Optimization of the Solidification Method of High-Level Waste for Increasing the Thermal Stability of the Magnesium Potassium Phosphate Compound
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Surrogate HLW Solution
2.2. Synthesis and Study of the MPP Compounds
3. Results and Discussion
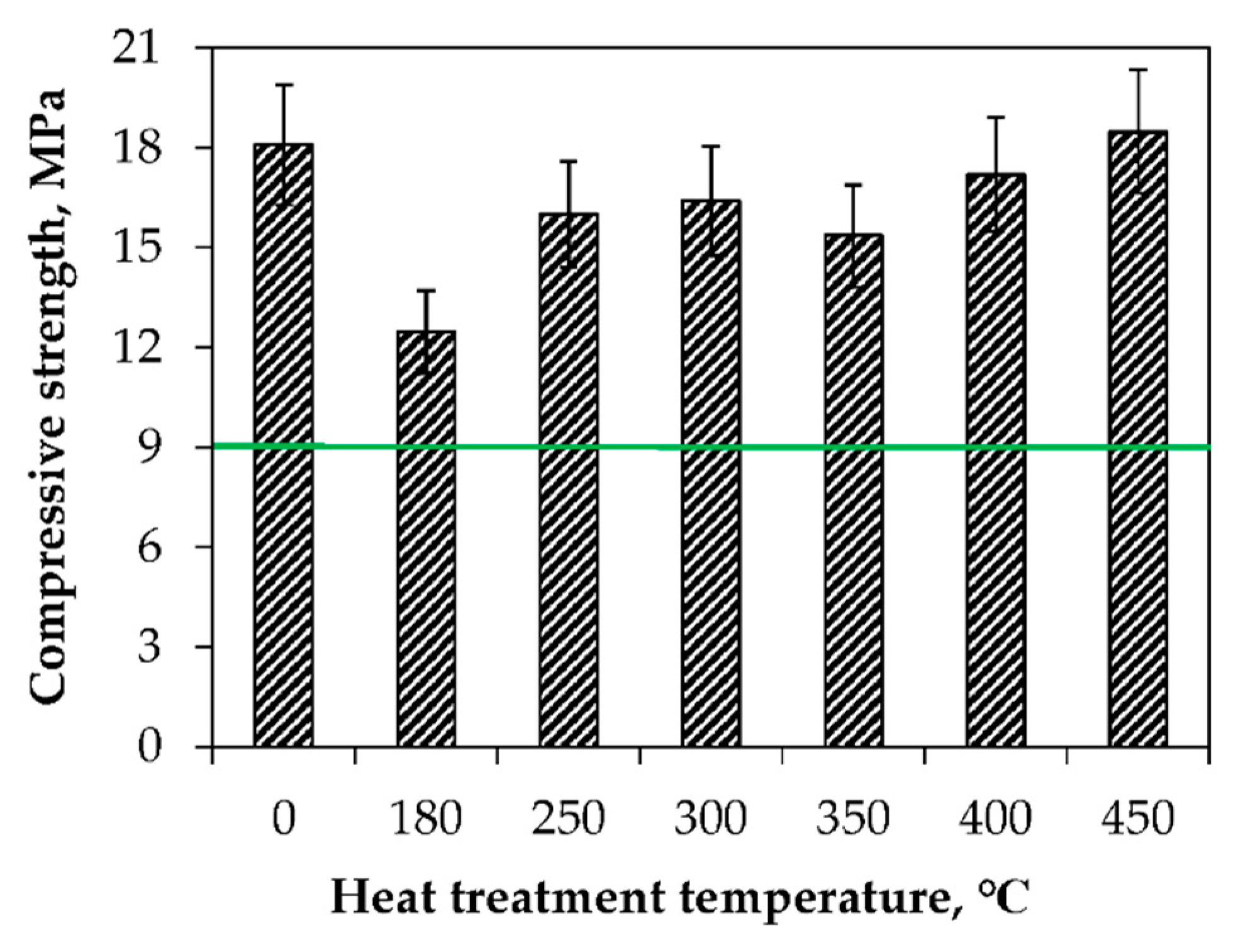
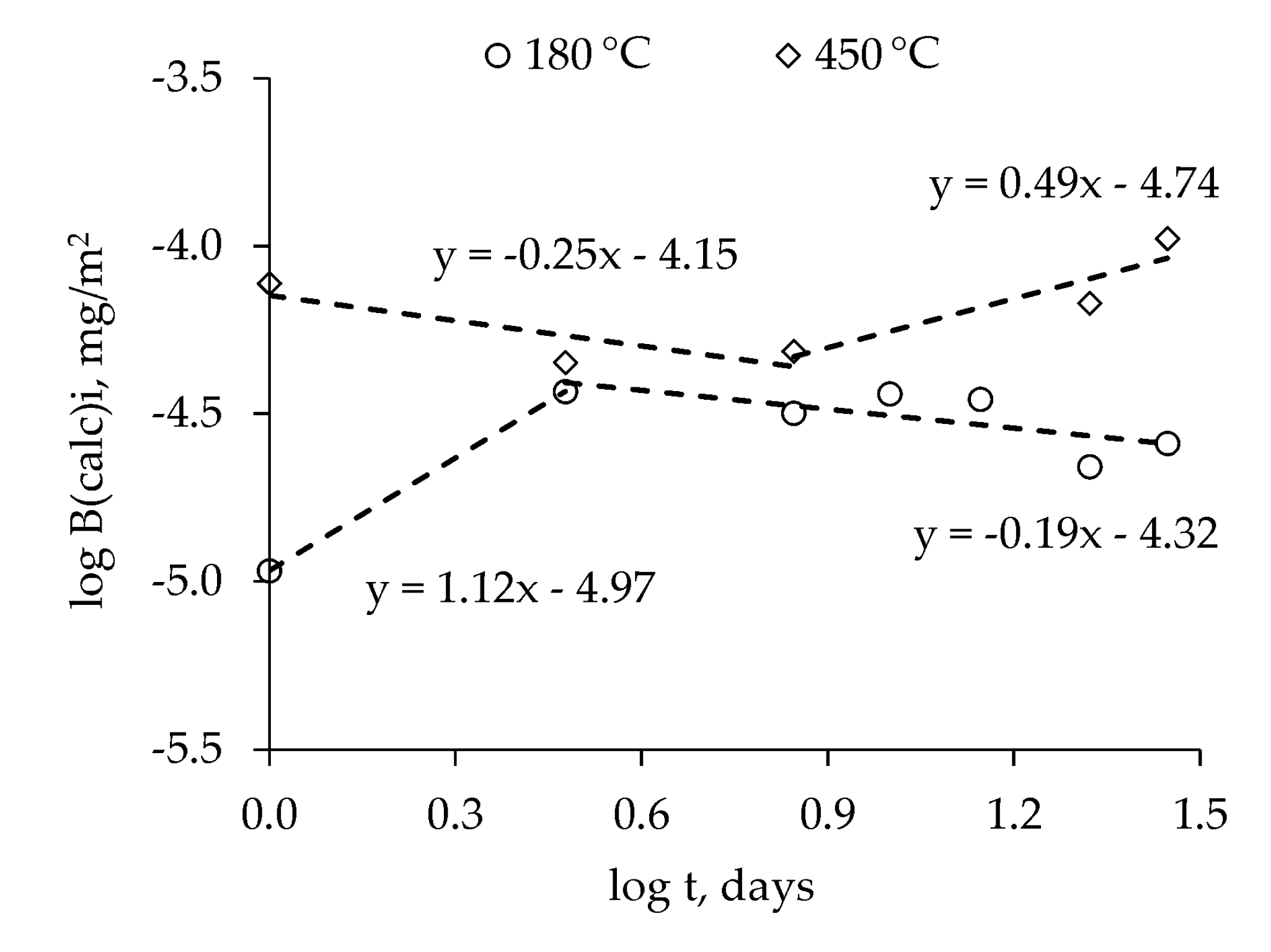
3.1. Thermal Stability of MPP-FKN-W Compound
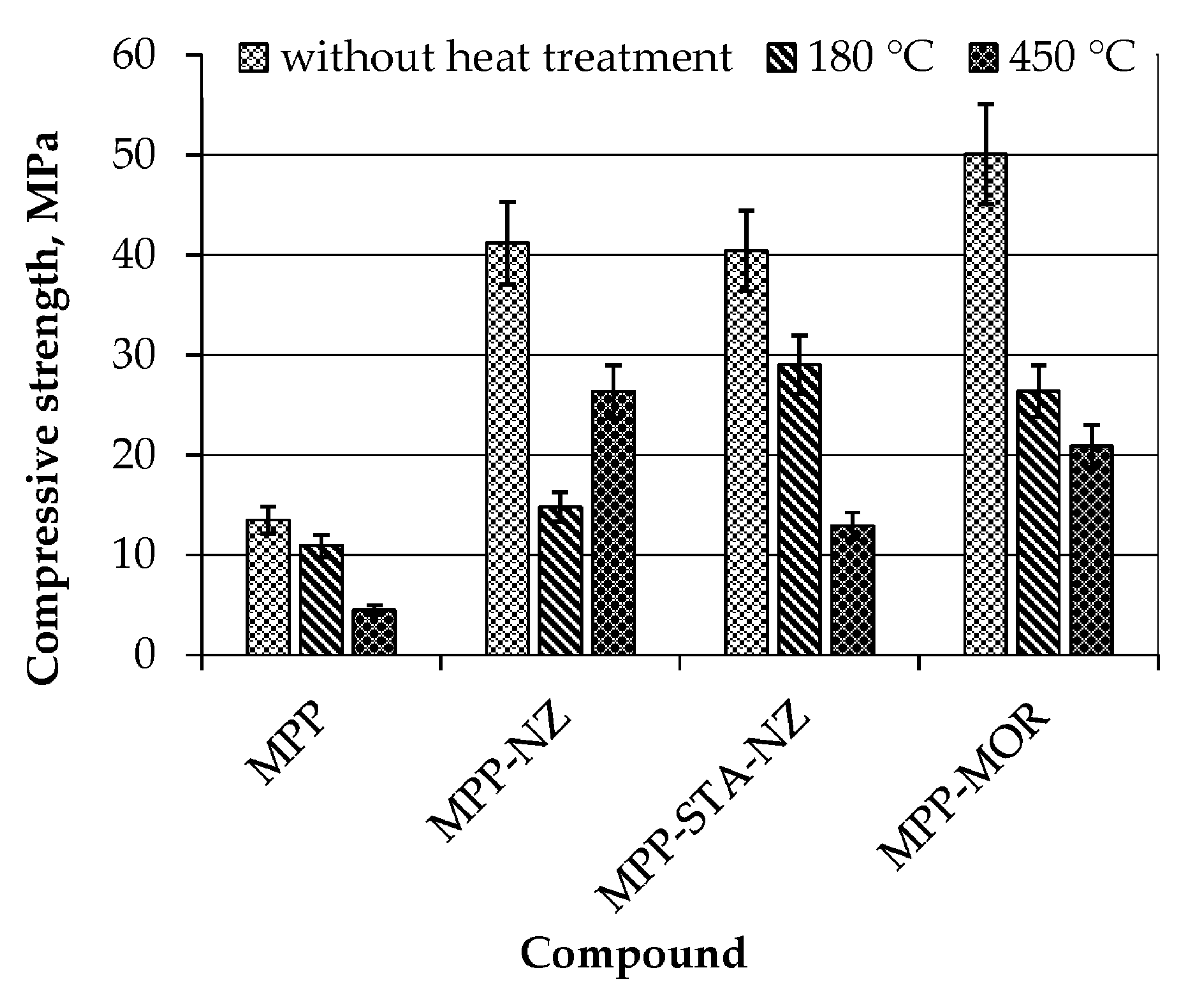
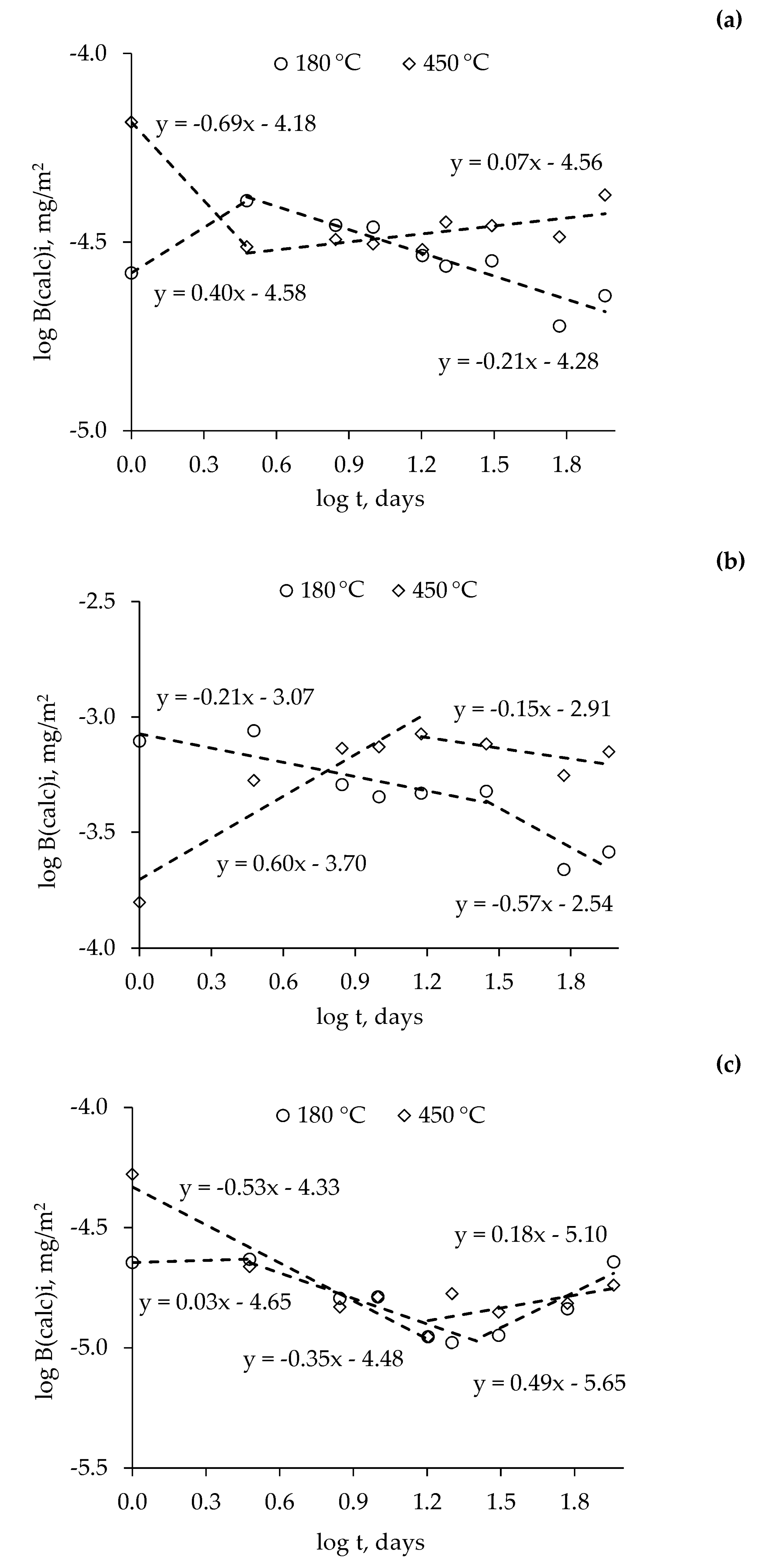
3.2. Thermal Stability of MPP-NZ, MPP-MOR, and MPP-STA-NZ Compounds
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Federal Norms and Rules in the Field of Atomic Energy Use. In “Collection, Processing, Storage and Conditioning of Liquid Radioactive Waste. Safety Requirements” (NP-019-15); Rostekhnadzor: Moscow, Russia, 2015; pp. 1–22.
- Wagh, A.S. Chemically Bonded Phosphate Ceramics; Chemically Bonded Phosphate Ceramics: Twenty-First Century Materials with Diverse Applications, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 1–422. ISBN 978-0-08-100380-0. [Google Scholar]
- Wagh, A.; Strãin, R.; Jeong, S.; Reed, D.; Krause, T.; Singh, D. Stabilization of Rocky Flats Pu-contaminated ash within chemically bonded phosphate ceramics. J. Nucl. Mater. 1999, 265, 295–307. [Google Scholar] [CrossRef]
- Singh, D.; Mandalika, V.; Parulekar, S.J.; Wagh, A. Magnesium potassium phosphate ceramic for 99Tc immobilization. J. Nucl. Mater. 2006, 348, 272–282. [Google Scholar] [CrossRef]
- Vinokurov, S.; Kulyako, Y.; Slyuntchev, O.; Rovny, S.; Myasoedov, B. Low-temperature immobilization of actinides and other components of high-level waste in magnesium potassium phosphate matrices. J. Nucl. Mater. 2009, 385, 189–192. [Google Scholar] [CrossRef]
- Vinokurov, S.E.; Kulikova, S.A.; Myasoedov, B.F. Magnesium Potassium Phosphate Compound for Immobilization of Radioactive Waste Containing Actinide and Rare Earth Elements. Materials 2018, 11, 976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vinokurov, S.E.; Kulikova, S.A.; Myasoedov, B.F. Hydrolytic and thermal stability of magnesium potassium phosphate compound for immobilization of high level waste. J. Radioanal. Nucl. Chem. 2018, 318, 2401–2405. [Google Scholar] [CrossRef]
- Vinokurov, S.E.; Kulikova, S.A.; Myasoedov, B.F. Solidification of high level waste using magnesium potassium phosphate compound. Nucl. Eng. Technol. 2019, 51, 755–760. [Google Scholar] [CrossRef]
- Dmitrieva, A.V.; Kalenova, M.Y.; Kulikova, S.A.; Kuznetsov, I.V.; Koshcheev, A.M.; Vinokurov, S.E. Magnesium-Potassium Phosphate Matrix for Immobilization of 14C. Russ. J. Appl. Chem. 2018, 91, 641–646. [Google Scholar] [CrossRef]
- Kulikova, S.A.; Belova, K.Y.; Tyupina, E.A.; Vinokurov, S.E. Conditioning of Spent Electrolyte Surrogate LiCl-KCl-CsCl Using Magnesium Potassium Phosphate Compound. Energies 2020, 13, 1963. [Google Scholar] [CrossRef]
- Shkuropatenko, V.A. High level wastes immobilization in ceramic and hydrated phosphate matrix. East Eur. J. Phys. 2016, 3, 49–60. [Google Scholar] [CrossRef]
- Zhenyu, L.; Hongtao, W.; Yang, H.; Tao, Y.; Zhongyuan, L.; Shuzhen, L.; Haibin, Z. Rapid solidification of Highly Loaded High-Level Liquid Wastes with magnesium phosphate cement. Ceram. Int. 2019, 45, 5050–5057. [Google Scholar] [CrossRef]
- Tao, Y.; Zhenyu, L.; Chunrong, R.; Yuanyuan, W.; Zhichao, H.; Xin, H.; Jie, W.; Mengliang, L.; Qiubai, D.; Khan, K.; et al. Study on solidification properties of chemically bonded phosphate ceramics for cesium radionuclides. Ceram. Int. 2020, 46, 14964–14971. [Google Scholar] [CrossRef]
- Graeser, S.; Postl, W.; Bojar, H.-P.B.; Armbruster, T.; Raber, T.; Ettinger, K.; Walter, F.; Berlepsch, P. Struvite-(K), KMgPO46H2O, the potassium equivalent of struvite a new mineral. Eur. J. Mineral. 2008, 20, 629–633. [Google Scholar] [CrossRef]
- Shahwan, T.; Akar, D.; Eroglu, A.E. Physicochemical characterization of the retardation of aqueous Cs+ ions by natural kaolinite and clinoptilolite minerals. J. Colloid Interface Sci. 2005, 285, 9–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belousov, P.; Semenkova, A.; Egorova, T.B.; Romanchuk, A.Y.; Zakusin, S.; Dorzhieva, O.; Tyupina, E.; Izosimova, Y.; Tolpeshta, I.; Chernov, M.; et al. Cesium Sorption and Desorption on Glauconite, Bentonite, Zeolite and Diatomite. Minerals 2019, 9, 625. [Google Scholar] [CrossRef] [Green Version]
- Abusafa, A.; Yücel, H. Removal of 137Cs from aqueous solutions using different cationic forms of a natural zeolite: Clinoptilolite. Sep. Purif. Technol. 2002, 28, 103–116. [Google Scholar] [CrossRef]
- Johan, E.; Yamada, T.; Munthali, M.W.; Kabwadza-Corner, P.; Aono, H.; Matsue, N. Natural Zeolites as Potential Materials for Decontamination of Radioactive Cesium. Procedia Environ. Sci. 2015, 28, 52–56. [Google Scholar] [CrossRef] [Green Version]
- Borai, E.H.; Harjula, R.; Malinen, L.; Paajanen, A. Efficient removal of cesium from low-level radioactive liquid waste using natural and impregnated zeolite minerals. J. Hazard. Mater. 2009, 172, 416–422. [Google Scholar] [CrossRef]
- Aono, H.; Kunimoto, T.; Takahashi, R.; Itagaki, Y.; Johan, E.; Matsue, N. Cs+ decontamination properties of mordenites and composite materials synthesized from coal fly ash and rice husk ash. J. Asian Ceram. Soc. 2018, 6, 213–221. [Google Scholar] [CrossRef]
- Aono, H.; Takeuchi, Y.; Itagaki, Y.; Johan, E. Synthesis of chabazite and merlinoite for Cs+ adsorption and immobilization properties by heat-treatment. Solid State Sci. 2020, 100, 106094. [Google Scholar] [CrossRef]
- Wang, J.; Zhuang, S.; Liu, Y. Metal hexacyanoferrates-based adsorbents for cesium removal. Coord. Chem. Rev. 2018, 374, 430–438. [Google Scholar] [CrossRef]
- Mimura, H.; Lehto, J.; Harjula, R. Ion Exchange of Cesium on Potassium Nickel Hexacyanoferrate (II)s. J. Nucl. Sci. Technol. 1997, 34, 484–489. [Google Scholar] [CrossRef] [Green Version]
- Michel, C.; Barre, Y.; De Dieuleveult, C.; Grandjean, A.; De Windt, L. Cs ion exchange by a potassium nickel hexacyanoferrate loaded on a granular support. Chem. Eng. Sci. 2015, 137, 904–913. [Google Scholar] [CrossRef]
- Tachikawa, H.; Haga, K.; Yamada, K. Mechanism of K+, Cs+ ion exchange in nickel ferrocyanide: A density functional theory study. Comput. Theor. Chem. 2017, 1115, 175–178. [Google Scholar] [CrossRef]
- Martin, I.; Patapy, C.; Boher, C.; Cyr, M. Investigation of caesium retention by potassium nickel hexacyanoferrate (II) in different pH conditions and potential effect on the selection of storage matrix. J. Nucl. Mater. 2019, 526, 151764. [Google Scholar] [CrossRef]
- Avramenko, V.; Bratskaya, S.; Zheleznov, V.; Sheveleva, I.; Voitenko, O.; Sergienko, V. Colloid stable sorbents for cesium removal: Preparation and application of latex particles functionalized with transition metals ferrocyanides. J. Hazard. Mater. 2011, 186, 1343–1350. [Google Scholar] [CrossRef]
- Voronina, A.; Noskova, A.Y.; Semenishchev, V.; Gupta, D. Decontamination of seawater from 137Cs and 90Sr radionuclides using inorganic sorbents. J. Environ. Radioact. 2020, 217, 106210. [Google Scholar] [CrossRef]
- Oh, S.-Y.; Kawai, K.; Kawamura, G.; Muto, H.; Matsuda, A. Characterization of mechanochemically synthesized MHSO4-H4SiW12O40 composites (M = K, NH4, Cs). Mater. Res. Bull. 2012, 47, 2931–2935. [Google Scholar] [CrossRef]
- Pesaresi, L.; Brown, D.; Lee, A.; Montero, J.; Williams, H.; Wilson, K. Cs-doped H4SiW12O40 catalysts for biodiesel applications. Appl. Catal. A Gen. 2009, 360, 50–58. [Google Scholar] [CrossRef]
- Zhu, S.; Gao, X.; Zhu, Y.; Zhu, Y.; Xiang, X.; Hu, C.; Li, Y. Alkaline metals modified Pt-H4SiW12O40ZrO2 catalysts for the selective hydrogenolysis of glycerol to 1,3-propanediol. Appl. Catal. B Environ. 2013, 140, 60–67. [Google Scholar] [CrossRef]
- Sawant, D.P.; Vinu, A.; Mirajkar, S.; Lefebvre, F.; Ariga, K.; Anandan, S.; Mori, T.; Nishimura, C.; Halligudi, S. Silicotungstic acid/zirconia immobilized on SBA-15 for esterifications. J. Mol. Catal. A Chem. 2007, 271, 46–56. [Google Scholar] [CrossRef]
- Haider, M.H.; Dummer, N.F.; Zhang, D.; Miedziak, P.; Davies, T.E.; Taylor, S.H.; Willock, D.J.; Knight, D.W.; Chadwick, D.; Hutchings, G.J. Rubidium-and caesium-doped silicotungstic acid catalysts supported on alumina for the catalytic dehydration of glycerol to acrolein. J. Catal. 2012, 286, 206–213. [Google Scholar] [CrossRef]
- Raveendra, G.; Rajasekhar, A.; Srinivas, M.; Prasad, P.S.S.; Lingaiah, N. Selective etherification of hydroxymethylfurfural to biofuel additives over Cs containing silicotungstic acid catalysts. Appl. Catal. A Gen. 2016, 520, 105–113. [Google Scholar] [CrossRef]
- Sayenko, S.Y.; Shkuropatenko, V.A.; Dikiy, N.P.; Tarasov, R.V.T.; Ulybkina, K.A.; Surkov, O.Y.; Litvinenko, L.M. Clinoptilolite with Cesium Immobilization to Potassium Magnesium Phosphate Matrix. East. Eur. J. Phys. 2017, 4, 37–43. [Google Scholar] [CrossRef] [Green Version]
- Kulikova, S.A.; Vinokurov, S.E. The Influence of Zeolite (Sokyrnytsya Deposit) on the Physical and Chemical Resistance of a Magnesium Potassium Phosphate Compound for the Immobilization of High-Level Waste. Molecules 2019, 24, 3421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vinokurov, S.E.; Kulyako, Y.M.; Slyunchev, O.M.; Rovnyi, S.I.; Wagh, A.S.; Maloney, M.D.; Myasoedov, B.F. Magnesium potassium phosphate matrices for immobilization of high-level liquid wastes. Radiochemistry 2009, 51, 65–72. [Google Scholar] [CrossRef]
- Vinokurov, S.E.; Kulikova, S.A.; Krupskaya, V.V.; Myasoedov, B.F. Magnesium Potassium Phosphate Compound for Radioactive Waste Immobilization: Phase Composition, Structure, and Physicochemical and Hydrolytic Durability. Radiochemistry 2018, 60, 70–78. [Google Scholar] [CrossRef]
- Vinokurov, S.E.; Kulikova, S.A.; Krupskaya, V.V.; Tyupina, E.A. Effect of Characteristics of Magnesium Oxide Powder on Composition and Strength of Magnesium Potassium Phosphate Compound for Solidifying Radioactive Waste. Russ. J. Appl. Chem. 2019, 92, 490–497. [Google Scholar] [CrossRef]
- GOST 310.4-81. Cements. Methods of Bending and Compression Strength Determination; Standardinform: Moscow, Russian, 1981; pp. 1–11. [Google Scholar]
- GOST R 52126-2003. Radioactive Waste. Long Time Leach Testing of Solidified Radioactive Waste Forms; Standardinform: Moscow, Russian, 2003; pp. 1–8. [Google Scholar]
- ANSI/ANS-16.1-1986. Measurement of the Leachability of Solidified Low-Level Radiactive Wastes by a Short-Term Test Procedure; American National Society: La Grande Park, IL, USA, 1986; pp. 1–30. [Google Scholar]
- De Groot, G.; Van Der Sloot, H. Determination of Leaching Characteristics of Waste Materials Leading to Environmental Product Certification. In Stabilization and Solidification of Hazardous, Radioactive, and Mixed Wastes, 2nd ed.; ASTM International: West Conshohocken, PA, USA, 2009; p. 149. [Google Scholar]
- Torras, J.; Buj-Corral, I.; Rovira, M.; De Pablo, J. Semi-dynamic leaching tests of nickel containing wastes stabilized/solidified with magnesium potassium phosphate cements. J. Hazard. Mater. 2011, 186, 1954–1960. [Google Scholar] [CrossRef]
- Mimura, H.; Lehto, J.; Harjula, R. Chemical and Thermal Stability of Potassium Nickel Hexacyanoferrate (II). J. Nucl. Sci. Technol. 1997, 34, 582–587. [Google Scholar] [CrossRef]
- Choi, J.; Um, W.; Choung, S. Development of iron phosphate ceramic waste form to immobilize radioactive waste solution. J. Nucl. Mater. 2014, 452, 16–23. [Google Scholar] [CrossRef]
- Kim, M.; Kim, H.G.; Kim, S.; Yoon, J.-H.; Sung, J.Y.; Jin, J.S.; Lee, M.-H.; Kim, C.-W.; Heo, J.; Hong, K.-S. Leaching behaviors and mechanisms of vitrified forms for the low-level radioactive solid wastes. J. Hazard. Mater. 2020, 384, 121296. [Google Scholar] [CrossRef] [PubMed]



| Compound | Sorbent (wt %) | Filler (wt %) | Solidified S-HLW (wt %) | Binders (wt %) | |
|---|---|---|---|---|---|
| MgO | KH2PO4 | ||||
| MPP-FKN-W | 1.5 * | 23.3 | 34.0 | 10.3 | 30.9 |
| MPP-NZ | 25.1 | - | 32.1 | 10.7 | 32.1 |
| MPP-MOR | 25.1 | - | 32.1 | 10.7 | 32.1 |
| MPP-STA-NZ | 5.0 | 25.0 | 30.0 | 10.0 | 30.0 |
| Sorbent | 137Cs Sorption Degree (%) |
|---|---|
| FKN | 99.5 |
| NZ | 93.0 |
| MOR | 98.5 |
| STA/NZ | 97.0/99.1 |
| Heat Treatment Temperature (°C) | Test Duration (days) | F (%) | D (cm2/s) | L | (L)av |
|---|---|---|---|---|---|
| 180 | 1 | 0.01 | 1.1 × 10−14 | 14.0 | 14.1 |
| 3 | 0.03 | 4.2 × 10−14 | 13.4 | ||
| 7 | 0.04 | 1.3 × 10−14 | 13.9 | ||
| 10 | 0.04 | 1.2 × 10−14 | 13.9 | ||
| 14 | 0.05 | 8.0 × 10−15 | 14.1 | ||
| 21 | 0.05 | 2.1 × 10−15 | 14.7 | ||
| 28 | 0.05 | 2.2 × 10−15 | 14.7 | ||
| 250 | 1 | 0.18 | 1.4 × 10−12 | 11.8 | 12.4 |
| 3 | 0.31 | 1.2 × 10−12 | 11.9 | ||
| 7 | 0.45 | 1.0 × 10−12 | 12.0 | ||
| 21 | 0.68 | 5.8 × 10−13 | 12.2 | ||
| 28 | 0.69 | 1.6 × 10−14 | 13.8 | ||
| 300 | 1 | 1.33 | 7.4 × 10−11 | 10.1 | 10.4 |
| 3 | 2.06 | 4.2 × 10−11 | 10.4 | ||
| 7 | 2.98 | 4.2 × 10−11 | 10.4 | ||
| 21 | 4.27 | 1.9 × 10−11 | 10.7 | ||
| 28 | 5.10 | 5.8 × 10−11 | 10.2 | ||
| 350 | 1 | 2.19 | 2.0 × 10−10 | 9.7 | 10.5 |
| 3 | 2.77 | 2.6 × 10−11 | 10.6 | ||
| 7 | 3.41 | 2.1 × 10−11 | 10.7 | ||
| 21 | 4.39 | 1.1 × 10−11 | 11.0 | ||
| 28 | 5.03 | 3.4 × 10−11 | 10.5 | ||
| 400 | 1 | 2.63 | 2.9 × 10−10 | 9.5 | 10.7 |
| 3 | 3.26 | 3.1 × 10−11 | 10.5 | ||
| 7 | 3.71 | 1.0 × 10−11 | 11.0 | ||
| 21 | 4.30 | 3.8 × 10−12 | 11.4 | ||
| 28 | 4.58 | 6.5 × 10−12 | 11.2 | ||
| 450 | 1 | 2.28 | 2.2 × 10−10 | 9.7 | 10.6 |
| 3 | 2.84 | 2.4 × 10−11 | 10.6 | ||
| 7 | 3.33 | 1.2 × 10−11 | 10.9 | ||
| 21 | 4.17 | 7.9 × 10−12 | 11.1 | ||
| 28 | 4.59 | 1.4 × 10−11 | 10.8 |
| Compound | Heat Treatment Temperature (°C) | Test Duration (Days) | F (%) | D (cm2/s) | L | (L)av |
|---|---|---|---|---|---|---|
| MPP-NZ | 180 | 1 | 3.48 | 5.1 × 10−10 | 9.3 | 10.3 |
| 3 | 5.77 | 4.1 × 10−10 | 9.4 | |||
| 7 | 7.37 | 1.3 × 10−10 | 9.9 | |||
| 10 | 8.13 | 8.9 × 10−11 | 10.1 | |||
| 16 | 8.94 | 3.9 × 10−11 | 10.4 | |||
| 20 | 9.32 | 2.8 × 10−11 | 10.6 | |||
| 31 | 10.06 | 1.9 × 10−11 | 10.7 | |||
| 59 | 10.75 | 4.5 × 10−12 | 11.3 | |||
| 91 | 11.34 | 4.2 × 10−12 | 11.4 | |||
| 450 | 1 | 9.88 | 4.1 × 10−9 | 8.4 | 10.0 | |
| 3 | 11.83 | 3.0 × 10−10 | 9.5 | |||
| 7 | 13.50 | 1.4 × 10−10 | 9.9 | |||
| 10 | 14.27 | 9.2 × 10−11 | 10.0 | |||
| 16 | 15.22 | 5.4 × 10−11 | 10.3 | |||
| 20 | 15.78 | 6.0 × 10−11 | 10.2 | |||
| 31 | 16.82 | 3.7 × 10−11 | 10.4 | |||
| 59 | 18.17 | 1.7 × 10−11 | 10.8 | |||
| 91 | 19.40 | 1.8 × 10−11 | 10.7 | |||
| MPP-STA-NZ | 180 | 1 | 4.23 | 7.5 × 10−10 | 9.1 | 10.7 |
| 3 | 6.22 | 3.1 × 10−10 | 9.5 | |||
| 7 | 7.17 | 4.5 × 10−11 | 10.3 | |||
| 10 | 7.56 | 2.5 × 10−11 | 10.6 | |||
| 15 | 8.02 | 1.8 × 10−11 | 10.8 | |||
| 28 | 8.71 | 9.8 × 10−12 | 11.0 | |||
| 59 | 9.08 | 9.8 × 10−13 | 12.0 | |||
| 91 | 9.35 | 9.0 × 10−13 | 12.0 | |||
| 450 | 1 | 0.78 | 2.5 × 10−11 | 10.6 | 10.6 | |
| 3 | 1.88 | 9.5 × 10−11 | 10.0 | |||
| 7 | 3.13 | 7.7 × 10−11 | 10.1 | |||
| 10 | 3.72 | 5.6 × 10−11 | 10.3 | |||
| 15 | 4.49 | 4.8 × 10−11 | 10.3 | |||
| 28 | 5.49 | 2.1 × 10−11 | 10.7 | |||
| 59 | 6.35 | 5.3 × 10−12 | 11.3 | |||
| 91 | 7.03 | 5.6 × 10−12 | 11.3 | |||
| MPP-MOR | 180 | 1 | 2.83 | 3.3 × 10−10 | 9.5 | 11.0 |
| 3 | 4.06 | 1.2 × 10−10 | 9.9 | |||
| 7 | 4.75 | 2.4 × 10−11 | 10.6 | |||
| 10 | 5.08 | 1.7 × 10−11 | 10.8 | |||
| 16 | 5.37 | 5.0 × 10−12 | 11.3 | |||
| 20 | 5.46 | 1.3 × 10−12 | 11.9 | |||
| 31 | 5.68 | 1.7 × 10−12 | 11.8 | |||
| 59 | 6.18 | 2.3 × 10−12 | 11.6 | |||
| 91 | 6.79 | 4.5 × 10−12 | 11.3 | |||
| 450 | 1 | 6.25 | 1.6 × 10−9 | 8.8 | 10.8 | |
| 3 | 7.34 | 9.3 × 10−11 | 10.0 | |||
| 7 | 7.95 | 1.8 × 10−11 | 10.7 | |||
| 10 | 8.26 | 1.6 × 10−11 | 10.8 | |||
| 16 | 8.54 | 4.5 × 10−12 | 11.3 | |||
| 20 | 8.75 | 8.3 × 10−12 | 11.1 | |||
| 31 | 9.08 | 3.8 × 10−12 | 11.4 | |||
| 59 | 9.58 | 2.3 × 10−12 | 11.6 | |||
| 91 | 10.00 | 2.1 × 10−12 | 11.7 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kulikova, S.A.; Danilov, S.S.; Belova, K.Y.; Rodionova, A.A.; Vinokurov, S.E. Optimization of the Solidification Method of High-Level Waste for Increasing the Thermal Stability of the Magnesium Potassium Phosphate Compound. Energies 2020, 13, 3789. https://doi.org/10.3390/en13153789
Kulikova SA, Danilov SS, Belova KY, Rodionova AA, Vinokurov SE. Optimization of the Solidification Method of High-Level Waste for Increasing the Thermal Stability of the Magnesium Potassium Phosphate Compound. Energies. 2020; 13(15):3789. https://doi.org/10.3390/en13153789
Chicago/Turabian StyleKulikova, Svetlana A., Sergey S. Danilov, Kseniya Yu. Belova, Anastasiya A. Rodionova, and Sergey E. Vinokurov. 2020. "Optimization of the Solidification Method of High-Level Waste for Increasing the Thermal Stability of the Magnesium Potassium Phosphate Compound" Energies 13, no. 15: 3789. https://doi.org/10.3390/en13153789







