and 18°C (night), relative humidity of 75% in the day and 55% at the night, 18 h/6 h day/night cycle) Addition of 5% nitric acid Exposition to contaminants: 14 days | Pb (25 mg/L and 125 mg/L) Cd (3.5 mg/L and 10.5 mg/L) |
| [120] |
| Plant | Phytormediation Process | References | Bio-Energy Production | References |
|---|---|---|---|---|
| Helianthus annuus | Phytoextraction of Zn and Cu Rhizofiltration of U | [122] | Bio-diesel Bio-gas | [262,263] |
| Zea mays | Phytoextraction of Pb, Cu, Zn, Cd, Ni | [127,128] | Bio-ethanol Bio-gas | [264,265] |
| Brassica juncea | Phytoextraction of Cd, Cr, Cu, Zn, Pb Rhizofiltration of U | [125] | Bio-diesel | [266] |
| Miscanthus | Phytostabilization of Cu, Zn, Pb, Cd | [92,93] | Bio-ethanol Bio-gas | [267,268] |
| Salix | Phytovolatilization of MTBE Rhizodegradation of perchlorate | [50,112,152] | Bio-ethanol Bio-gas | [269,270] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grzegórska, A.; Rybarczyk, P.; Rogala, A.; Zabrocki, D. Phytoremediation—From Environment Cleaning to Energy Generation—Current Status and Future Perspectives. Energies 2020, 13, 2905. https://doi.org/10.3390/en13112905
Grzegórska A, Rybarczyk P, Rogala A, Zabrocki D. Phytoremediation—From Environment Cleaning to Energy Generation—Current Status and Future Perspectives. Energies. 2020; 13(11):2905. https://doi.org/10.3390/en13112905
Chicago/Turabian StyleGrzegórska, Anna, Piotr Rybarczyk, Andrzej Rogala, and Dawid Zabrocki. 2020. "Phytoremediation—From Environment Cleaning to Energy Generation—Current Status and Future Perspectives" Energies 13, no. 11: 2905. https://doi.org/10.3390/en13112905
APA StyleGrzegórska, A., Rybarczyk, P., Rogala, A., & Zabrocki, D. (2020). Phytoremediation—From Environment Cleaning to Energy Generation—Current Status and Future Perspectives. Energies, 13(11), 2905. https://doi.org/10.3390/en13112905







