Inorganic Chromium Speciation in Geothermal Water of the Podhale Trough (Southern Poland) Used for Recreational Purposes
Abstract
:1. Introduction
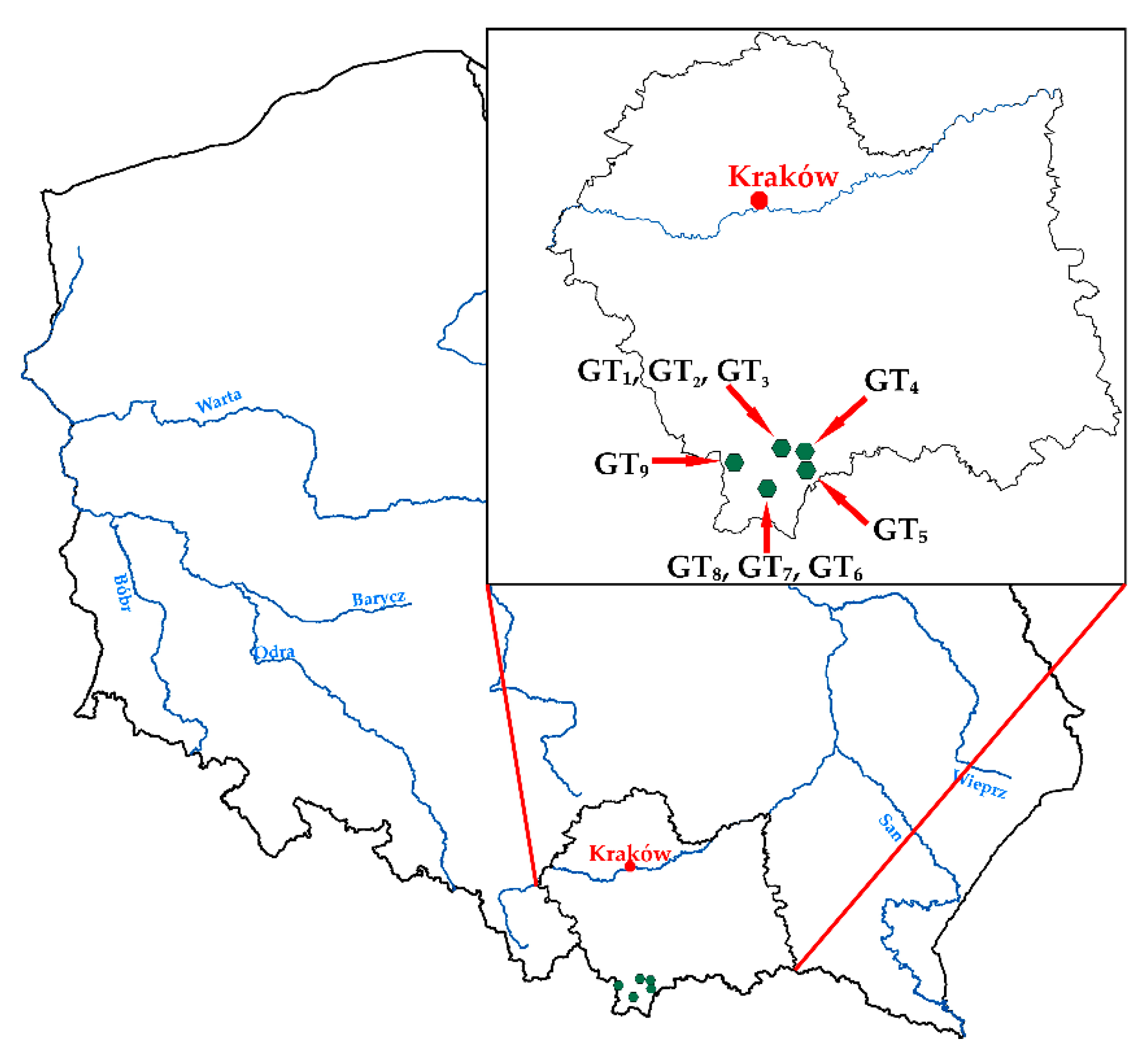
2. Materials and Methods
- Nine normal unfiltered water samples were used for the determination of bicarbonates and chlorides by titration methods.
- Nine normal water samples were filtered with a membrane filter of 0.45 µm pore diameter and acidified with concentrated nitric acid (1 mL HNO3 per 100 mL of water sample) to allow for the control of the chemical analyses by ionic charge balance; these samples were used to analyze the concentrations of major ions, total chromium and other trace elements, considering detailed characteristics.
- Two duplicate control samples were collected in the same way as the normal ones from randomly selected intakes; these samples were used to check the repeatability, expressed as relative percentage difference (RPD) between chemical analyses.
- One blank (field) control sample was used to verify the influence of the transport process on the contamination of water samples and to compare with the laboratory blank.
- Nine normal samples and one blank sample were spiked with a known amount (10 µg·L−1) of total chromium to check the accuracy (recovery) of the chemical analyses.
2.1. Reagents, Solutions and Reference Materials
2.2. Instrumentation
2.3. Speciation Modeling
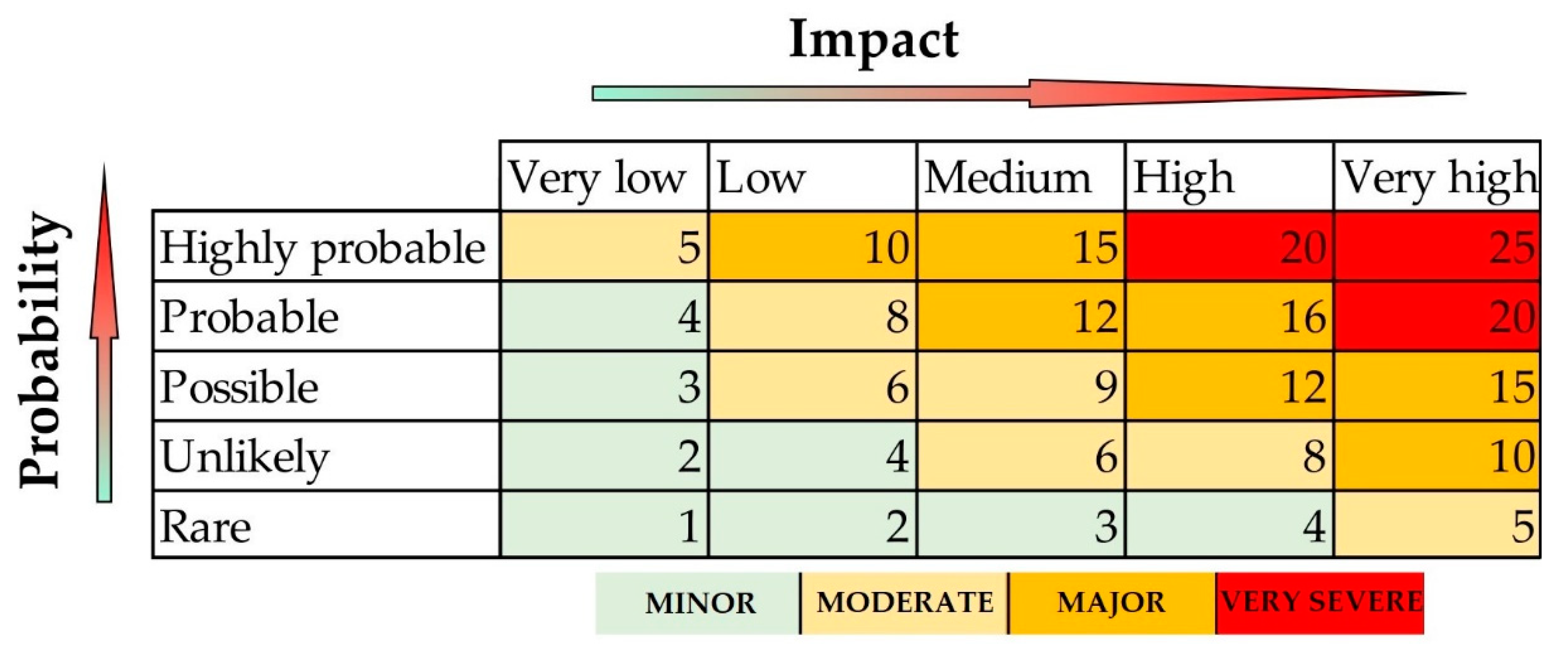
2.4. Risk Assessment
3. Results and Discussion
3.1. Speciation Modeling Results
3.2. Analysis of the Risk of Exposure to Chromium
- Geothermal water injection into formation (L/F = 5, SC = 1).
- Discharge of geothermal water to surface watercourses in the case of fish farming (L/F = 3, SC = 4).
- Discharge of geothermal water to surface watercourses in the case of surface water chemical status changes (L/F = 3, SC = 2).
- Oxidation of Cr(III) to toxic Cr(VI) due to changes in redox conditions (L/F = 5, SC = 5).
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Nriagu, J.O.; Nieboer, E. (Eds.) Chromium in the Natural and Human Environments; John Wiley & Sons: New York, NY, USA, 1988; Volume 20. [Google Scholar]
- Stefánsson, A.; Gunnarsson, I.; Kaasalainen, H.; Arnórsson, S. Chromium geochemistry and speciation in natural waters, Iceland. Appl. Geochem. 2015, 62, 200–206. [Google Scholar] [CrossRef]
- Kraemer, D.; Frei, R.; Viehmann, S.; Bau, M. Mobilization and isotope fractionation of chromium during water-rock interaction in presence of siderophores. Appl. Geochem. 2019, 102, 44–54. [Google Scholar] [CrossRef]
- Şahan, S.; Saçmacı, Ş.; Kartal, Ş.; Saçmacı, M.; Şahin, U.; Ülgen, A. Development of a new on-line system for the sequential speciation and determination of chromium species in various samples using a combination of chelating and ion exchange resins. Talanta 2014, 120, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Saputro, S.; Yoshimura, K.; Matsuoka, S.; Takehara, K.; Aizawa, J.; Tennichi, Y. Speciation of dissolved chromium and the mechanisms controlling its concentration in natural water. Chem. Geol. 2014, 364, 33–41. [Google Scholar] [CrossRef]
- Vignati, D.A.L.; Ferrari, B.J.; Roulier, J.L.; Coquery, M.; Szalinska, E.; Bobrowski, A.; Czaplicka, A.; Kownacki, A.; Dominik, J. Chromium bioavailability in aquatic systems impacted by tannery wastewaters. Part 1: Understanding chromium accumulation by indigenous chironomids. Sci. Total Environ. 2019, 653, 401–408. [Google Scholar] [CrossRef] [Green Version]
- Witczak, S.; Kania, J.; Kmiecik, E. Guidebook on Selected Physical and Chemical Indicators of Groundwater Contamination and Methods of Their Determination, 1st ed.; Inspekcja Ochrony Środowiska, Biblioteka Monitoringu Środowiska: Warszawa, Poland, 2013. (In Polish) [Google Scholar]
- Eisen-Cuadra, A.; Christian, A.D.; Dorval, E.; Broadaway, B.; Herron, J.; Hannigan, R.E. Metal geochemistry of a Brackish lake: Étang Saumâtre, Haiti. In Medical Geochemistry, 1st ed.; Censi, P., Darrah, T., Erel, Y., Eds.; Springer: Dordrecht, The Netherlands, 2013; pp. 149–166. [Google Scholar]
- Pędziwiatr, A.; Kierczak, J.; Waroszewski, J. Nickel, chromium and cobalt–bearing minerals in various ultrabasic rocks of lower Silesia (southwestern Poland). In Proceedings of the 4th Central European Mineralogical Conference, Skalský Dvůr, Czech Republic, 23–26 April 2014. [Google Scholar]
- Selinus, O.; Alloway, B.; Centeno, J.A.; Finkelman, R.B.; Fuge, R.; Lindh, U.; Smedley, P. (Eds.) Essentials of Medical Geology; Revised Edition; Springer: New York, NY, USA, 2013. [Google Scholar]
- Cohen, M.D.; Kargacin, B.; Klein, C.B.; Costa, M. Mechanisms of chromium carcinogenicity and toxicity. Crit. Rev. Toxicol. 1993, 23, 255–281. [Google Scholar] [CrossRef]
- Mertz, W.; Toepfer, E.W.; Roginski, E.E.; Polansky, M.M. Present knowledge of the role of chromium. Fed. Proc. 1974, 33, 2275–2280. [Google Scholar]
- Pradhan, D.; Sukla, L.B.; Sawyer, M.; Rahman, P.K. Recent bioreduction of hexavalent chromium in wastewater treatment: A review. J. Ind. Eng. Chem. 2017, 55, 1–20. [Google Scholar] [CrossRef] [Green Version]
- European Comission. Council Directive 98/83/EC of 3 November 1998 On the Quality of Water Intended for Human Consumption; European Comission: Brussels, Belgium, 1998. [Google Scholar]
- WHO. Guidelines for Drinking-Water Quality, 4th ed.; 1. Potable Water—Standards. 2. Water—Standards. 3. Water Quality—Standards. 4. Guidelines; World Health Organization: Geneva, Switzerland, 2011; p. 541. [Google Scholar]
- RMH. Regulation of the Minister of the Health of Poland, of 11 December on the Scope of Water Intended for Human Consumption. J. Laws 2017. [Google Scholar]
- Kępińska, B. Przegląd stanu wykorzystania energii geotermalnej w Polsce w latach 2016–2018. Technika Poszukiwań Geologicznych 2018, 57, 11–26. (In Polish) [Google Scholar]
- RMH. Regulation of the Minister of Health of Poland, of 9 November 2015 on the Requirements that Should Be Fulfill by the Water at the Swimming Pools. J. Laws 2015. [Google Scholar]
- Mika, A.; Kmiecik, E.; Krawiec, A.; Wątor, K.; Zamfir, M.; Chochorek, A. Legionella pneumophila in Thermal Spa–Methodological Aspects of Sampling and Analysis. Geomicrobiol. J. 2019, 36, 460–467. [Google Scholar] [CrossRef]
- Bujakowski, W.; Tomaszewska, B.; Miecznik, M. The Podhale geothermal reservoir simulation for long-term sustainable production. Renew. Energ. 2016, 99, 420–430. [Google Scholar] [CrossRef]
- Kmiecik, E.; Tomaszewska, B.; Wątor, K.; Bodzek, M. Selected problems with boron determination in water treatment processes. Part I: Comparison of the reference methods for ICP-MS and ICP-OES determinations. Environ. Sci. Pollut. Res. 2016, 23, 11658–11667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomaszewska, B.; Kmiecik, E.; Wątor, K. Evaluating the stability of iodine in bottled mineral waters. J. Geochem. Explor. 2016, 168, 20–25. [Google Scholar] [CrossRef]
- Tomaszewska, B.; Rajca, M.; Kmiecik, E.; Bodzek, M.; Bujakowski, W.; Wątor, K.; Tyszer, M. The influence of selected factors on the effectiveness of pre-treatment of geothermal water during the nanofiltration process. Desalination 2017, 406, 74–82. [Google Scholar] [CrossRef]
- Tomaszewska, B.; Kmiecik, E.; Wątor, K.; Tyszer, M. Use of numerical modelling in the prediction of membrane scaling. Reaction between antiscalants and feedwater. Desalination 2017, 427, 27–34. [Google Scholar] [CrossRef]
- Tomaszewska, B.; Bundschuh, J.; Pająk, L.; Dendys, M.; Delgado Quezada, V.; Bodzek, M.; Armienta, A.A.; Ormachea Munioz, M.; Kasztelewicz, A. Use of low-enthalpy and waste geothermal energy sources to solve arsenic problems in freshwater production in selected regions of Latin America using a process membrane distillation-research into model solutions. Sci. Total Environ. 2020, 714, 136853. [Google Scholar] [CrossRef]
- Tomaszewska, B.; Dendys, M. Zero-waste initiatives—Waste geothermal water as a source of medicinal raw material and drinking water. Desalin. Water Treat. 2020, 112, 12–18. [Google Scholar] [CrossRef] [Green Version]
- Rusiniak, P.; Wątor, K.; Plata, J. Deterministic and probabilistic approaches to the denomination of the hydrochemical type of potentially medicinal groundwater from the “Zdrój Główny” intake (Krzeszowice, Poland). Geol. Geophys. Environ. 2017, 43, 303–309. [Google Scholar] [CrossRef]
- Wątor, K.; Kmiecik, E.; Tomaszewska, B. Assessing medicinal qualities of groundwater from the Busko-Zdrój area (Poland) using the probabilistic method. Environ. Earth Sci. 2016, 75, 804. [Google Scholar] [CrossRef] [Green Version]
- Wątor, K.; Kmiecik, E.; Rusiniak, P. An Influence of Research Methodology on the Results of Determination of a Chemical Composition of Curative Water. Acta Balneologica 2018, 60, 272–276. (In Polish) [Google Scholar]
- Wątor, K.; Kmiecik, E.; Lipiec, I. The use of principal component analysis for the assessment of the spatial variability of curative waters from the Busko-Zdrój and Solec-Zdrój region (Poland)–preliminary results. Water Supp. 2019, 19, 1137–1143. [Google Scholar] [CrossRef]
- RMH. Regulation of the Minister of Health of Poland, of 13 April 2006 on the scope of the studies required to determine the medicinal properties of natural medicinal resources and medicinal properties of climate, the criteria for their evaluation and a specimen certificate confirming these properties. J. Laws 2006. (565). [Google Scholar]
- Loos, R.; Marinov, D.; Sanseverino, I.; Napierska, D.; Lettieri, T. Review of the 1st Watch List under the Water Framework Directive and Recommendations for the 2nd Watch List; Publications Office of the European Union: Luxemburg, 2018. [Google Scholar]
- Chowaniec, J. “Hot raw materials” of the Podhale trough (Poland and Slovakia) versus other peri-tatric troughs. Biul. Państw. Inst. Geol. 2012, 448, 229–238. (In Polish) [Google Scholar]
- Kępińska, B. The role of the Podhale geothermal system’s research for geothermal water exploitation. Tech. Poszuk. Geologicznych Geoterm. Zrównoważony Rozw. 2009, 48, 29–48. (In Polish) [Google Scholar]
- Pająk, L.; Tomaszewska, B.; Bujakowski, W.; Bielec, B.; Dendys, M. Review of the Low-Enthalpy Lower Cretaceous Geothermal Energy Resources in Poland as an Environmentally Friendly Source of Heat for Urban District Heating Systems. Energies 2020, 13, 1302. [Google Scholar] [CrossRef] [Green Version]
- Kępińska, B.; Ciągło, J. Possibilities of use of the Podhale geothermal waters for balneotherapeutical and recreational purposes. Geologia 2008, 34, 541–559. (In Polish) [Google Scholar]
- Sekuła, K. Hydrogeochemical Characteristic of Geothermal Water in Bańska Niżna. Ph.D. Thesis, AGH University of Science and Technology, Kraków, Poland, January 2016. [Google Scholar]
- Rusiniak, P.; Ruszczyńska, A.; Wątor, K.; Bulska, E.; Kmiecik, E. Methodological aspects concerning sampling and determination of total selenium and selenium species in geothermal waters. Bull. Geogr. Phys. Geogr. Ser. 2020, 18, 5–16. [Google Scholar] [CrossRef]
- ISO. ISO 5667-11:2009—Water Quality—Sampling—Part 11: Guidance on Sampling of Groundwaters; International Organization for Standarization: Geneva, Switzerland, 2009. [Google Scholar]
- Kmiecik, E.; Wątor, K.; Tomaszewska, B.; Sekuła, K.; Mika, A. Methodological aspects of pH and EC measurements in geothermal water. Bull. Geogr. Phys. Geogr. Ser. 2020, 17, 39–47. [Google Scholar] [CrossRef] [Green Version]
- Zdechlik, R.; Drzymała, M.; Wątor, K. Practical aspects of water sampling in groundwater monitoring. Biul. Państwowego Inst. Geologicznego 2013, 456, 659–664. [Google Scholar]
- PN-EN. PN-EN 27888:1999 Water Quality—Determination of Electrical Conductivity; Polish Committee for Standardization: Warsaw, Poland, 1999. (In Polish) [Google Scholar]
- ISO. ISO 10523:2008 Water Quality—Determination of pH; International Organization for Standarization: Geneva, Switzerland, 2008. [Google Scholar]
- ISO. ISO 11885:2007 Water Quality—Determination of Selected Elements by Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES); International Organization for Standarization: Geneva, Switzerland, 2007. [Google Scholar]
- ISO. ISO 9297:1989 Water Quality—Determination of Chloride—Silver Nitrate Titration with Chromate Indicator (Mohr’s Method); International Organization for Standarization: Geneva, Switzerland, 1989. [Google Scholar]
- ISO. ISO 9963-1:1994 Water Quality—Determination of Alkalinity—Part 1: Determination of Total and Composite Alkalinity; International Organization for Standarization: Geneva, Switzerland, 1994. [Google Scholar]
- ISO. ISO 17294:2016-2 Water Quality—Application of Inductively Coupled Plasma Mass Spectrometry (ICP-MS)—Part 2: Determination of Selected Elements Including Uranium Isotopes; International Organization for Standarization: Geneva, Switzerland, 2016. [Google Scholar]
- US Environmental Protection Agency (USEPA). Method 200.7: Trace Elements in Water, Solids, and Biosolids by Inductively Coupled Plasma-Atomic Emission Spectrometry; US Environmental Protection Agency: Washington, DC, USA, 2001.
- Parkhurst, D.L.; Appelo, C.A.J. Description of Input and Examples for PHREEQC Version 3: A Computer Program for Speciation, Batch-Reaction, ONE-dimensional Transport, and Inverse Geochemical Calculations (No. 6-A43); US Geological Survey: Reston, VA, USA, 2013.
- Dobrzyński, D.; Kmiecik, E.; Wątor, K. Oxidation Reduction Potential-An Informative and Unused Indicator of Curative and Mineral Water Quality. Acta Balneologica 2018, 60, 233–238. [Google Scholar]
- Wątor, K.; Dobrzyński, D.; Sugimori, K.; Kmiecik, E. Redox potential research in the field of balneochemistry: Case study on equilibrium approach to bioactive elements in therapeutic waters. Int. J. Biometeorol. 2020, 64, 815–826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fallahzadeh, R.A.; Khosravi, R.; Dehdashti, B.; Ghahramani, E.; Omidi, F.; Adli, A.; Miri, M. Spatial distribution variation and probabilistic risk assessment of exposure to chromium in ground water supplies; a case study in the east of Iran. Food Chem. Toxicol. 2018, 115, 260–266. [Google Scholar] [CrossRef] [PubMed]
- US Environmental Protection Agency (USEPA). Regional Screening Levels (RSLs)—User’s Guide; USEPA: Washington, DC, USA, 2016.
- Peeters, W.; Peng, Z. An approach towards global standardization of the risk matrix. J. Soc. Sci. Educ. 2015, 2, 31–38. [Google Scholar] [CrossRef]
- PN-EN. PN-EN 15975-2:2013-12—Safety of Drinking Water Supply—Crisis Management and Risk Guidelines—Part 2: Risk Management; Polish Committee for Standardization: Warsaw, Poland, 2013. (In Polish) [Google Scholar]
- Bielec, B.; Operacz, A. Newest recognition of exploitation parameters based on Chochołów PIG-1 borehole in the aspect of temperature effect. Inżynieria Ekologiczna 2018, 19, 145–152. [Google Scholar] [CrossRef]
- Kmiecik, K.; Korzec, K. Uncertainty associated with the sampling of geothermal water. In Proceedings of the World Geothermal Congress, Melbourne, Australia, 19–25 April 2015. [Google Scholar]
- Małecka, D. The thermal waters of Podhale, southern Poland: History of research, genesis and utility. Geol. Q. 2003, 47, 195–210. [Google Scholar]
- Mika, A.; Korzec, K. Analysis of the concentration stability of metasilic acid in the thermal water from the Bańska PGP-1 well. Tech. Poszukiwań Geologicznych 2015, 54, 89–96. (In Polish) [Google Scholar]
- Operacz, A. Variability of basic geothermal water parameters in Chochołów PIG-1 borehole in the western part of the Podhale Basin. Infrastrukt. Ekol. Teren. Wiej. 2018, 4, 961–972. (In Polish) [Google Scholar] [CrossRef]
- GML. Geological and mining law of 9 June 2011. J. Laws 2011. (981) (In Polish) [Google Scholar]
- Fleming, J.; Albus, H.; Neidhart, B.; Wegscheider, W. Glossary of analytical terms (VII). Accreditation and Quality Assurance. J. Qual. Comp. Reliab. Chem. Meas. 1997, 2, 51–52. [Google Scholar]
- Magnusson, B.; Örnemark, U. (Eds.) Eurachem Guide: The Fitness for Purpose of Analytical Methods—A Laboratory Guide to Method Validation and Related Topics, 2nd ed.; 2014; ISBN 978-91-87461-59-0. Available online: www.eurachem.org (accessed on 7 July 2020).
- Stefánsson, A.; Arnórsson, S. Gas pressures and redox reactions in geothermal fluids in Iceland. Chem. Geol. 2002, 190, 251–271. [Google Scholar] [CrossRef]
- Stefánsson, A.; Arnórsson, S.; Sveinbjörnsdóttir, Á.E. Redox reactions and potentials in natural waters at disequilibrium. Chem. Geol. 2005, 221, 289–311. [Google Scholar] [CrossRef]
- Tomaszewska, B.; Bodzek, M. Desalination of geothermal waters using a hybrid UF-RO process. Part I: Boron removal in pilot-scale tests. Desalination 2013, 319, 99–106. [Google Scholar] [CrossRef]
- Tomaszewska, B.; Bodzek, M.; Kmiecik, E. Boron removal from geothermal water using DOW chemical high separation BWRO membrane. Desalin. Water Treat. 2016, 57, 27477–27484. [Google Scholar] [CrossRef]
- Baba, A.; Şaroğlu, F.; Akkuş, I.; Özel, N.; Yeşilnacar, M.İ.; Nalbantçılar, M.T.; Demir, M.M.; Gökçena, G.; Arslan, Ş.; Dursun, N.; et al. Geological and hydrogeochemical properties of geothermal systems in the southeastern region of Turkey. Geothermics 2019, 78, 255–271. [Google Scholar] [CrossRef]
- Kaasalainen, H.; Stefánsson, A.; Giroud, N.; Arnórsson, S. The geochemistry of trace elements in geothermal fluids, Iceland. Appl. Geochem. 2015, 62, 207–223. [Google Scholar] [CrossRef]
- Godgul, G.; Sahu, K.C. Chromium contamination from chromite mine. Environ. Geol. 1995, 25, 251–257. [Google Scholar] [CrossRef]
- Bartlett, R.J.; James, B.R. Mobility and bioavailability of chromium in soils. In Chromium in the Natural and Human Environments; Jerome, O., Ed.; Wiley: New York NY, USA, 1988; Volume 20, pp. 267–304. [Google Scholar]
- Kasassi, A.; Rakimbei, P.; Karagiannidis, A.; Zabaniotou, A.; Tsiouvaras, K.; Nastis, A.; Tzafeiropoulou, K. Soil contamination by heavy metals: Measurements from a closed unlined landfill. Bioresour. Technol. 2008, 99, 8578–8584. [Google Scholar] [CrossRef]
- Gowd, S.S.; Reddy, M.R.; Govil, P.K. Assessment of heavy metal contamination in soils at Jajmau (Kanpur) and Unnao industrial areas of the Ganga Plain, Uttar Pradesh, India. J. Hazard. Mater. 2010, 174, 113–121. [Google Scholar] [CrossRef]
- Ye, T.; Li, H.; Wang, Z.X.; Huang, R.; Yu, Y.J.; Yang, Z.; Gao, C.; Xie, C. Transport and fate of hexavalent chromium in slag–soil system. Environ. Earth Sci. 2019, 78, 239. [Google Scholar] [CrossRef]
- Pađan, J.; Marcinek, S.; Cindrić, A.M.; Layglon, N.; Lenoble, V.; Salaün, P.; Garnier, C.; Omanović, D. Improved voltammetric methodology for chromium redox speciation in estuarine waters. Anal. Chim. Acta 2019, 1089, 40–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, B.; Zhang, Y.; Zhang, X.X.; Cheng, S.P. Health risk assessment of polycyclic aromatic hydrocarbons in the source water and drinking water of China: Quantitative analysis based on published monitoring data. Sci. Total Environ. 2011, 410, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.R.; Scott, P.K.; Sheehan, P.J.; Paustenbach, D.J. A probabilistic assessment of household exposures to MTBE from drinking water. Hum. Ecol. Risk Assess. 2000, 6, 827–849. [Google Scholar] [CrossRef]
- Huang, D.; Yang, J.; Wei, X.; Qin, J.; Ou, S.; Zhang, Z.; Zou, Y. Probabilistic risk assessment of Chinese residents’ exposure to fluoride in improved drinking water in endemic fluorosis areas. Environ. Pollut. 2017, 222, 118–125. [Google Scholar] [CrossRef]
- Leung, A.O.; Duzgoren-Aydin, N.S.; Cheung, K.C.; Wong, M.H. Heavy metals concentrations of surface dust from e-waste recycling and its human health implications in southeast China. Environ. Sci. Technol. 2008, 42, 2674–2680. [Google Scholar] [CrossRef]
- Sharma, S.; Nagpal, A.K.; Kaur, I. Appraisal of heavy metal contents in groundwater and associated health hazards posed to human population of Ropar wetland, Punjab, India and its environs. Chemosphere 2019, 227, 179–190. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, J.; Shi, W.; Zhang, D.; Zhu, T.; Li, X. Establishing a human health risk assessment methodology for metal species and its application of Cr6+ in groundwater environments. Chemosphere 2017, 189, 525–537. [Google Scholar] [CrossRef]
- Magesh, N.S.; Chandrasekar, N.; Elango, L. Trace element concentrations in the groundwater of the Tamiraparani river basin, South India: Insights from human health risk and multivariate statistical techniques. Chemosphere 2017, 185, 468–479. [Google Scholar] [CrossRef]
- Tseng, C.H.; Lee, I.H.; Chen, Y.C. Evaluation of hexavalent chromium concentration in water and its health risk with a system dynamics model. Sci. Total Environ. 2019, 669, 103–111. [Google Scholar] [CrossRef]
- Wen, X.; Lu, J.; Wu, J.; Lin, Y.; Luo, Y. Influence of coastal groundwater salinization on the distribution and risks of heavy metals. Sci. Total Environ. 2019, 652, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Fryer, M.; Collins, C.D.; Ferrier, H.; Colvile, R.N.; Nieuwenhuijsen, M.J. Human exposure modelling for chemical risk assessment: A review of current approaches and research and policy implications. Environ. Sci. Policy 2006, 9, 261–274. [Google Scholar] [CrossRef]
- Gao, J.; Liu, Q.; Song, L.; Shi, B. Risk assessment of heavy metals in pipe scales and loose deposits formed in drinking water distribution systems. Sci. Total Environ. 2019, 652, 1387–1395. [Google Scholar] [CrossRef] [PubMed]
- Kavcar, P.; Sofuoglu, A.; Sofuoglu, S.C. A health risk assessment for exposure to trace metals via drinking water ingestion pathway. Int. J. Hyg. Environ. Health 2009, 212, 216–227. [Google Scholar] [CrossRef] [Green Version]
- Yin, J.; Wang, L.; Chen, Y.; Zhang, D.; Hegazy, A.M.; Zhang, X. A comparison of accumulation and depuration effect of dissolved hexavalent chromium (Cr6+) in head and muscle of bighead carp (Aristichthys nobilis) and assessment of the potential health risk for consumers. Food Chem. 2019, 286, 388–394. [Google Scholar] [CrossRef]
- Shakeri, A.; Fard, M.S.; Mehrabi, B.; Mehr, M.R. Occurrence, origin and health risk of arsenic and potentially toxic elements (PTEs) in sediments and fish tissues from the geothermal area of the Khiav River, Ardebil Province (NW Iran). J. Geochem. Explor. 2020, 208, 106347. [Google Scholar] [CrossRef]
- Bonmatin, J.M.; Noome, D.A.; Moreno, H.; Mitchell, E.A.; Glauser, G.; Soumana, O.S.; Bijleveld van Lexmond, M.; Sánchez-Bayo, F. A survey and risk assessment of neonicotinoids in water, soil and sediments of Belize. Environ. Pollut. 2019, 249, 949–958. [Google Scholar] [CrossRef]
- Buchner, E.M.; Happel, O.; Schmidt, C.K.; Scheurer, M.; Schmutz, B.; Kramer, M.; Knauer, M.; Gartiser, S.; Hollert, H. Approach for analytical characterization and toxicological assessment of ozonation products in drinking water on the example of acesulfame. Water Res. 2019, 153, 357–368. [Google Scholar] [CrossRef]
- Cheng, M.; Liu, T.K.; Olenin, S.; Su, P.X. Risk assessment model based on expert’s perspective for ballast water management. Ocean Coast. Manag. 2019, 171, 80–86. [Google Scholar] [CrossRef]
- Domínguez, C.R.; Martínez, I.V.; Peña, P.M.P.; Ochoa, A.R. Analysis and evaluation of risks in underground mining using the decision matrix risk-assessment (DMRA) technique, in Guanajuato, Mexico. J. Sustain. Min. 2019, 18, 52–59. [Google Scholar] [CrossRef]
- Faber, J.H.; Marshall, S.; Van den Brink, P.J.; Maltby, L. Priorities and opportunities in the application of the ecosystem services concept in risk assessment for chemicals in the environment. Sci. Total Environ. 2019, 651, 1067–1077. [Google Scholar] [CrossRef] [PubMed]
- Gul, M.; Guneri, A.F. A fuzzy multi criteria risk assessment based on decision matrix technique: A case study for aluminum industry. J. Loss Prevent. Proc. 2016, 40, 89–100. [Google Scholar] [CrossRef]
- Guo, X.; Feng, C.; Gu, E.; Tian, C.; Shen, Z. Spatial distribution, source apportionment and risk assessment of antibiotics in the surface water and sediments of the Yangtze Estuary. Sci. Total Environ. 2019, 671, 548–557. [Google Scholar] [CrossRef]
- Matthes, C.S.; Woerner, D.F.; Hendricks, T.J. Risk management for dynamic Radioisotope Power Systems. J. Soc. Sci. Environ. 2018, 5, 3–8. [Google Scholar] [CrossRef]
- Mohtar, W.H.M.W.; Maulud, K.N.A.; Muhammad, N.S.; Sharil, S.; Yaseen, Z.M. Spatial and temporal risk quotient based river assessment for water resources management. Environ. Pollut. 2019, 248, 133–144. [Google Scholar] [CrossRef]
- Moss, S.; Ulber, L.; den Hoed, I. A herbicide resistance risk matrix. Crop. Prot. 2019, 115, 13–19. [Google Scholar] [CrossRef]
- Praveena, S.M.; Shaifuddin, S.N.M.; Sukiman, S.; Nasir, F.A.M.; Hanafi, Z.; Kamarudin, N.; Ismail, T.H.T.; Aris, A.Z. Pharmaceuticals residues in selected tropical surface water bodies from Selangor (Malaysia): Occurrence and potential risk assessments. Sci. Total Environ. 2018, 642, 230–240. [Google Scholar] [CrossRef]
- Schaefer, T.; Udenio, M.; Quinn, S.; Fransoo, J.C. Water risk assessment in supply chains. J. Clean. Prod. 2019, 208, 636–648. [Google Scholar] [CrossRef]
- Xiao, J.; Wang, L.; Deng, L.; Jin, Z. Characteristics, sources, water quality and health risk assessment of trace elements in river water and well water in the Chinese Loess Plateau. Sci. Total Environ. 2019, 650, 2004–2012. [Google Scholar] [CrossRef]
- Jeleński, T.; Dendys, M.; Tomaszewska, B.; Pająk, L. The potential of RES in the reduction of air pollution: The SWOT analysis of smart energy management solutions for Krakow Functional Area (KrOF). Energies 2020, 13, 1754. [Google Scholar] [CrossRef] [Green Version]
- Benekos, I.D.; Shoemaker, C.A.; Stedinger, J.R. Probabilistic risk and uncertainty analysis for bioremediation of four chlorinated ethenes in groundwater. Stoch. Environ. Res. Risk 2007, A21, 375–390. [Google Scholar] [CrossRef]
- Rezaei, A.; Hassani, H.; Hayati, M.; Jabbari, N.; Barzegar, R. Risk assessment and ranking of heavy metals concentration in Iran’s Rayen groundwater basin using linear assignment method. Stoch. Environ. Res. Risk 2018, A32, 1317–1336. [Google Scholar] [CrossRef]

| Geothermal Water Well | pH | EH (mV) | EC (mS/cm) | T (°C) | TDS (mg·L−1) | F− (mg·L−1) | H2SiO3 (mg·L−1) | Hydrochemical Type |
|---|---|---|---|---|---|---|---|---|
| GW1 | 6.64 | −5.9 | 3.41 | 84.9 | 2192.9 | 2.62 | 69.25 | SO4-Cl-Na-Ca, F |
| GW2 | 6.67 | 103.8 | 3.38 | 83.8 | 2215.8 | 2.62 | 69.00 | SO4-Cl-Na-Ca, F |
| GW3 | 6.63 | −118.1 | 3.42 | 67.4 | 2245.6 | 2.66 | 70.79 | SO4-Cl-Na-Ca, H2SiO3, F |
| GW4 | 6.91 | −117.6 | 2.53 | 74.5 | 1648.8 | 2.47 | 63.73 | SO4-Cl-Na-Ca, F |
| GW5 | 6.91 | −182.5 | 2.04 | 60.5 | 1446.5 | 2.64 | 48.79 | SO4-Na-Ca, F |
| GW6 | 7.56 | −135.9 | 0.42 | 34.7 | 253.5 | 0.346 | 19.89 | HCO3-Ca-Mg |
| GW7 | 7.55 | −72.2 | 0.40 | 23.3 | 222.0 | 0.223 | 7.98 | HCO3-Ca-Mg |
| GW8 | 7.53 | −129.7 | 0.330 | 25.4 | 186.6 | 0.178 | 5.73 | HCO3-Ca-Mg |
| GW9 | 6.65 | −153.7 | 1.39 | 76.9 | 1077.8 | 1.48 | 64.66 | SO4-Ca |
| Geothermal Water Well | Crtot ± U (µg·L−1) |
|---|---|
| GW1 | 1.20 ± 0.45 |
| GW2 | 1.06 ± 0.40 |
| GW3 | 1.10 ± 0.41 |
| GW4 | 1.40 ± 0.52 |
| GW5 | 2.03 ± 0.76 |
| GW6 | 1.19 ± 0.45 |
| GW7 | 1.89 ± 0.71 |
| GW8 | 1.05 ± 0.39 |
| GW9 | 2.18 ± 0.82 |
| Geothermal Water Well | Crtot (µg·L−1) | Cr(III) (µg·L−1) | Cr(VI) (µg·L−1) |
|---|---|---|---|
| GW1 | 1.20 | 1.20 | 1.30 × 10−19 |
| GW2 | 1.06 | 1.06 | 7.56 × 10−22 |
| GW3 | 1.10 | 1.10 | 5.44 × 10−24 |
| GW4 | 1.40 | 1.40 | 3.91 × 10−22 |
| GW5 | 2.03 | 2.03 | 2.62 × 10−29 |
| GW6 | 1.19 | 1.19 | 1.72 × 10−27 |
| GW7 | 1.89 | 1.89 | 3.66 × 10−27 |
| GW8 | 1.05 | 1.05 | 4.88 × 10−31 |
| GW9 | 2.18 | 2.18 | 3.51 × 10−23 |
| Parameter | Age Group | ||
|---|---|---|---|
| Adults | Teenagers | Children | |
| Deterministic approach | |||
| EDIderm (mg·kg−1·day−1) | 5.45 × 10−11 | 5.51 × 10−11 | 7.44 × 10−11 |
| HQderm | 3.6 × 10−6 | 3.7 × 10−6 | 5.0 × 10−6 |
| Approach including measurement uncertainty | |||
| EDIderm (mg·kg−1·day−1) | 7.49 × 10−11 | 7.57 × 10−11 | 1.02 × 10−10 |
| HQderm | 5.0 × 10−6 | 5.0 × 10−6 | 6.8 × 10−6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rusiniak, P.; Wątor, K.; Kmiecik, E. Inorganic Chromium Speciation in Geothermal Water of the Podhale Trough (Southern Poland) Used for Recreational Purposes. Energies 2020, 13, 3531. https://doi.org/10.3390/en13143531
Rusiniak P, Wątor K, Kmiecik E. Inorganic Chromium Speciation in Geothermal Water of the Podhale Trough (Southern Poland) Used for Recreational Purposes. Energies. 2020; 13(14):3531. https://doi.org/10.3390/en13143531
Chicago/Turabian StyleRusiniak, Piotr, Katarzyna Wątor, and Ewa Kmiecik. 2020. "Inorganic Chromium Speciation in Geothermal Water of the Podhale Trough (Southern Poland) Used for Recreational Purposes" Energies 13, no. 14: 3531. https://doi.org/10.3390/en13143531