Quantitative Evaluation of the Emissions of a Transport Engine Operating with Diesel-Biodiesel
Abstract
1. Introduction
2. Materials and Methods
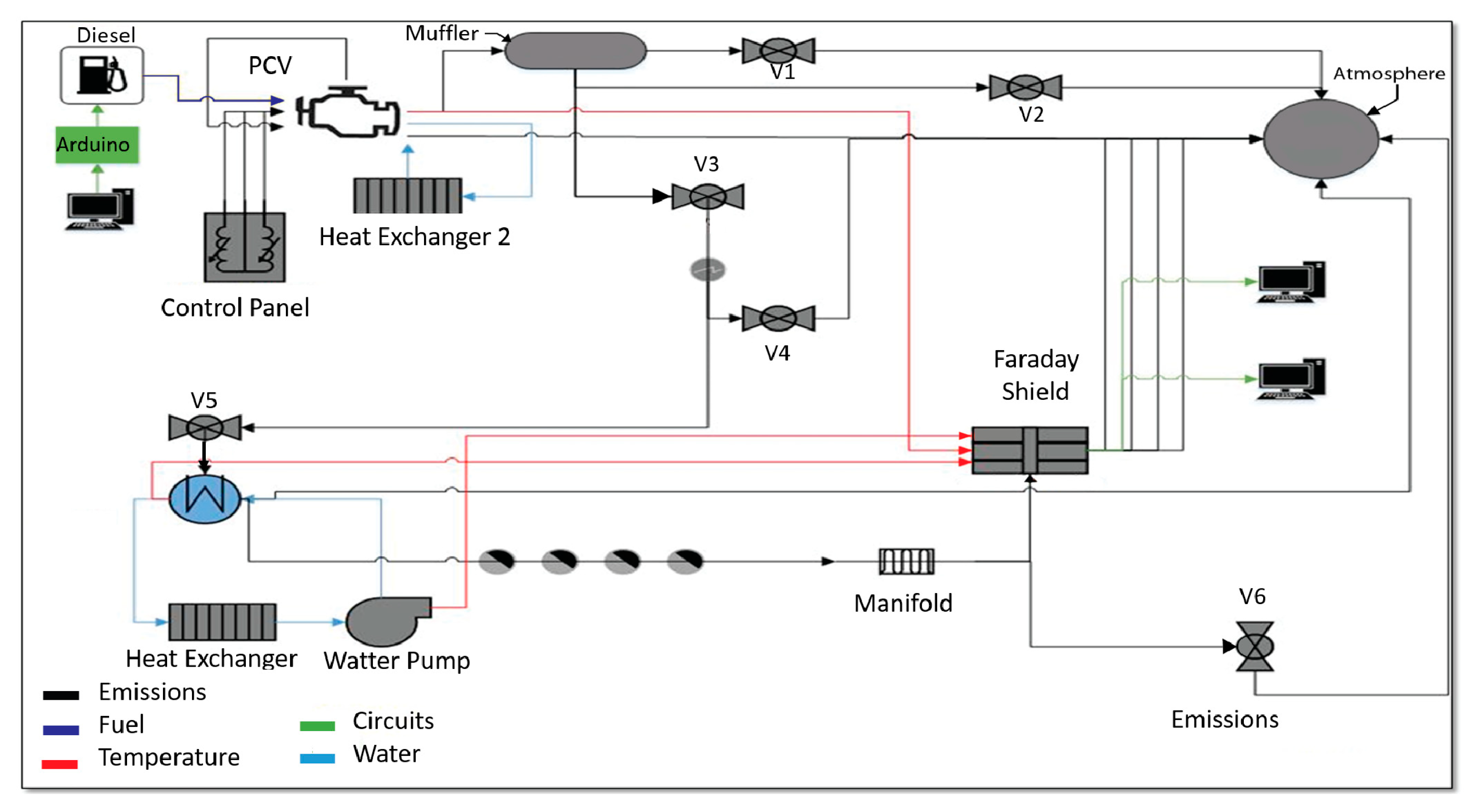
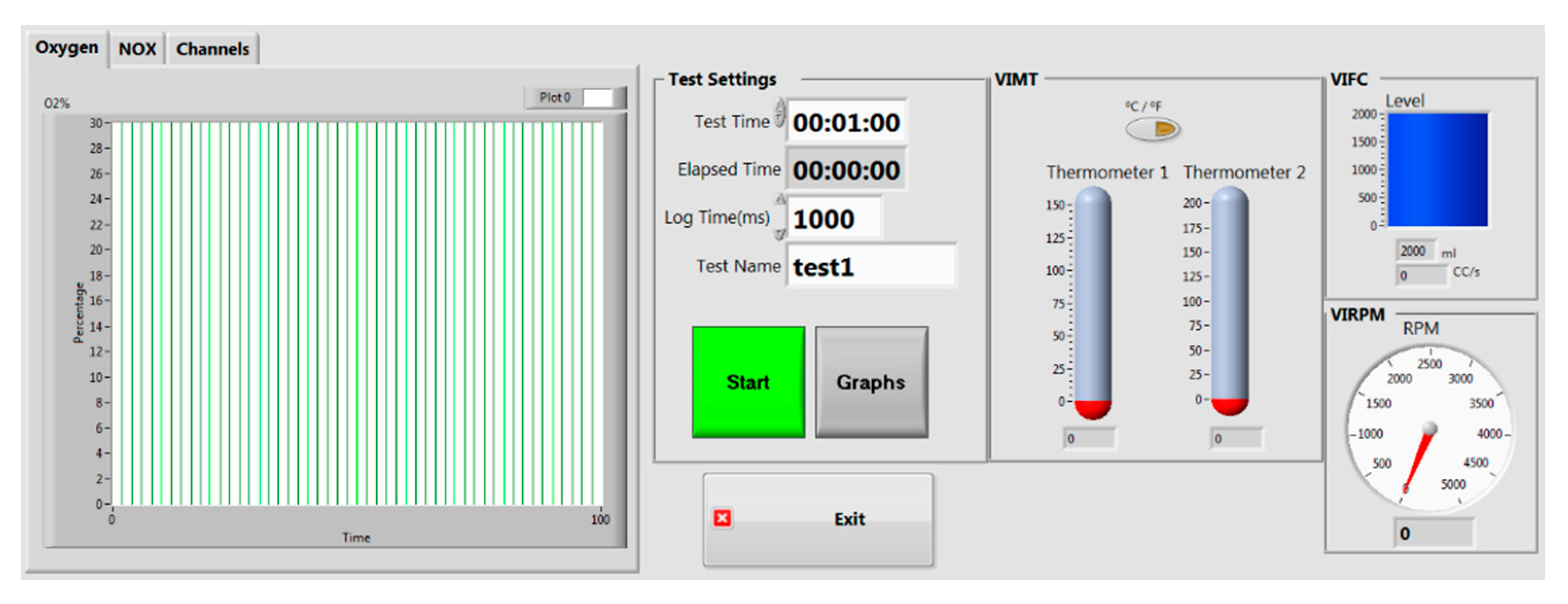
2.1. Virtual Instrument for Measuring Engine Parameters
2.2. Fuel and Its Mixtures Elaboration
2.3. Operation Conditions of CI-Engine
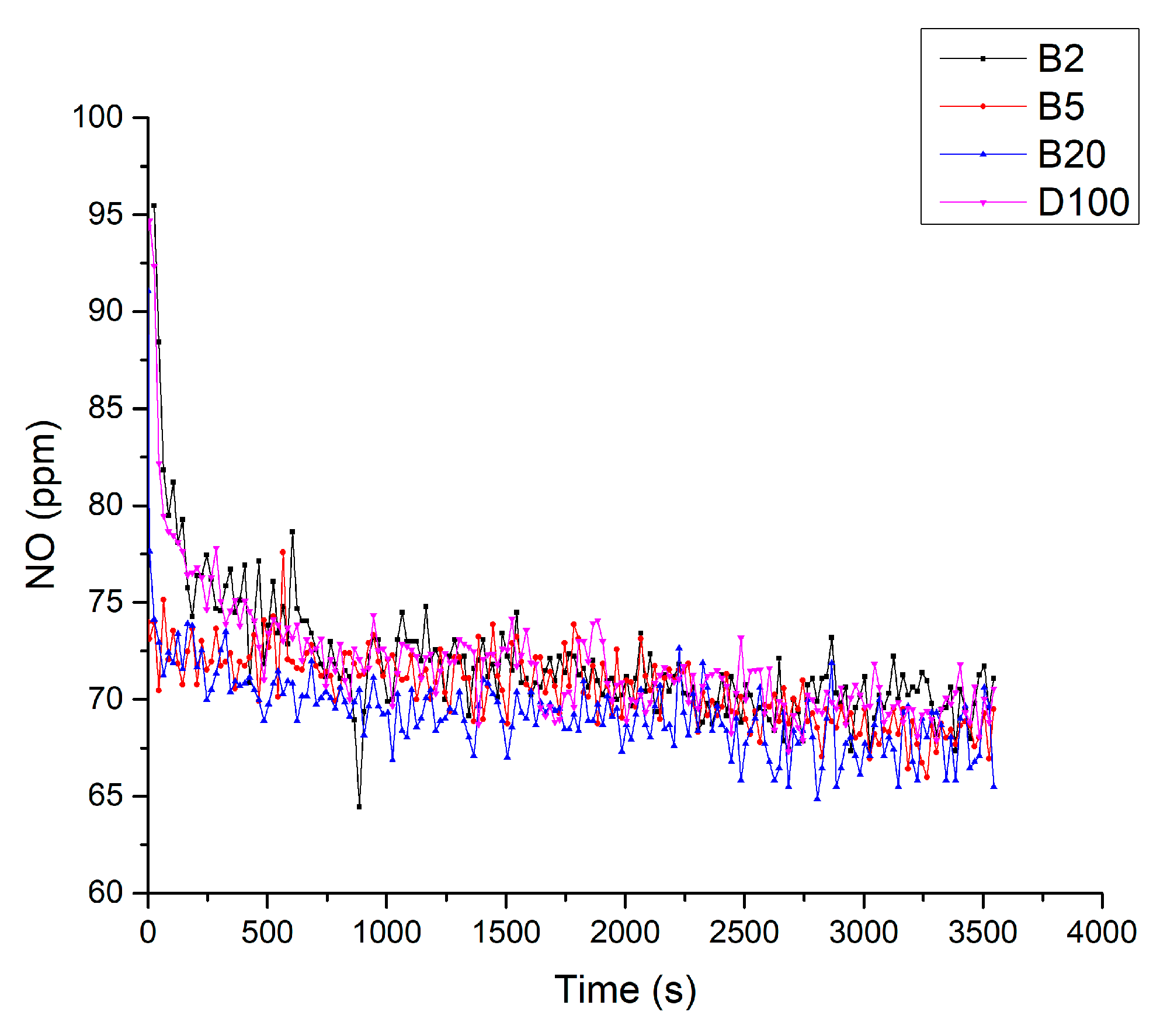
3. Results and Discussions
3.1. Specific Viscosity of the Mixtures
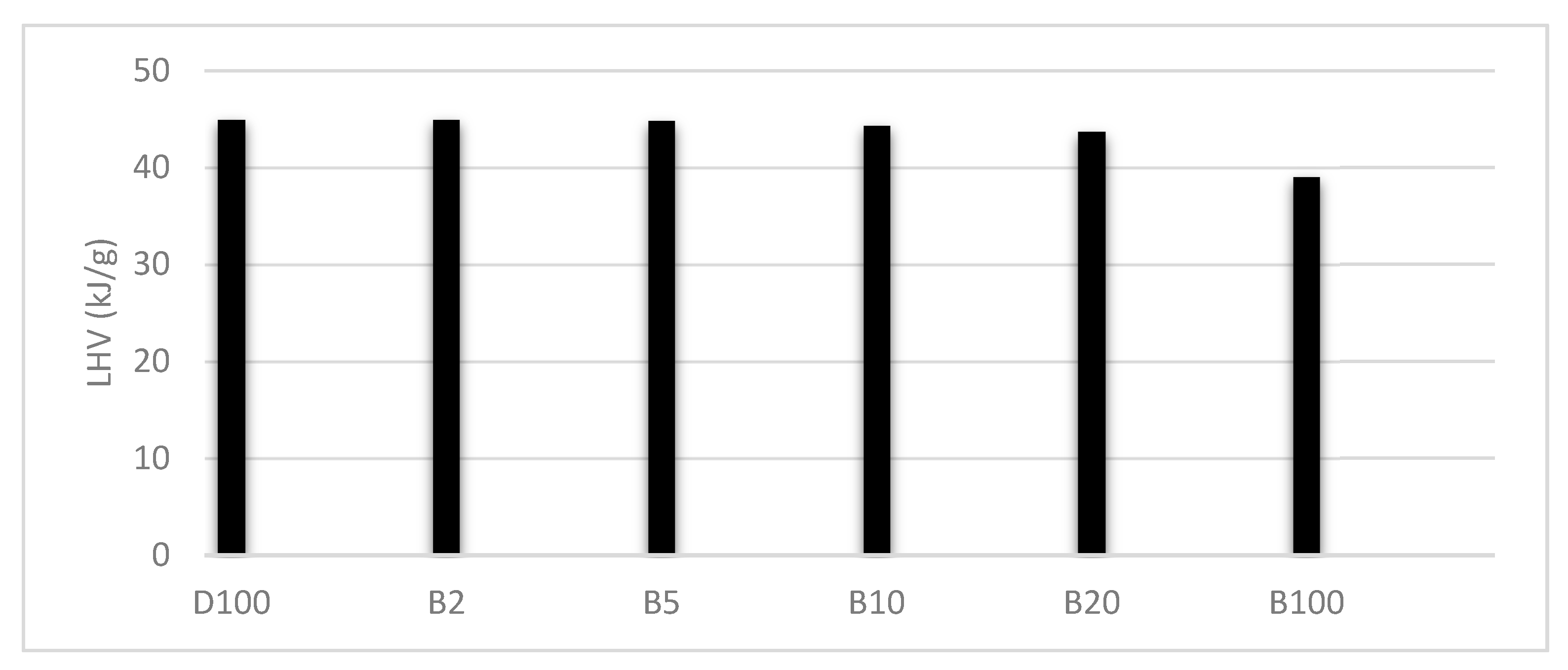
3.2. Low Calorific Power of Fuels
3.3. Raman Analysis
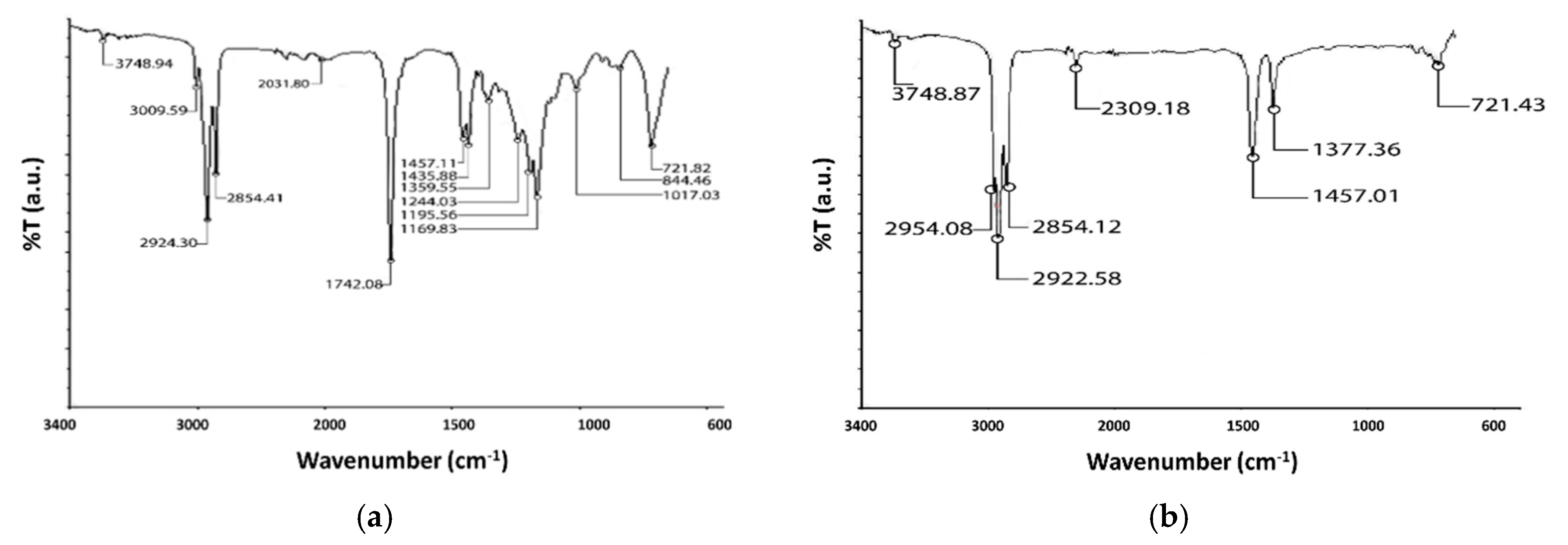
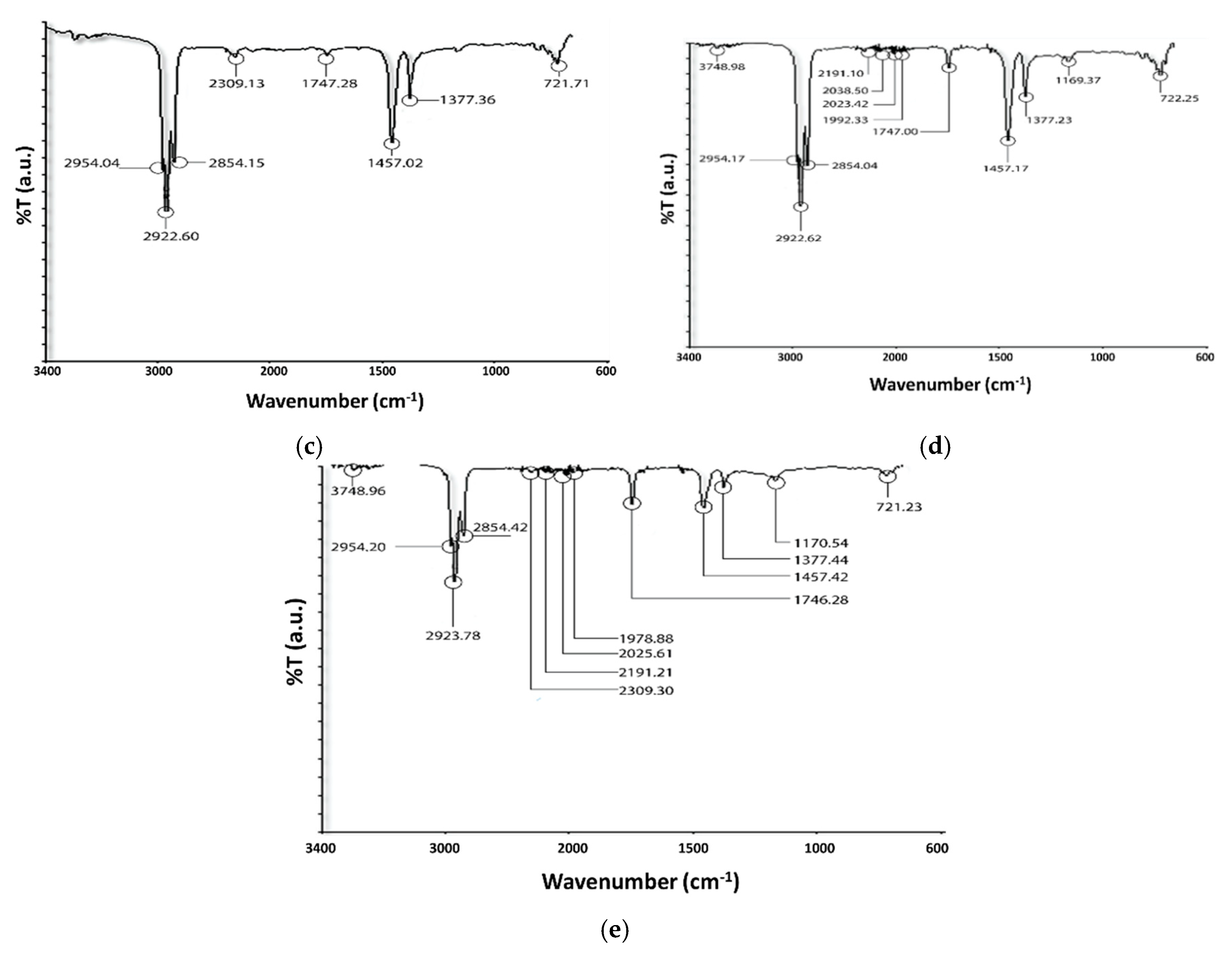
3.4. FTIR Analysis
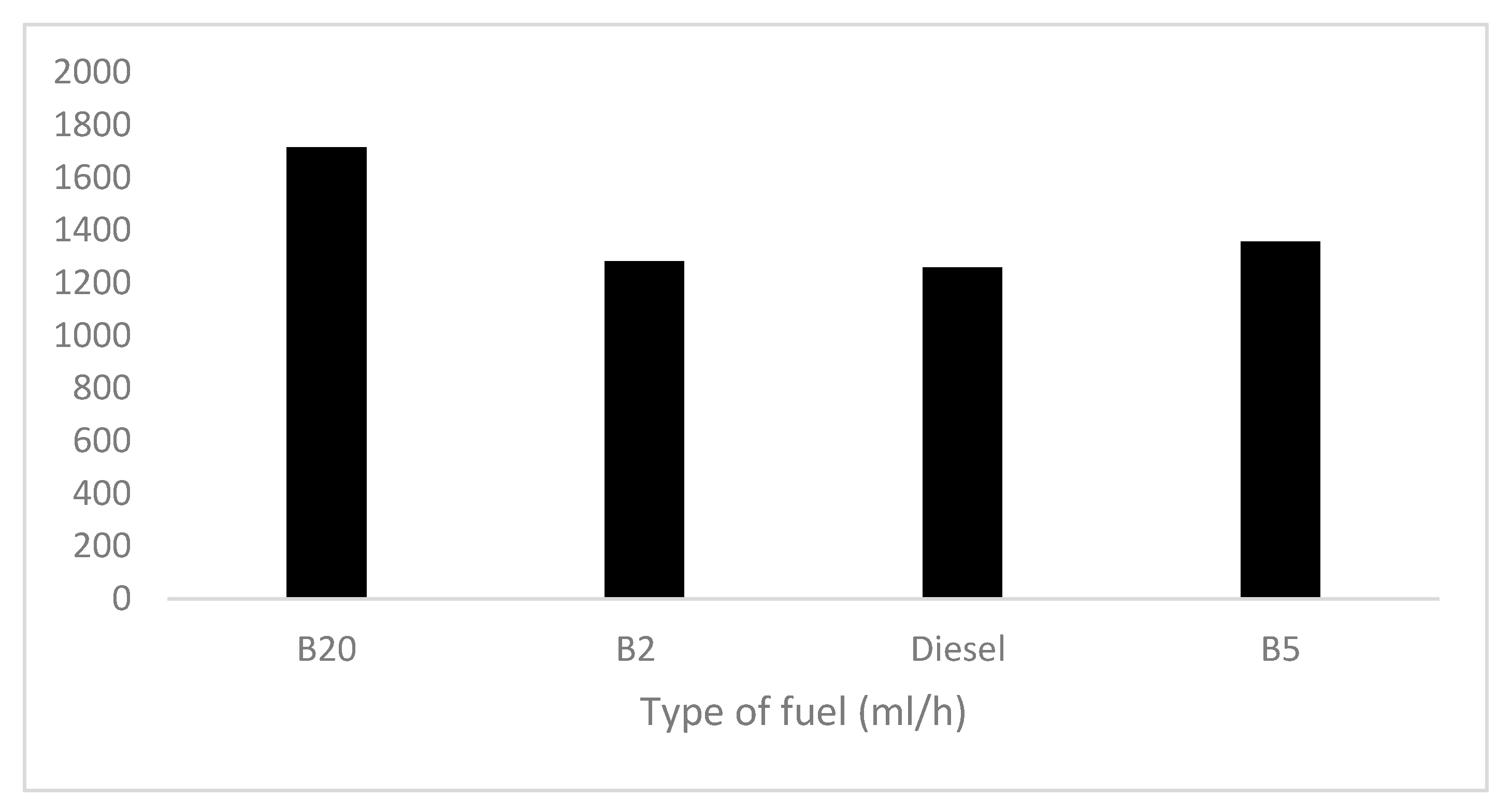
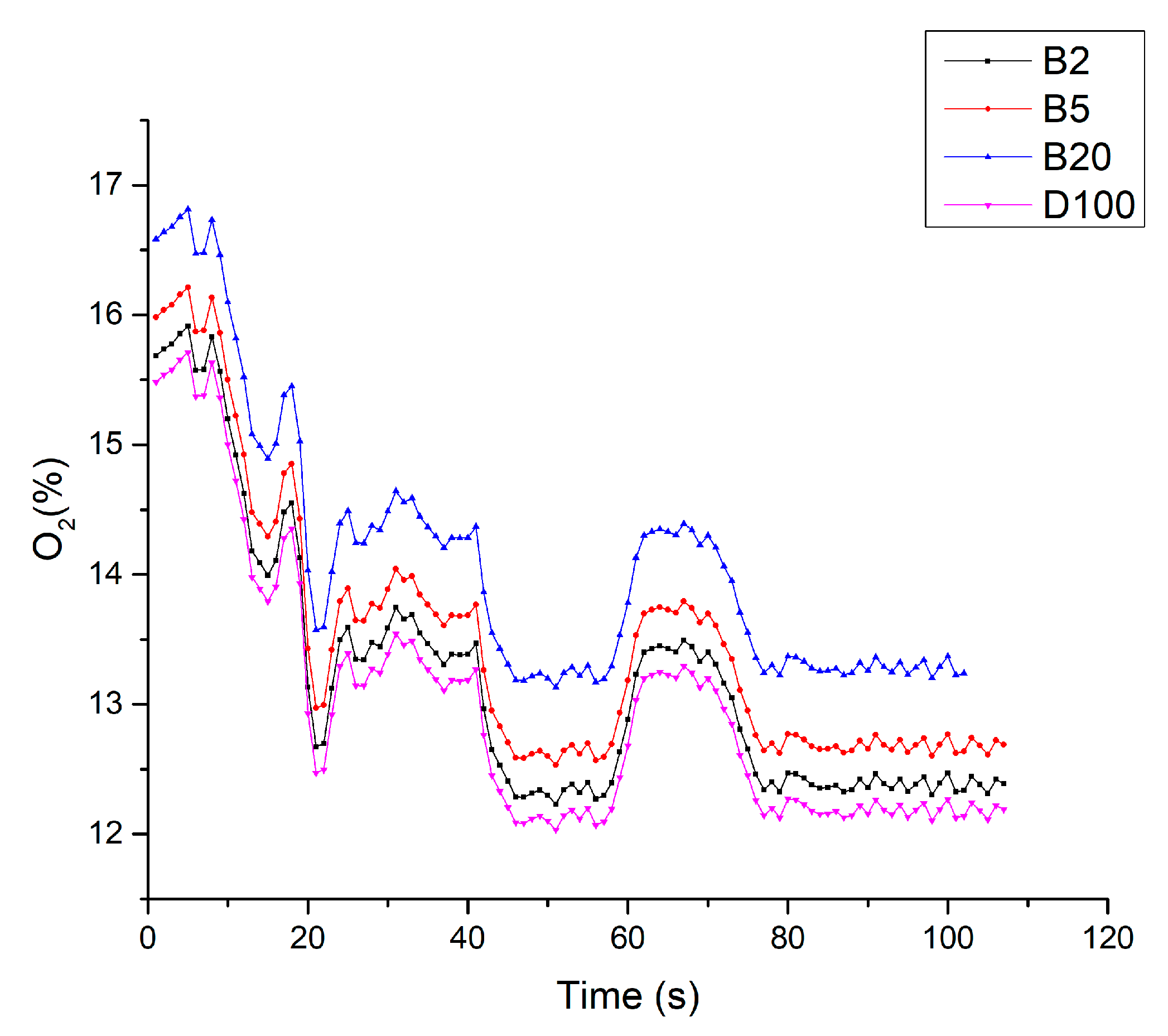
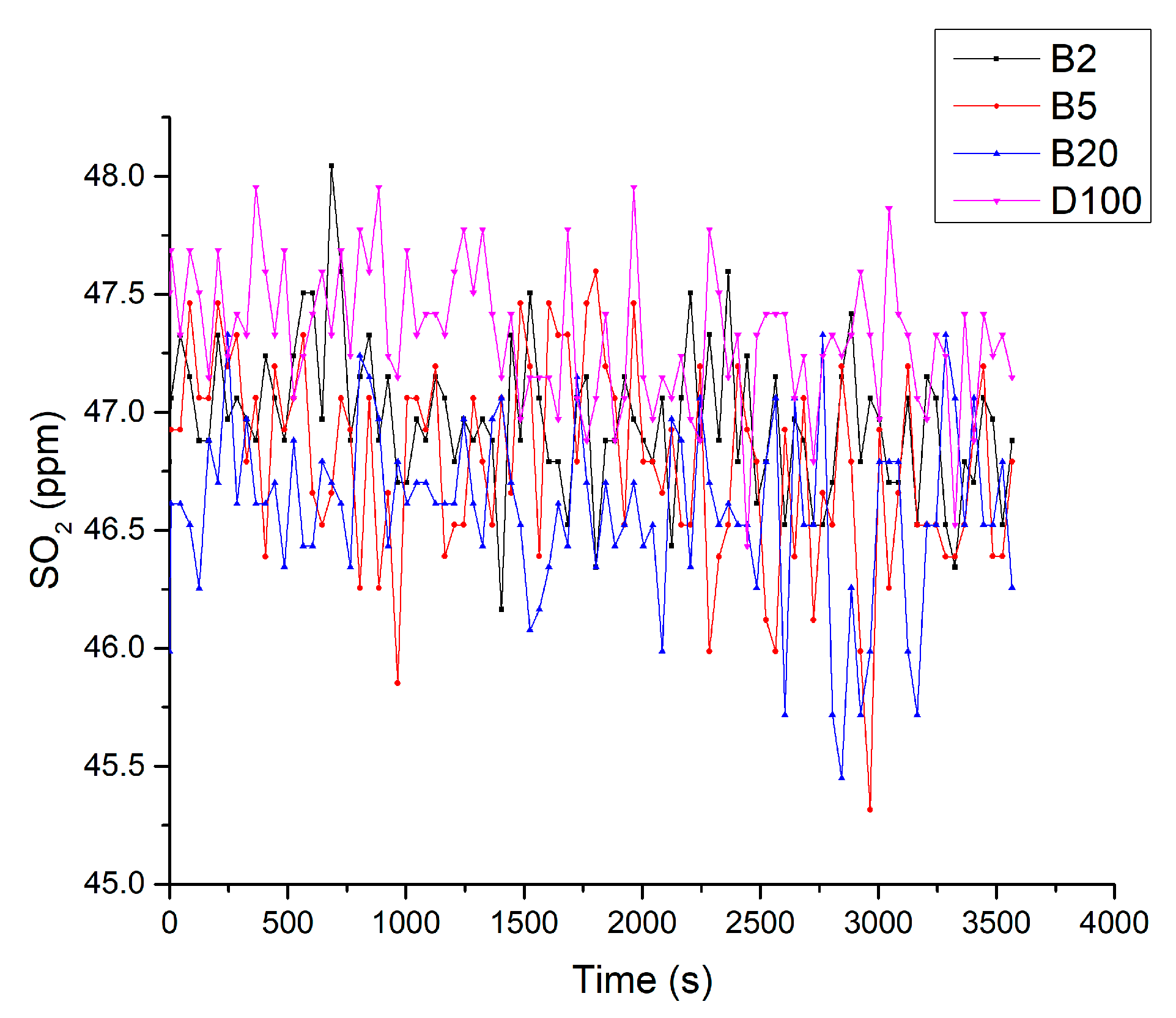
3.5. Results of Fuel Tests
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Asif, M.; Muneer, T. Energy supply, its demand and security issues for developed and emerging economies. Renew. Sustain. Energy Rev. 2007, 11, 1388–1413. [Google Scholar] [CrossRef]
- Dincer, I. Environmental impacts of energy. Energy Policy 1999, 27, 845–854. [Google Scholar] [CrossRef]
- Kalam, M.A.; Hassan, M.; Jayed, M.; Liaquat, A. Emission and performance characteristics of an indirect ignition diesel engine fuelled with waste cooking oil. Energy 2011, 36, 397–402. [Google Scholar] [CrossRef]
- BP. BP Statistical Review of World Energy June 2017; BP: London, UK, 2017. [Google Scholar]
- International Renewable Energy Agency (IRENA). Global Energy Transformation: A Roadmap to 2050; IRENA: Abu Dhabi, UAE, 2018. [Google Scholar] [CrossRef]
- Lee, H.V.; Juan, J.C.; Taufiq-Yap, Y.H.; Kong, P.S.; Rahman, N.A. Advancement in heterogeneous base catalyzed technology: An efficient production of biodiesel fuels. J. Renew. Sustain. Energy 2015, 7, 032701. [Google Scholar] [CrossRef]
- Ali, O.M.; Mamat, R.; Abdullah, N.R.; Abdullah, A.A. Analysis of blended fuel properties and engine performance with palm biodiesel–diesel blended fuel. Renew. Energy 2016, 86, 59–67. [Google Scholar] [CrossRef]
- Ma, F.; A Hanna, M. Biodiesel production: A review. Bioresour. Technol. 1999, 70, 1–15. [Google Scholar] [CrossRef]
- Espinosa, E.A.M.; Piloto-Rodríguez, R.; Goyos-Pérez, L.; Sierens, R.; Verhelst, S. Emulsification of animal fats and vegetable oils for their use as a diesel engine fuel: An overview. Renew. Sustain. Energy Rev. 2015, 47, 623–633. [Google Scholar] [CrossRef]
- Igbum, O.G.; Eloka-Eboka, A.C.; Ubwa, S.T.; Inambao, F.L. Evaluation of environmental impact and gaseous emissions of biodiesel fuels and blends of selected feed-stocks. Int. J. Glob. Warm. 2014, 6, 99. [Google Scholar] [CrossRef]
- Dorado, M.P.; Ballesteros, E.; Arnal, J.M.; Gómez, J.; López, F.J. Exhaust emissions from a Diesel engine fueled with transesterified waste olive oil⋆. Fuel 2003, 82, 1311–1315. [Google Scholar] [CrossRef]
- Atadashi, I.; Aroua, M.K.; Aziz, A.A. High quality biodiesel and its diesel engine application: A review. Renew. Sustain. Energy Rev. 2010, 14, 1999–2008. [Google Scholar] [CrossRef]
- Rakopoulos, C.D.; Antonopoulos, K.; Rakopoulos, D.; Hountalas, D.; Giakoumis, E.G. Comparative performance and emissions study of a direct injection Diesel engine using blends of Diesel fuel with vegetable oils or bio-diesels of various origins. Energy Convers. Manag. 2006, 47, 3272–3287. [Google Scholar] [CrossRef]
- Graboski, M.S.; McCormick, R.L. Combustion of fat and vegetable oil derived fuels in diesel engines. Prog. Energy Combust. Sci. 1998, 24, 125–164. [Google Scholar] [CrossRef]
- Claxton, L. The history, genotoxicity, and carcinogenicity of carbon-based fuels and their emissions. Part 3: Diesel and gasoline. Mutat. Res. Mutat. Res. 2015, 763, 30–85. [Google Scholar] [CrossRef] [PubMed]
- Borillo, G.C.; Tadano, Y.S.; Godoi, A.F.L.; Pauliquevis, T.; Sarmiento, H.; Rempel, D.; Yamamoto, C.I.; Marchi, M.R.; Potgieter-Vermaak, S.; Godoi, R. Polycyclic Aromatic Hydrocarbons (PAHs) and nitrated analogs associated to particulate matter emission from a Euro V-SCR engine fuelled with diesel/biodiesel blends. Sci. Total. Environ. 2018, 644, 675–682. [Google Scholar] [CrossRef]
- Testo 340—Flue gas analyzer for industry | Metal and steel | Emissions | Industry | Target groups. Available online: https://www.testo.com/en-US/testo-340/p/0632-3340 (accessed on 13 June 2019).
- Gas Analyzer Enerac 700—Portable Combustion Analyzer. Available online: http://www.enerac.com/gas-analyzer-enerac-700/ (accessed on 13 June 2019).
- Bonilla, D.; Gil Samaniego, M.; Gil-Samaniego, M.; Campbell, H. Practical and low-cost monitoring tool for building energy management systems using virtual instrumentation. Sustain. Cities Soc. 2018, 39, 155–162. [Google Scholar] [CrossRef]
- Goldberg, H. What is virtual instrumentation? IEEE Instrum. Meas. Mag. 2000, 3, 10–13. [Google Scholar] [CrossRef]
- Manigandan, S.; Gunasekar, P.; Devipriya, J.; Nithya, S. Emission and injection characteristics of corn biodiesel blends in diesel engine. Fuel 2019, 235, 723–735. [Google Scholar] [CrossRef]
- Moore, K. Testing Automotive Exhaust Emissions—Application Note; National Instruments: Austin, TX, USA, 2013. [Google Scholar]
- Pérez, A.; Ramos, R.; Montero, G.; Coronado, M.; García-González, C.; Pérez, R. Virtual Instrument for Emissions Measurement of Internal Combustion Engines. J. Anal. Methods Chem. 2016, 2016, 1–13. [Google Scholar] [CrossRef]
- Frijters, P.; Baert, R. Oxygenated fuels for clean heavy-duty diesel engines. Int. J. Veh. Des. 2006, 41, 242. [Google Scholar] [CrossRef]
- Sahoo, P.; Das, L.; Babu, M.; Arora, P.; Singh, V.; Kumar, N.; Varyani, T. Comparative evaluation of performance and emission characteristics of jatropha, karanja and polanga based biodiesel as fuel in a tractor engine. Fuel 2009, 88, 1698–1707. [Google Scholar] [CrossRef]
- Chauhan, B.; Kumar, N.; Cho, H.M. A study on the performance and emission of a diesel engine fueled with Jatropha biodiesel oil and its blends. Energy 2012, 37, 616–622. [Google Scholar] [CrossRef]
- Tesfa, B.; Gu, F.; Mishra, R.; Ball, A.D. Emission Characteristics of a CI Engine Running with a Range of Biodiesel Feedstocks. Energies 2014, 7, 334–350. [Google Scholar] [CrossRef]
- Hansen, A.C.; Gratton, M.R.; Yuan, W. Diesel Engine Performance and NOX Emissions From Oxygenated Biofuels and Blends with Diesel Fuel. Trans. ASABE 2006, 49, 589–595. [Google Scholar] [CrossRef]
- Canakci, M. NOx emissions of biodiesel as an alternative diesel fuel. Int. J. Veh. Des. 2009, 50, 213. [Google Scholar] [CrossRef]
- Kim, H.; Choi, B. The effect of biodiesel and bioethanol blended diesel fuel on nanoparticles and exhaust emissions from CRDI diesel engine. Renew. Energy 2010, 35, 157–163. [Google Scholar] [CrossRef]
- Xue, J.; Grift, T.E.; Hansen, A.C. Effect of biodiesel on engine performances and emissions. Renew. Sustain. Energy Rev. 2011, 15, 1098–1116. [Google Scholar] [CrossRef]
- Cardoso, C.C.; Mendes, B.M.; Pasa, V.M.D. Production and characterization of cold-flow quality biofuel from soybean oil using different alky and benzyl alcohols. J. Environ. Chem. Eng. 2018, 6, 2241–2247. [Google Scholar] [CrossRef]
- Martínez, A.; Mijangos, G.E.; Romero-Ibarra, I.; Hernández-Altamirano, R.; Mena-Cervantes, V.Y. In-situ transesterification of Jatropha curcas L. seeds using homogeneous and heterogeneous basic catalysts. Fuel 2019, 235, 277–287. [Google Scholar] [CrossRef]
- Reyero, I.; Arzamendi, M.C.; Zabala, S.; Gandía, L.M. Kinetics of the NaOH-catalyzed transesterification of sunflower oil with ethanol to produce biodiesel. Fuel Process. Technol. 2015, 129, 147–155. [Google Scholar] [CrossRef]
- Versión, N. PEMEX DIESEL Hoja de Datos de Seguridad SECCIÓN I. DATOS GENERALES. n.d. Available online: https://www.pemex.com/negocio/gasolineras/nuestros-productos/Documents/HDS-Pemex%20Diesel.pdf (accessed on 18 February 2019).
- Pullen, J.; Saeed, K. Factors affecting biodiesel engine performance and exhaust emissions—Part I: Review. Energy 2014, 72, 1–16. [Google Scholar] [CrossRef]
- Benavides, A.; Benjumea, P.; Pashova, V. El biodiesel de aceite de higuerilla como combustible alternativo para motores diesel. Dyna 2007, 74, 141–150. [Google Scholar]
- Mattarelli, E.; Rinaldini, C.A.; Savioli, T. Combustion Analysis of a Diesel Engine Running on Different Biodiesel Blends. Energies 2015, 8, 3047–3057. [Google Scholar] [CrossRef]
- Heise, H.M.; Fritzsche, J.; Tkatsch, H.; Waag, F.; Karch, K.; Henze, K.; Delbeck, S.; Budde, J. Recent advances in mid- and near-infrared spectroscopy with applications for research and teaching, focusing on petrochemistry and biotechnology relevant products. Eur. J. Phys. 2013, 34, S139–S159. [Google Scholar] [CrossRef]
- Soares, I.P.; Rezende, T.F.; Pereira, R.D.C.C.; Dos Santos, C.G.; Fortes, I.C.P. Determination of biodiesel adulteration with raw vegetable oil from ATR-FTIR data using chemometric tools. J. Braz. Chem. Soc. 2011, 22, 1229–1235. [Google Scholar] [CrossRef]
- Aliske, M.A.; Zagonel, G.F.; Costa, B.J.; Veiga, W.; Saul, C. Measurement of biodiesel concentration in a diesel oil mixture. Fuel 2007, 86, 1461–1464. [Google Scholar] [CrossRef]
- Aryee, A.N.A.; van de Voort, F.R.; Simpson, B.K. Process Biochemistry; Elsevier Science: Amsterdam, The Netherlands, 2009; Volume 44. [Google Scholar]
- Buyukkaya, E. Effects of biodiesel on a DI diesel engine performance, emission and combustion characteristics. Fuel 2010, 89, 3099–3105. [Google Scholar] [CrossRef]
- Knothe, G.; Krahl, J.; Van Gerpen, J.H. The Biodiesel Handbook; AOCS Press: Urbana, IL, USA, 2010. [Google Scholar]
- Di, Y.; Cheung, C.S.; Huang, Z. Experimental investigation on regulated and unregulated emissions of a diesel engine fueled with ultra-low sulfur diesel fuel blended with biodiesel from waste cooking oil. Sci. Total. Environ. 2009, 407, 835–846. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-Y.; Lin, H.-A. Diesel engine performance and emission characteristics of biodiesel produced by the peroxidation process. Fuel 2006, 85, 298–305. [Google Scholar] [CrossRef]
- Lin, Y.-F.; Wu, Y.-P.G.; Chang, C.-T. Combustion characteristics of waste-oil produced biodiesel/diesel fuel blends. Fuel 2007, 86, 1772–1780. [Google Scholar] [CrossRef]
- Canakci, M. Combustion characteristics of a turbocharged DI compression ignition engine fueled with petroleum diesel fuels and biodiesel. Bioresour. Technol. 2007, 98, 1167–1175. [Google Scholar] [CrossRef]
- Lapuerta, M.; Fernández, J.R.; Agudelo, J.R. Diesel particulate emissions from used cooking oil biodiesel. Bioresour. Technol. 2008, 99, 731–740. [Google Scholar] [CrossRef]
- Heywood, J.B. Internal Combustion Engine Fundamentals; McGraw-Hill Inc: New York, NY, USA, 1998. [Google Scholar]
- Norris, J. EMStec-Unclassified an In-Service Emissions Test for Spark Ignition (SI) Petrol Engines-PPAD 9/107/09; Phase 2b Report Identification of options for alternative test procedures; A report produced for DfT; 2002. Available online: https://uk-air.defra.gov.uk/assets/documents/reports/cat15/0408171322_SI_Phase2aReport.pdf (accessed on 22 September 2019).
- Graboski, M.S.; Mccormick, R.L.; Alleman, T.L.; Herring, A.M. The Effect of Biodiesel Composition on Engine Emissions from a DDC Series 60 Diesel Engine: Final Report. Report 2 in a Series of 6. 2003. 2003. Available online: https://pdfs.semanticscholar.org/7bd3/35e0bd81f41601152303fd4093b697f24d59.pdf (accessed on 14 April 2019).
- Varatharajan, K.; Cheralathan, M.; Velraj, R. Mitigation of NOx emissions from a jatropha biodiesel fuelled DI diesel engine using antioxidant additives. Fuel 2011, 90, 2721–2725. [Google Scholar] [CrossRef]
- Hess, M.A.; Haas, M.J.; Foglia, T.A.; Marmer, W.N. Effect of Antioxidant Addition on NOxEmissions from Biodiesel. Energy Fuels 2005, 19, 1749–1754. [Google Scholar] [CrossRef]
- McCormick, R.L.; Alvarez, J.R.; Graboski, M.S. NOx Solutions for Biodiesel: Final Report; Report 6 in a Series of 6; Office of Scientific and Technical Information (OSTI): Washington, DC, USA, 2003; p. 6.
- Varatharajan, K.; Cheralathan, M. Effect of aromatic amine antioxidants on NOx emissions from a soybean biodiesel powered DI diesel engine. Fuel Process. Technol. 2013, 106, 526–532. [Google Scholar] [CrossRef]
- Ileri, E.; Kocar, G. Experimental investigation of the effect of antioxidant additives on NOx emissions of a diesel engine using biodiesel. Fuel 2014, 125, 44–49. [Google Scholar] [CrossRef]
- Palash, S.; Kalam, M.A.; Hassan, M.; Masum, B.; Fattah, I.M.R.; Mofijur, M. Impacts of biodiesel combustion on NOx emissions and their reduction approaches. Renew. Sustain. Energy Rev. 2013, 23, 473–490. [Google Scholar] [CrossRef]
- Sun, J.; Caton, J.A.; Jacobs, T.J. Oxides of nitrogen emissions from biodiesel-fuelled diesel engines. Prog. Energy Combust. Sci. 2010, 36, 677–695. [Google Scholar] [CrossRef]




| Parameter | Value | Unit |
|---|---|---|
| Number of cylinders | In-line four cylinders | - |
| Cylinder diameter | 102 | mm |
| Stroke | 130 | mm |
| Displacement volume | 4.25 | lt |
| Compression ratio | 17.4:1 | - |
| Rated power at 2300 rpm | 141.7 | kW |
| Maximum torque at 1200–1600 rpm | 705.02 | Nm |
| Intake valve | 2 | - |
| Exhaust valve | 1 | - |
| Decompression valve | 1 | - |
| Redline | 2300 | - |
| Injection | Direct injection | - |
| Sensor Properties | Ranges | |||
|---|---|---|---|---|
| O2 | CO | SO2 | NO | |
| Concentration ranges (ppm) | 0–30 | 0–500 | 0–2000 | 0–2000 |
| Sensitivity (ppm) | N/A | 55–85 | 8–20 | 320–480 |
| Maximum overload | 100% | 1500 ppm | N/A | 1000 ppm |
| Resolution | 0.1% | 1 ppm | 5 ppm | 0 to 4.5 ppm |
| Response time (s) | 15–90 | 30–90 | 60–90 | 35–90 |
| Temperature range (°C) | −20 to +55 | −20 to +50 | −20 to +50 | −20 to +50 |
| Relative humidity (%) | 15–95 | 15–90 | 15–90 | 15–90 |
| Pressure range (kPa) | 90–110 | 90–110 | 90–110 | 90–110 |
| Measured Parameter | Measurement Range | Accuracy | Measurement Sensor | Percentage Uncertainty (%) |
|---|---|---|---|---|
| Engine speed (rpm) | 0–10,000 | ±1 | Magnetic pickup speed transducer | ±0.1 |
| Time (s) | - | ±0.1 | - | ±0.2 |
| Fuel flow (mL/min) | 20–6000 | ±10 | Flow Sensor Meter | ±2 |
| Viscometer (Poise) | 0.2–15,000 Poise | <1 mL | Speed of Rotation 5–1000 RPM | ±0.5 |
| Calorimeter (J) | 0–40,000 | ±1 | Temperature sensor | ±0.2 |
| RAMAN spectroscopy | 95–3500 cm−1 Raman shift | 4 cm−1 FWHM Pear resolution and 1 cm−1 Pixel resolution | High sensitivity open electrode CCD detector, 1024 × 256 pixels sensor hermetically sealed vacuum | |
| Fourier-Transform Infrared spectroscopy | 350–7800 cm−1 | 0.5 to 64 cm−1 | 785 nm laser at room temperature was used |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez, A.; Mateos, D.; García, C.; Caraveo, C.; Montero, G.; Coronado, M.; Valdez, B. Quantitative Evaluation of the Emissions of a Transport Engine Operating with Diesel-Biodiesel. Energies 2020, 13, 3594. https://doi.org/10.3390/en13143594
Pérez A, Mateos D, García C, Caraveo C, Montero G, Coronado M, Valdez B. Quantitative Evaluation of the Emissions of a Transport Engine Operating with Diesel-Biodiesel. Energies. 2020; 13(14):3594. https://doi.org/10.3390/en13143594
Chicago/Turabian StylePérez, Armando, David Mateos, Conrado García, Camilo Caraveo, Gisela Montero, Marcos Coronado, and Benjamín Valdez. 2020. "Quantitative Evaluation of the Emissions of a Transport Engine Operating with Diesel-Biodiesel" Energies 13, no. 14: 3594. https://doi.org/10.3390/en13143594
APA StylePérez, A., Mateos, D., García, C., Caraveo, C., Montero, G., Coronado, M., & Valdez, B. (2020). Quantitative Evaluation of the Emissions of a Transport Engine Operating with Diesel-Biodiesel. Energies, 13(14), 3594. https://doi.org/10.3390/en13143594









