1. Introduction
The development of a country is intrinsically connected with the energy demand; therefore, the scarcity of energy sources justifies the improvement of studies that enable better use of the sources that are still available to humanity. Regarding the total energy generated in power plants, 70% is released into the environment as heat [
1]. In buildings, the use of heating and cooling systems increases electricity consumption, causing peaks that vary considerably during the day and night. The scarcity and intermittency of renewable energy sources, such as wind and solar energy, and the pollution produced by the use of fuels, such as oil, which is the fundamental base of the global energy matrix, motivates the search for ways to optimize energy processes.
In this sense, the development of a thermal energy storage system provides an important contribution in the search for reductions in the difference between the global energy supply and demand, as well as in the search for greater thermal efficiency in thermodynamic processes. This storage can occur through the heat from reversible chemical reactions; sensitive heat, which allows liquid or solid media to increase its internal energy by raising its temperature; and latent heat, which is a characteristic of some materials such that when subjected to thermal changes, the state of the matter that composes them is modified, increasing their internal energy and keeping their temperature almost constant.
Sensitive heat storage is easy to implement through the heating or cooling of such materials; however, the low thermal storage capacity and the high weight or volume required for this type of storage limit its applications. The storage of chemical energy has not been always applied in practice due to issues surrounding the technical aspects necessary for its application, as well as economic issues. [
2]. Therefore, energy storage in the form of latent heat has been applied due to its high heat transfer rates associated with relatively low volumes. In this context, it has been observed that certain materials can absorb or release large amounts of energy in certain periods and under specific operating conditions. These materials are called phase change materials (PCMs), which capable of storing 5 to 14 times more energy per unit volume than materials that store energy via sensitive heat, such as water, concrete, or rocks, and present specific phase change temperatures that tend to remain constant during the matter transformation [
3].
Depending on the type of PCM, the storage process can be described in terms of different types of phase transitions, such as solid–solid (changes in the material crystalline structure, which characterizes the storage or release energy), solid–liquid, liquid–gas, or solid-gas. However, liquid–gas and solid–gas transformations are not applied to construction materials, due to their high variations in volume and pressure during phase change processes. Transformations of the solid–solid type are limited due to the difficulty found in mixing them with other construction materials, such as cement and plaster. Therefore, solid–liquid-transformation PCMs are the most common type in latent thermal storage systems [
4].
However, several factors influence the application of solid–liquid PCMs: (a) on the energy side, PCMs must have adequate phase transition temperatures, high latent heat values per unit weight, high conductivity, and high thermal capacity; (b) on the physical side, it must have a favorable balance phase for their application, high density, minimum variation in volume during the phase transition, and low vapor pressure at the operating temperature; (c) regarding the chemical aspect, it must have little or no subcooling during the solidification process, a sufficient crystallization rate, and melting and solidification at the same temperature and phase separation. Furthermore, it must also have chemical properties that are capable of completing the melting/solidification cycles; have a high chemical stability; be compatible with other materials; not show degradation after a long period of thermal cycles; and must not be corrosive, toxic, or flammable [
4].
Among the mentioned requirements, it is possible to highlight the low thermal conductivity, making it difficult to transfer heat, which is a process that is fundamental to the application of these materials. To solve this problem, several procedures have been proposed: the construction of thermal intensification systems heat exchangers with finned surfaces (pin-fins, radial fins, and longitudinal fins) [
5,
6], the application of heat pipes [
7] or multiple PCMs with different melting points [
8], and techniques used to increase thermal conductivity (manufacture of composite materials composed of both PCMs) and high thermal conductivity materials (metal foams, honeycomb structures, carbon nanofillers) [
9,
10,
11], or the PCM encapsulation technique [
12,
13,
14,
15]). In this sense, studies that enable the correct understanding of these materials’ behaviors over a diverse range of applications are necessary. In this context, solar energy storage systems for heating water [
3] or air [
16], and for maintaining temperatures in buildings [
17,
18], are cases in which phase change materials can be used as thermal energy capacitors.
Al-Abidi et al. [
19] numerically studied the increase in heat transfer between a heat exchange fluid (water—H
2O) and the commercial paraffin RT82. The heat exchange process was carried out using a triplex tube heat exchanger (TTHX) that was manufactured with copper (Cu), aluminum (Al), and carbon steel tubes, and was finned on the surfaces such that it was directly in contact with the PCMs with different longitudinal fin configurations. A two-dimensional numerical model was developed using the Ansys FLUENT
® 6.3.26 software by considering pure heat conduction and natural heat convection through the phase change material. The influence of the number of fins and their dimensions and material on the complete melting time of the PCM was investigated. Experiments were conducted to validate the proposed model and the simulated results agreed well with those obtained experimentally. The results revealed that the geometry of the TTHX with eight fins achieved a 34.7% reduction in the time required for the complete PCM fusion compared with that of the triplex tube without fins, that the complete melting time required for the case with 42 mm length in the fins with a configuration of eight fins produced a reduction of 37.5% relative to the case with 10 mm fins, and that the use of copper instead steel reduced the complete melting time by 42.8%.
Kong et al. [
20] numerically evaluated the reduction in energy consumption and the improvement in thermal comfort of a building located in the city of Tianjin (China) due to the application of two panel systems with PCMs: one system used capric acid and was installed on the external surface of the walls and ceiling of the building (phase change material on the outer walls—PCMOW), while the other system used a compound of 95% capric acid and 5% 1-dodecanol that was installed on the internal surfaces of the walls and ceiling (phase change material on the inside walls—PCMIW). A one-dimensional and transient mathematical model based on temperature was developed to predict the thermal behavior of environments with a PCMOW or PCMIW system in comparison with a reference environment where a thermal storage system was not applied. The numerical solution was validated by comparing the results with experimental data, which presented errors of less than 5%. The authors found that the temperature variations of the wall and ceiling in the reference environment were greater than those of environments with a PCMOW or PCMIW. The low thermal conductivity of the PCMs reduced the transmission of heat to the internal atmosphere of the environment. In rooms with a PCMIW, the heat transmitted through the walls, windows, and doors in the room could be absorbed or released by the PCMs, which was different for the environment with a PCMOW, which only reduced the heat transmitted through the walls. The lower temperature fluctuations indicated that the operating times of air-cooling systems or heaters can be reduced through the use of PCMs, which represents an opportunity to reduce energy consumption.
Jmal and Baccar [
21] studied the solidification process of C
18 paraffin through the use of a TTHX with radial fins distributed along the surfaces that were in contact with the PCM. On this occasion, the researchers used air as a heat exchange fluid, which flowed through the volumes, including between the outer and intermediate tubes and inside the inner tube. The work presented two-dimensional numerical modeling based on the continuity, momentum, and energy equations by using the finite volume discretization method. Experimental results were used to validate the model, which showed good agreement. The authors concluded that an increase in the number of fins accelerates the solidification process up to a maximum limit of 9 fins, at which point, the increase of these fins becomes negligible due to the restriction of the fluid movement; the insertion of the fins also represents limits on the process of thermal exchange to heat transfers by natural convection.
Mastani Joybari et al. [
22] carried out a numerical study to evaluate the performance of a TTHX that was subject to simultaneous loading and unloading processes. The proposal consisted of the analysis of a continuous operating condition with an energy storage and release system such that at some point in the thermal cycles, the PCM will be melting in a part of its volume and being solidified elsewhere; this type of system is called simultaneous charging and discharging (SCD). The governing equations were numerically solved using Ansys FLUENT
® 16.2 software. The effect of natural convection on the heat transfer process was investigated, as well as the modes of operation that consider heating from the outer surface of the intermediate tube and cooling, from the inner surface of the inner tube, and those that consider heating from the internal surface of the inner tube and cooling from the outer surface of the intermediate tube. The results were validated with experimental data, which considered the loading and unloading processes separately and showed good agreement. The analysis indicated that the initial condition of the PCM (completely melted or completely solidified) had a great impact on the final configuration of the material’s solid–liquid interface. It was observed that the purely diffusive model could be applied with low levels of error for the initial condition of the completely melted PCM, but in the initial condition of the completely solidified material while disregarding natural convection, relevant errors were generated in the description of the process.
Youssef et al. [
23] carried out numerical investigations of a phase change heat exchanger (PCM heat exchanger—HX) built with eight tubes with external surfaces connected to spiral copper wires, which interact with organic paraffin A16, which melts at 16 °C, inside a metallic container. In this system, the tubes were connected to form a serpentine structure, through which the heat exchange fluid (air), coming from a heat pump system, assisted by solar energy, entered one end of the exchanger to heat or cool the PCM through the existing thermal cycles at the site. The authors proposed a detailed three-dimensional model to evaluate the performance of the HX using the Ansys FLUENT
® software to solve the conservation equations and validated the numerical results with the experimental data. It was observed that, within the analyzed operating conditions, the loading period was shorter than the PCM discharge, which was attributed to the effect of heat transfer by natural convection, which was more effective during the melting of the material. It was found that both the heat transfer fluid (HTF) inlet velocity, as well as the difference between their temperature and the PCM, was inversely proportional to the time required for loading and unloading the material.
The thermal comfort of environments is achieved when the air is conditioned to levels that are capable of covering the demanded sensitive and latent thermal loads. In the case of environments configured using large latent loads, due to the high energy necessary for using conventional air-conditioning systems, such as vapor compression, liquid desiccant air conditioning systems (LDAC) are recommended. An LDAC system works by applying a liquid desiccant solution (water + mineral salts) that interacts directly or indirectly with an air stream by absorbing or releasing water vapor to or from it to adjust the moisture content and pressure of the surface air stream. During the LDAC operations, the desiccant solution is heated and cooled to allow for dehumidification of the air flow and control its temperature [
24]. The energy accumulated in a PCM latent thermal system can be used in a liquid desiccant air-conditioning system to control a room’s temperature and relative humidity (or absolute humidity). Many other PCM applications in buildings have been reported in the literature, such as a PCM–air heat exchanger system for the heating, ventilation, and air conditioning of buildings [
25]; latent heat thermal energy storage using PCM [
26]; and solar radiation in PCM cells [
27].
Given the operating conditions of a thermodynamic air conditioning system using LDAC, thermal energy storage can be used in the form of a phase change material that is associated with a thermal solar energy absorption system, which occurs during periods of high solar incidence, to store the energy demanded by an LDAC system [
19].
Based on this, to complement the cited studies, this work aimed to perform a numerical analysis of the PCM melting process that was heated by a flow of hot water through the pipes of a triplex tube type heat exchanger. This water was heated due to its interaction with solar panels during periods of high solar incidence. Through turbulence, energy, and phase change models applied using the Ansys Fluent® 15.07 software (Canonsburg, Pennsylvania, USA), it was possible to describe the material loading process.
2. Methodology
2.1. Physical Problem and Geometries
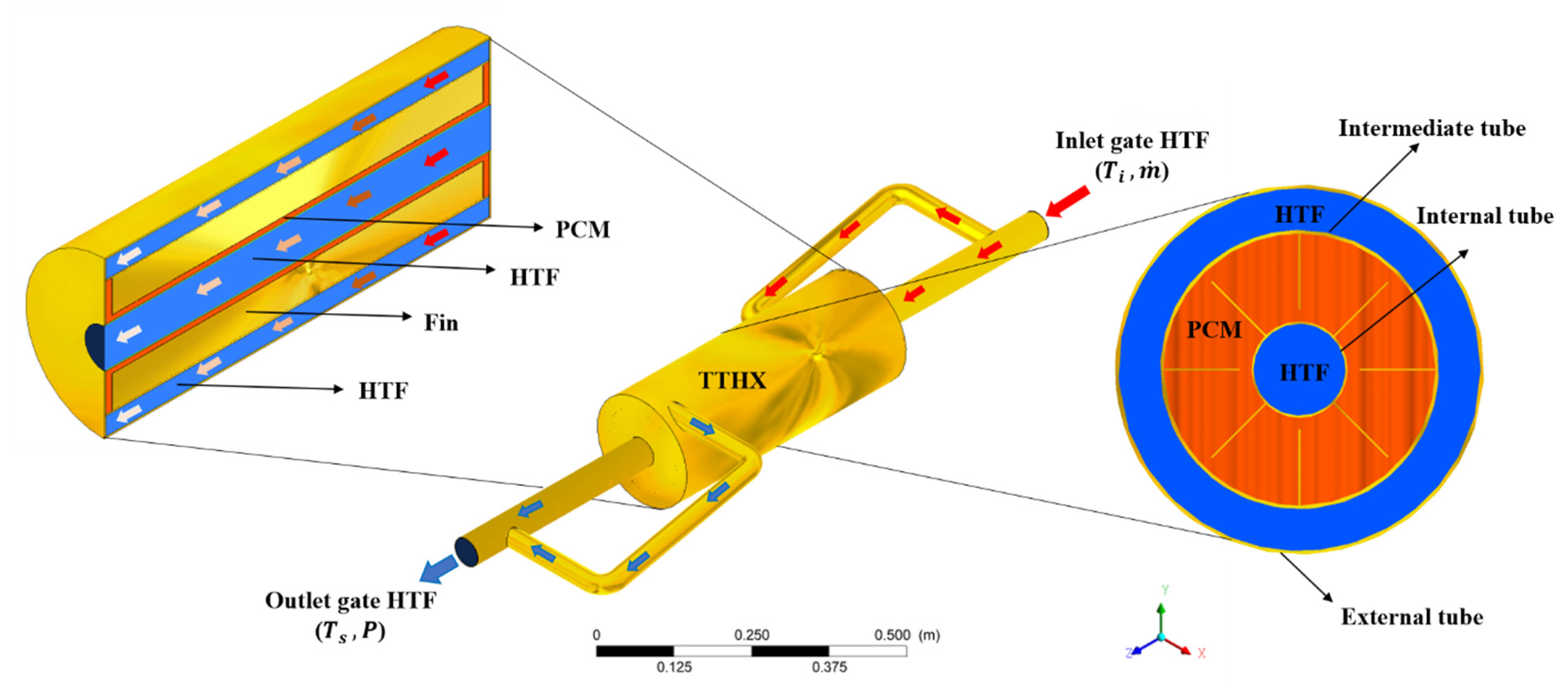
The proposed physical study was the analysis of the PCM (RT82) fusion process, which exchanges heat with the water (the HTF) through a TTHX made of copper tubes with eight longitudinal fins with a 1 mm thickness and 42 mm length installed on the tubular surfaces, and was directly in contact with the PCM. As illustrated in
Figure 1, the TTHX was described using five physical domains, namely, the fluid volume (water) circulating in the pipes, the solid volumes (internal, intermediate, and external copper tubes), and the PCM (the volume between the intermediate tube inner surface and the inner tube outer surface). In the energy storage process, water entered the heat exchanger with a pre-established temperature
Ti and mass flow rate
through a copper tube with a 50.8 mm diameter and a thickness of 1.2 mm. The mass flow rate is divided by a branch in the inlet pipe, which was 32 mm in diameter and 1.2 mm thick. Thus, the HTF transferred energy to the PCM through the intermediate tube’s outer surface and the inner tube’s internal surface. Then, the heat was conducted through the finned pipes to the PCM. As the HTF released energy to the system, its temperature at the outlet
Ts was reduced. The TTHX external surfaces were considered to be isolated such that the heat transfer with the external environment was neglected.
In
Figure 2,
Figure 3,
Figure 4,
Figure 5,
Figure 6 and
Figure 7, the frontal and lateral views of the TTHX, frontal and isometric views of the fluid domain, of the internal and intermediate tubes, and the PCM with its dimensions are presented, respectively.
To simplify, the domain referring to the external tube was not considered in the simulations due to its small thickness and the insulation considered on the TTHX external surface. In this way, only the domains referring to the HTF, PCM, and internal and intermediate pipes were considered.
As can be seen in
Figure 5, there was a variation of 16 mm between the tube length and the fins’ length. Adding the thickness (2 mm) of the neglected external tube, which lined the complete heat exchanger, this difference grew to 20 mm, with 10 mm at each end of the heat exchanger. Another simplification used was to consider only the 480 mm of the TTHX length, corresponding to the fin length.
Thus, the simplified physical problem took the form showed in
Figure 7, where the five planes along the length of the exchanger, which were treated in the results presented in this work and were located at
z = 0, 100, 240, 380, and 480 mm, are presented. These simplifications allowed for the production of meshes with considerable quality. The presented geometry was performed in ANSYS DesignModeler
® software (Canonsburg, Pennsylvania, USA).
The heat exchange area (finned pipe surfaces in contact with the PCM) was 0.308832 m2. The volume occupied by the PCM was 0.00725707 m3. Converting these values to mass, depending on the PCM density, there was 6.89 kg of material stored in the equipment.
2.2. Numerical Mesh
In this work, the finite volume discretization method was used. Hybrid unstructured meshes (tetra and hexahedral elements) unstructured were developed within the scope of the Ansys Meshing
® software. This type of mesh made it possible to associate the molding capacity with the complex geometries of the tetrahedral elements allocated in the HTF domain, where good quality the results were related to the hexahedral elements in the finned structures and the PCM domain, which maintained the mesh quality close to the wall regions for that geometry.
Figure 8,
Figure 9,
Figure 10,
Figure 11,
Figure 12 and
Figure 13 illustrate the meshes used for the domains treated in the physical problem.
Figure 8 and
Figure 9 show the isometric and front view of the mesh used for the heat exchanger with all domains assembled.
Figure 10 presents the details of the mesh used for HTF, which had tetrahedral elements inside the volume and layers of hexahedral elements on the walls due to the hydrodynamic and thermal boundary layers developed in these regions.
Figure 11,
Figure 12 and
Figure 13 show the domain of the internal and intermediate finned pipes, as well as the PCM. Through the procedure used, it was possible to maintain element uniformity in the pipes, while in the PCM, the elements had an irregular shape that could track the PCM geometry.
2.3. Mathematical Modeling
The conservation laws that described the operation of the TTHX are presented in Equations (1)–(10). When modeling, the analysis must include the fluid dynamics, and thermal and phase transition processes, which occur simultaneously during PCM loading.
(a) Mass conservation:
where
is the density of the fluid,
t is the time variable,
X is the position vector,
u is the velocity vector, and the sub-indications
i and
j represent the components (
x,
y, and
z) of the coordinate axes such that
i or
j = 1 represents the
x-direction,
i or
j = 2 represents the
y-direction, and
i or
j = 3 represents the
z-direction.
(b) Linear momentum conservation:
where
μ is the dynamic viscosity,
P is the pressure, and the term and (
represents the Reynolds stresses, which were derived from the turbulent flow.
(c) Turbulence through the shear–stress transport k-ω SST model
The stress transport model (shear–stress transport
k-
ω shear stress transport (SST) applied in the Ansys FLUENT
® software was developed by Menter [
28] to combine the robustness and accuracy of the results close to the wall using the standard
k-
ω model [
29] with the accuracy and simplicity of the
k-
ε model [
30] for the regions far from the wall. To this end, coupling functions are used to activate the
k-
ω and
k-
ε models in the cells near and far from the walls, respectively, of the computational domain. The variable
k represents the turbulent kinetic energy and the variable
ω represents the dissipation rate of this energy. Equations (3) and (4) describe the transport of these variables.
where
and
represent the generation of
k and
ω,
and
are the effective diffusivity,
and
represent the dissipation of
k and
ω, the term
represents the cross-diffusion, and
and
represent the source terms of the referred equations. Equations (1)–(4) are applied in domains that present flow: the HTF that is injected into the heat exchanger flows in a turbulent, laminar, or transition flow regime depending on the analyzed region, and the PCM presents a low-speed flow during the phase change. In this way, the
k-
ω SST turbulence model was used with corrections for low Reynolds (
Re) numbers, which enabled the flow analysis in all referred regimes.
(d) Energy conservation:
where
T is the temperature,
is the energy source term,
E is the total energy (Equation (6)), and
is the effective thermal conductivity, described in Equation (8):
where
h is the sensible enthalpy, defined as:
where
href is the reference enthalpy,
Tref is the reference temperature, and
cp is the specific heat of the fluid under constant pressure. In the FLUENT
® standard scheme,
href and
Tref are 0 J/kg and 15 °C, respectively.
where
is the turbulent thermal conductivity, described in Equation (9):
where
is the turbulent viscosity and
is the Prandtl number.
The term
is the effective stress tensor given by:
where:
Equation (5) is applied to all domains to describe the heat transfer process.
(e) Phase change model
To solve the PCM melting transient problem the enthalpy–porosity model developed by Voller and Prakash [
31] was chosen. In this technique, the solid–liquid interface is not explicitly tracked, where the calculated liquid fraction is the one that indicates its position throughout the phase change processes. The heat transfer in the PCM domain occurs due to the thermal diffusion mechanism and natural convection due to density variations, which is a function of the temperature and phase change levels. Equations (12)–(16) were used for this stage of the process:
where:
and:
where:
where
is the liquid fraction;
H is the total enthalpy of the material, which is computed as the sum of the sensitive enthalpies
h and the latent enthalpy variation of the material ∆
H;
L is the latent heat of fusion;
Tsol is the solidification temperature; and
Tliq is the temperature at which all the material is in a liquid state.
The PCM density (
) was calculated using Equation (16). This equation couples the Boussinesq natural convection model [
32] for
T ≥ Tl and the mixture model for
Tliq ≥ T ≥ Tsol. If the material is below
Tsol the density (
) remains constant.
where
and
are the densities of the material in the solid and liquid states, respectively, and
is the thermal expansion coefficient. As the PCM is heated, it shows variations in its density, which primarily occur in the regions closer to the pipe walls. These density variations, when subjected to the gravitational force, are converted into buoyant forces, which move the PCM inside the heat exchanger at a low velocity.
2.4. Boundary Conditions
The prescribed mass flow rate was defined at the TTHX inlet. The mass flow rate specification allowed for the total pressure to vary in response to the numerical solution. In this boundary condition, the absolute reference system, the direction of flow normal to the inlet surface, the turbulence intensity
I (Equation (17)) of 5%, and the turbulent viscosity ratio of
Rμ = 10 (Equation (18)) were previously established. The HTF at 90 °C was injected from the inlet gate with a volumetric flow rate of 8.3 L/min.
The outflow boundary conditions were established at the TTHX outlet gate. In this model, the necessary information (velocities, pressure, and temperature) were extrapolated from the conditions within the physical domain. Two main features were activated in the solver as soon as the outflow boundary condition was enabled: (1) null diffusive flow, which means that the outlet conditions were extrapolated from the internal domain and that these conditions had no influence on the upstream flow; and (2) for all flow variables, a general mass balance correction was made. The wall conditions were used to connect the fluid and solid regions and to incorporate the insulation on the HTF external surfaces. Non-slip boundary conditions were applied to the pipe walls and the fluid surfaces of the domains, which were taken as stationary surfaces with a negligible roughness.
Regarding the heat transfer, two distinct conditions were used: zero flux for the external surfaces of the HTF domain and the coupled condition for the solid–liquid interfaces existing between the external surface of the intermediate tube and the internal surface of the inner tube with HTF, as well as between the inner surface of the intermediate tube and the outer surface of the inner tube with the PCM. Under these conditions, the heat flux was calculated at the interfaces depending on the temperatures of the neighboring cells of both domains.
Table 1 describes the border regions of the physical problem and its boundary conditions.
2.5. Numerical Solution Methods
Different numerical treatments were used to solve the governing equations: a) for the pressure–velocity coupling, the coupled method was used; b) for the spatial discretization, the least-squares cell-based method [
33] was used to determine the gradient ∇
Φ; c) the quadratic upwind implicit differencing convective kinematics (QUICK) method [
34] was used for the discretization of linear momentum equations and turbulent kinetic energy generation; d) the first- and second-order upwind methods [
35] were used to discretize the turbulent kinetic energy and thermal energy dissipation equations, respectively; e) the PRESTO! method [
32] was used for pressure discretization; and finally, f) for the temporal discretization, the implicit first-order method was used in the simulations.
2.6. Simulation Strategies
Given the long simulation time required to simulate the PCM transient fusion process, the cases were initialized in a steady state, without the energy and phase change models being enabled. This procedure allowed for the pressure and velocity fields in the flow in the HTF to be established. As the steady-state process reached convergence, the fluid dynamic results were used as an initial condition for the transient simulation with the energy and phase change models enabled.
The relaxation factors used are described in
Table 2.
The convergence criteria used in the simulations was 2 × 10−4 for velocities in the x- and y-directions, 10−4 for velocities in the z-direction, mass conservation, balance of turbulent generation energy, and balance of turbulent dissipation energy, and 10−5 for energy conservation.
2.7. Physical Properties
The physical properties of the materials used in the simulations are described in
Table 3, which were taken from Al-Abidi et al. [
36].
It was considered that in the initial condition, the entire domain regarding the heat exchange fluid was completely filled with HTF at 90 °C, and that the PCM and the pipes were at a temperature of 27 °C. The time step used in the transient simulation was 0.5 s.