Thermal Performance Combined with Cooling System Parameters Study for a Roller Kiln Based on Energy-Exergy Analysis
Abstract
1. Introduction
- To analyze and quantify the energy and exergy of each stream based on energy-exergy analysis model, and identify key factors affecting the thermal efficiency of the process.
- To analyze and quantify the main sources of energy and exergy loss, and identify the evitable and inevitable exergy destruction and the evitable exergy loss for the roller kiln.
- To carry out the parametric studies of the cooling system for investigating the influence on thermal performance, fuel-saving, cost-saving and environmental impact for the roller kiln.
2. Description of the Roller Kiln
3. Theoretical Analysis
3.1. Mass and energy balances
3.2. Exergy Balance
3.3. Cost and Environmental Analysis
3.4. Uncertainty Analysis
4. Results and Discussion
4.1. Mass Balance
4.2. Energy Analysis
4.2.1. Energy Balance
4.2.2. Reaction Enthalpy
4.2.3. Kiln Wall Heat Loss
4.3. Exergy Analysis
4.3.1. Exergy Balance
4.3.2. Exergy Destruction
Exergy Destruction of Fuel Combustion
Exergy Destruction of Calcination Reaction
Exergy Destruction of the Heat and Mass Transfer
5. Parametric Study of the Cooling System
5.1. Thermodynamic and Fuel-Saving Analysis
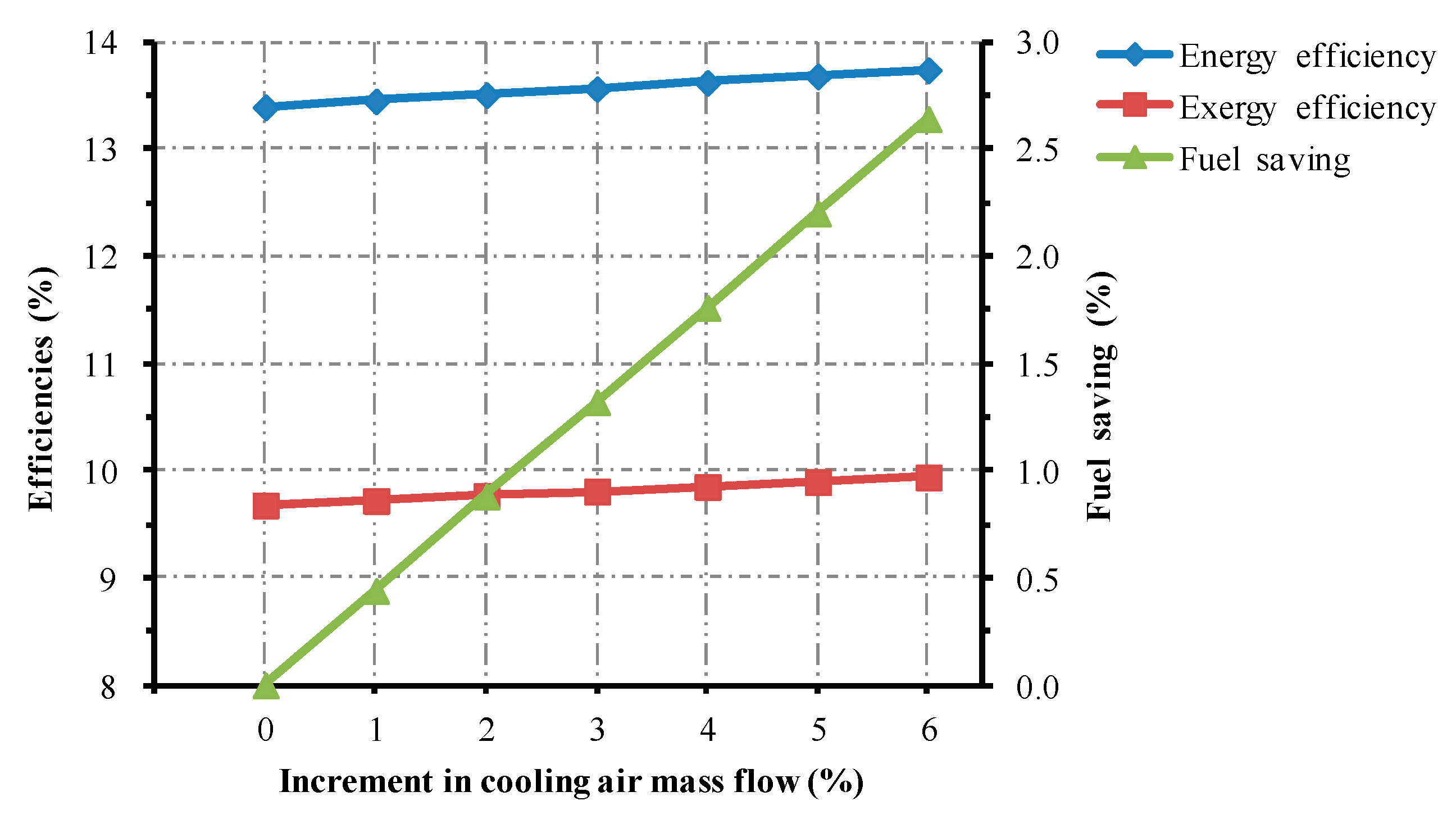
5.1.1. Change in Cooling Air Mass Flow
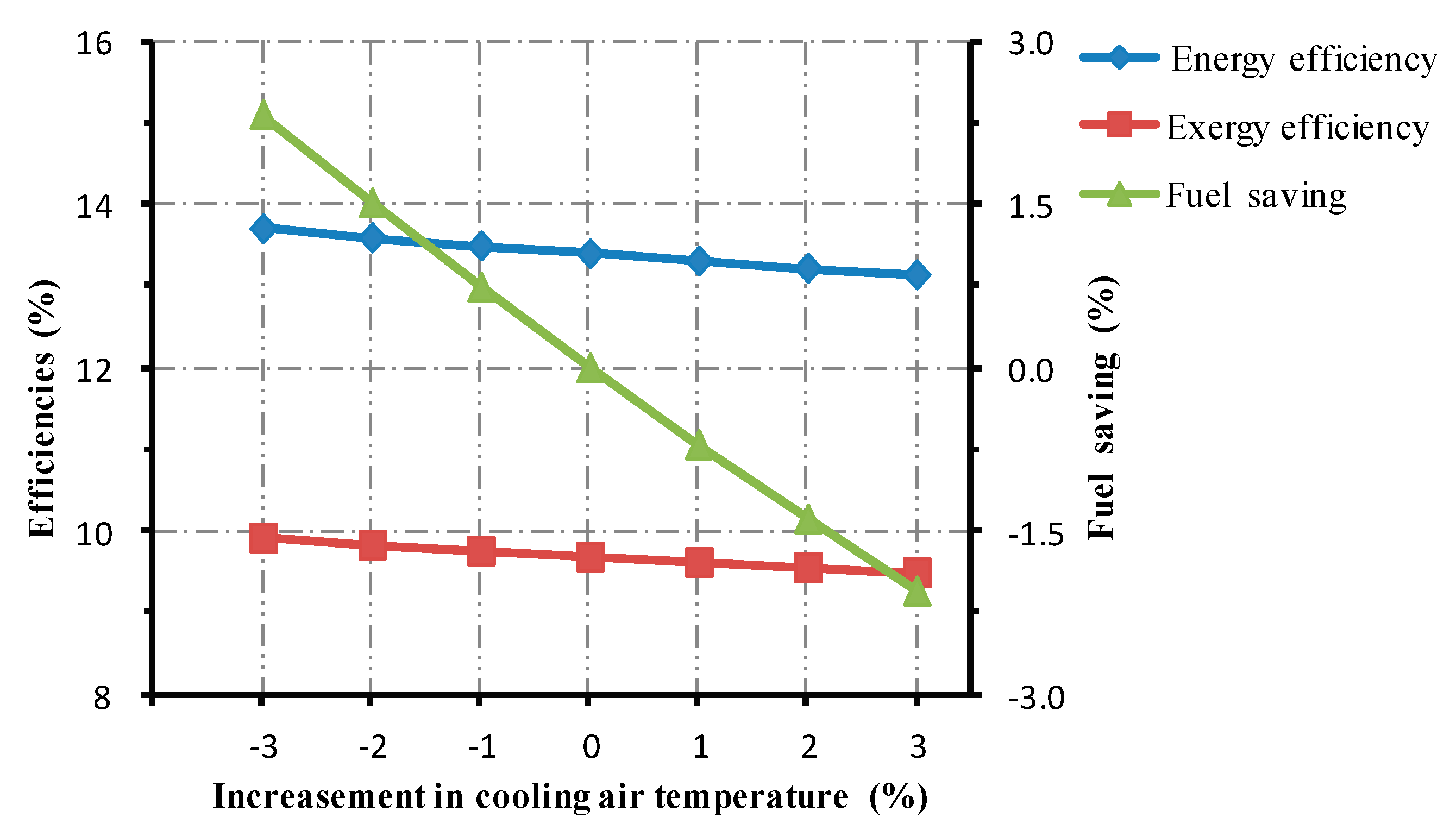
5.1.2. Change in Cooling Air Temperature
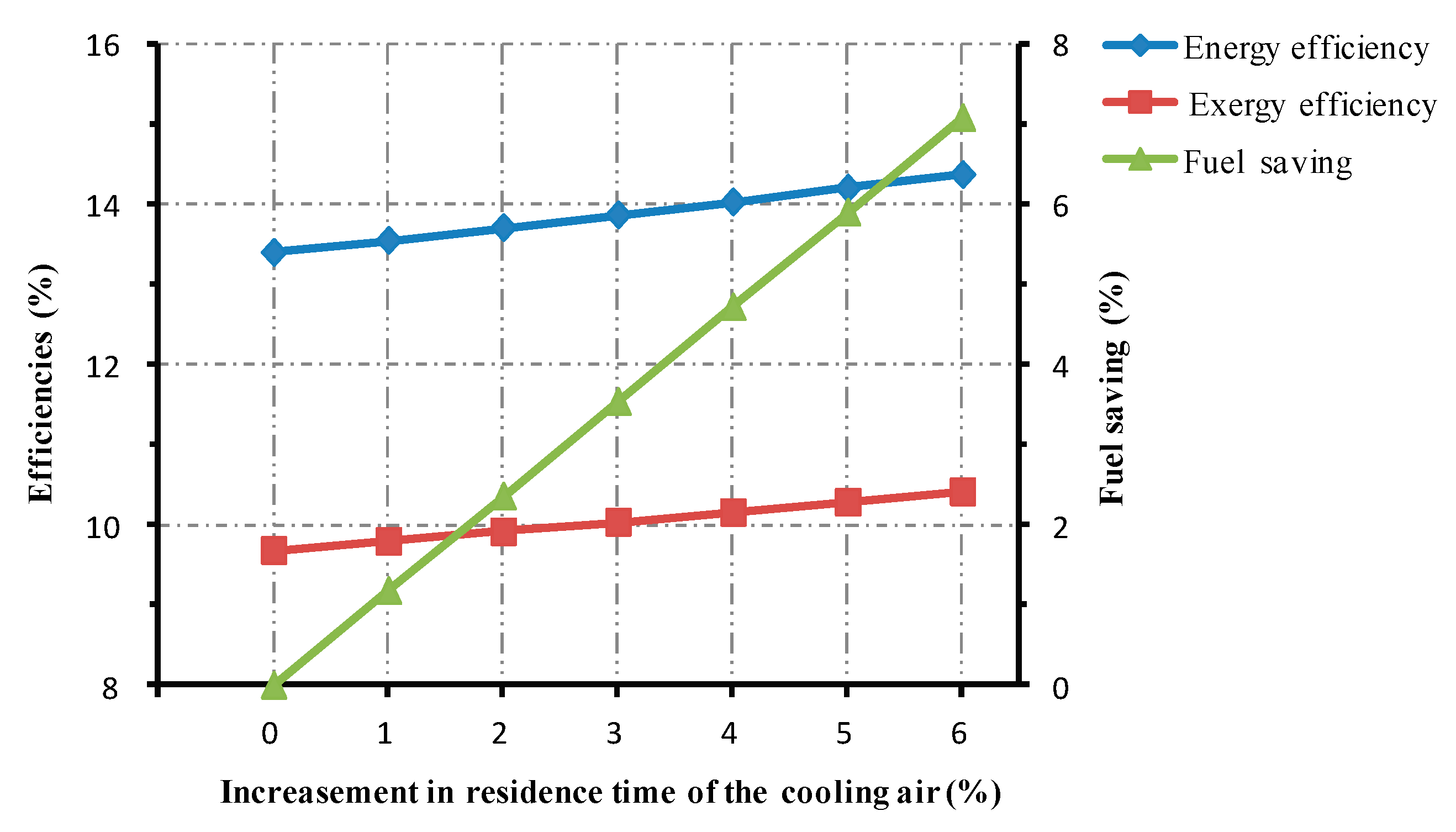
5.1.3. The Residence Time of the Cooling Air
5.2. Cost and Environmental Analysis
6. Conclusions
- The targeted physical-chemical reactions only account for 13.4% of the total energy input, while the exergy value is 9.7%. These results indicate that the system has a very poor thermal performance.
- The main sources of exergy destruction generated from the heat and mass transfer and fuel combustion are about 14,615,289 kJ/h, accounting for 75.4% of the exergy input—50.8% of the exergy destruction is due to the heat and mass transfer, while 37.9% of the exergy destruction is caused by fuel combustion. The results also show that the heating system is the subsystem with greater exergy destruction.
- Changing operational parameters in the cooling system could affect the thermal performance of the roller kiln. For every 1% increase in cooling air mass flow, the energy and the exergy efficiencies of the kiln increase roughly by 0.06% and 0.04%. Increasing the temperature of the cooling gas by 1%, the energy efficiency experiences an approximate drop of 0.09%, while the associated exergy efficiency drops by 0.07%. Prolonging the residence time of the cooling gas by 1%, the studied kiln experiences approximately 0.16% and 0.12% rises in energy and exergy efficiencies, respectively.
- The cooling air residence time has the main impact on the cost-saving and carbon dioxide emission reduction, followed by cooling air mass and cooling air temperature.
Author Contributions
Funding
Conflicts of Interest
Nomenclatures
| Nomenclature | |
| A | area (m2) |
| CP | specific heat (kJ/(kg·K)) |
| CS | cost-saving (RMB/h) |
| ex | specific exergy (kJ/kg) |
| En | energy (kJ) |
| n | energy rate (kJ/h) |
| Ex | exergy (kJ) |
| x | exergy rate (kJ/h) |
| EC | energy cost (RMB/MJ) |
| EER | environmental emission reduction (kg/h) |
| ES | energy-saving (MJ/h) |
| FE | emission per kg of fuel |
| FC | fuel-saving (kg/h) |
| H | enthalpy (kJ) |
| LHV | low heating value (kJ/kg) |
| m | mass (kg) |
| mass rate (kg/h) | |
| P | pressure (Pa) |
| q | reaction heat (kJ/kg) |
| Q | heat (kJ) |
| heat rate (kJ/h) | |
| r | latent heat of vaporization (kJ/kg) |
| R | thermal resistance (K/W) or the universal gas constant (kJ/kmol·K) |
| S | entropy (kJ) |
| T | temperature (K) |
| Greek Letters | |
| convective radiation heat transfer coefficient (W/m2·K) | |
| thickness (m) | |
| conductivity(W/m·K) | |
| emissivity | |
| efficiency (%) | |
| the relation between the chemical exergy and the LHV | |
| Subscripts | |
| ca | cooling air |
| cg | cooling gas |
| ch | chemical |
| com | combustion |
| coma | combustion air |
| comg | combustion gas |
| cr | chemical reaction |
| d | drying |
| D | destruction |
| en | energy |
| ex | exhausted |
| f | flue |
| fg | flue gas |
| ft | fired tile |
| gen | generation |
| gm | gas mixture |
| hmt | heat and mass transfer |
| in | input |
| kw | kiln wall |
| la | leakage air |
| lh | latent heat |
| out | output |
| p | product |
| ph | physical |
| pt | phase-transition |
| r | reactant |
| reac | reaction |
| rec | recovered cooling gas |
| T | total |
| uft | unfired tile |
| wa | water |
| wo | wall outer |
| 0 | ambient |
References
- Montfort, E.; Mezquita, A.; Granel, R.; Vaquer, E.; Escrig, A.; Miralles, A.; Zaera, V. Analysis of energy consumption and carbon dioxide emissions in ceramic tile manufacture. Bol. Soc. Esp. Ceram. Vidr. 2010, 49, 303–310. [Google Scholar]
- Milani, M.; Montorsi, L.; Stefani, M.; Saponelli, R.; Lizzano, M. Numerical analysis of an entire ceramic kiln under actual operating conditions for the energy efficiency improvement. J. Environ. Manag. 2017, 203, 1026–1037. [Google Scholar] [CrossRef]
- Delpech, B.; Milani, M.; Montorsi, L.; Boscardin, D.; Chauhan, A.; Almahmoud, S.; Axcell, B.; Jouhara, H. Energy efficiency enhancement and waste heat recovery in industrial processes by means of the heat pipe technology: Case of the ceramic industry. Energy 2018, 158, 656–665. [Google Scholar] [CrossRef]
- Caglayan, H.; Caliskan, H. Investigation of the energy recovery in the burners of the ceramic factory kiln. Energy Procedia 2018, 144, 118–124. [Google Scholar] [CrossRef]
- Mezquita, A.; Boix, A.; Monfort, E.; Mallol, G. Energy saving in ceramic tile kilns: Cooling gas heat recovery. Appl. Therm. Eng. 2014, 65, 102–110. [Google Scholar] [CrossRef]
- BoroumandJazi, G.; Rismanchi, B.; Saidur, R. A review on exergy analysis of industrial sector. Renew. Sustain. Energy Rev. 2013, 27, 198–203. [Google Scholar] [CrossRef]
- Koroneos, C.; Roumbas, G.; Moussiopoulos, N. Exergy analysis of cement production. Int. J. Exergy 2005, 2, 55–68. [Google Scholar] [CrossRef]
- Utlu, Z.; Sogut, Z.; Hepbasli, A.; Oktay, Z. Energy and exergy analyses of a raw mill in cement production. Appl. Therm. Eng. 2006, 26, 2479–2489. [Google Scholar] [CrossRef]
- Amiri Rad, E.; Mohammadi, S. Energetic and exergetic optimized Rankine cycle for waste heat recovery in a cement factory. Appl. Therm. Eng. 2018, 132, 410–422. [Google Scholar] [CrossRef]
- Söğüt, M.Z. A research on exergy consumption and potential of total CO2 emission in the Turkish cement sector. Energy Convers. Manag. 2012, 56, 37–45. [Google Scholar] [CrossRef]
- Karellas, S.; Leontaritis, A.D.; Panousis, G.; Bellos, E.; Kakaras, E. Energetic and exergetic analysis of waste heat recovery systems in the cement industry. Energy 2013, 58, 147–156. [Google Scholar] [CrossRef]
- Renó, M.L.G.; Torres, F.M.; da Silva, R.J.; Santos, J.J.C.S.; Melo, M.L.N.M. Exergy analyses in cement production applying waste fuel and mineralizer. Energy Convers. Manag. 2013, 75, 98–104. [Google Scholar] [CrossRef]
- Ustaoglu, A.; Alptekin, M.; Akay, M.E. Thermal and exergetic approach to wet type rotary kiln process and evaluation of waste heat powered ORC (Organic Rankine Cycle). Appl. Therm. Eng. 2017, 112, 281–295. [Google Scholar] [CrossRef]
- Camdali, U.; Tunc, M.; Dikec, F. A thermodynamic analysis of a steel production step carried out in the ladle furnace. Appl. Therm. Eng. 2001, 21, 643–655. [Google Scholar] [CrossRef]
- Mert, M.S.; Dilmaç, O.F.; Özkan, S.; Karaca, F.; Bolat, E. Exergoeconomic analysis of a cogeneration plant in an iron and steel factory. Energy 2012, 46, 78–84. [Google Scholar] [CrossRef]
- Wu, J.; Wang, R.; Pu, G.; Qi, H. Integrated assessment of exergy, energy and carbon dioxide emissions in an iron and steel industrial network. Appl. Energy 2016, 183, 430–444. [Google Scholar] [CrossRef]
- Rong, W.; Li, B.; Liu, P.; Qi, F. Exergy assessment of a rotary kiln-electric furnace smelting of ferronickel alloy. Energy 2017, 138, 942–953. [Google Scholar] [CrossRef]
- Rong, W.; Li, B.; Qi, F.; Cheung, S.C.P. Energy and exergy analysis of an annular shaft kiln with opposite burners. Appl. Therm. Eng. 2017, 119, 629–638. [Google Scholar] [CrossRef]
- Gutiérrez, A.S.; Martínez, J.B.C.; Vandecasteele, C. Energy and exergy assessments of a lime shaft kiln. Appl. Therm. Eng. 2013, 51, 273–280. [Google Scholar] [CrossRef]
- Gürtürk, M.; Oztop, H.F. Energy and exergy analysis of a rotary kiln used for plaster production. Appl. Therm. Eng. 2014, 67, 554–565. [Google Scholar] [CrossRef]
- Gürtürk, M.; Oztop, H.F. Exergoeconomic analysis of a rotary kiln used for plaster production as building materials. Appl. Therm. Eng. 2016, 104, 486–496. [Google Scholar] [CrossRef]
- Karamarkovic, V.; Marasevic, M.; Karamarkovic, R.; Karamarkovic, M. Recuperator for waste heat recovery from rotary kilns. Appl. Therm. Eng. 2013, 54, 470–480. [Google Scholar] [CrossRef]
- Utlu, Z.; Hepbasl, A. Exergoeconomic analysis of energy utilization of drying process in a ceramic production. Appl. Therm. Eng. 2014, 70, 748–762. [Google Scholar] [CrossRef]
- Kandilli, C.; Ayna, O.M.; Sahin, M. Evaluation of the performance of a hydrogen enriched combustion system for ceramic sector. Int. J. Hydrog. Energy 2015, 40, 11195–11206. [Google Scholar] [CrossRef]
- Caglayan, H.; Caliskan, H. Energy, exergy and sustainability assessments of a cogeneration system for ceramic industry. Appl. Therm. Eng. 2018, 136, 504–515. [Google Scholar] [CrossRef]
- Ferrer, S.; Mezquita, A.; Aguilella, V.M.; Monfort, E. Beyond the energy balance: Exergy analysis of an industrial roller kiln firing porcelain tile. Appl. Therm. Eng. 2019, 150, 1002–1015. [Google Scholar] [CrossRef]
- Çengel, Y.A.; Boles, M.A. Thermodynamics: An Engineering Approach, 5th ed.; McGraw-Hill: New York, NY, USA, 2006. [Google Scholar]
- Atmaca, A.; Yumrutas, R. Analysis of the parameters affecting energy consumption of a rotary kiln in cement industry. Appl. Therm. Eng. 2014, 66, 435–444. [Google Scholar] [CrossRef]
- Hu, G.L. Build Ceramic Industrial Roller Kiln; Light Industry Press: Beijing, China, 1998. (In Chinese) [Google Scholar]
- Kotas, T.J. The Exergy Method of Thermal Plant Analysis; Anchor Brendon Ltd.: London, UK, 1985. [Google Scholar]
- Kotas, T.J. The Exergy Method of Thermal Plant Analysis; Krieger Publishing: Malabar, FL, USA, 1995. [Google Scholar]
- Wajs, J.; Golabek, A.; Bochniak, R.; Mikielewicz, D. Air-cooled photovoltaic roof tile as an example of the BIPVT system An experimental study on the energy and exergy performance. Energy 2020, 197, 117–255. [Google Scholar] [CrossRef]
- Shoeibi, S.; Rahbar, N.; Esfahlani, A.A.; Kargarsharifabad, H. Application of simultaneous thermoelectric cooling and heating to improve the performance of a solar still: An experimental study and exergy analysis. Appl. Energy 2020, 263, 114581. [Google Scholar] [CrossRef]
- Yu, L.D.; Chen, Q.B. Thermal Balance Calculation of Ceramic Equipment; Light Industry Press: Beijing, China, 1990. (in Chinese) [Google Scholar]
- Barin, I. Thermalchemical Data of Pure Substances; Wiley-VCH: Weinheim, Germany, 1997. [Google Scholar]
- Lin, C.X.; Bai, Z.H.; Zhang, Z.R. Thermodynamic Data Manual for Minerals and the Related Compounds; Science Press: Beijing, China, 1985. (in Chinese) [Google Scholar]
- Luis, P.; Van der Bruggen, B. Exergy analysis of energy-intensive production processes: Advancing towards a sustainable chemical industry. J. Chem. Technol. Biotechnol. 2014, 89, 1288–1303. [Google Scholar] [CrossRef]
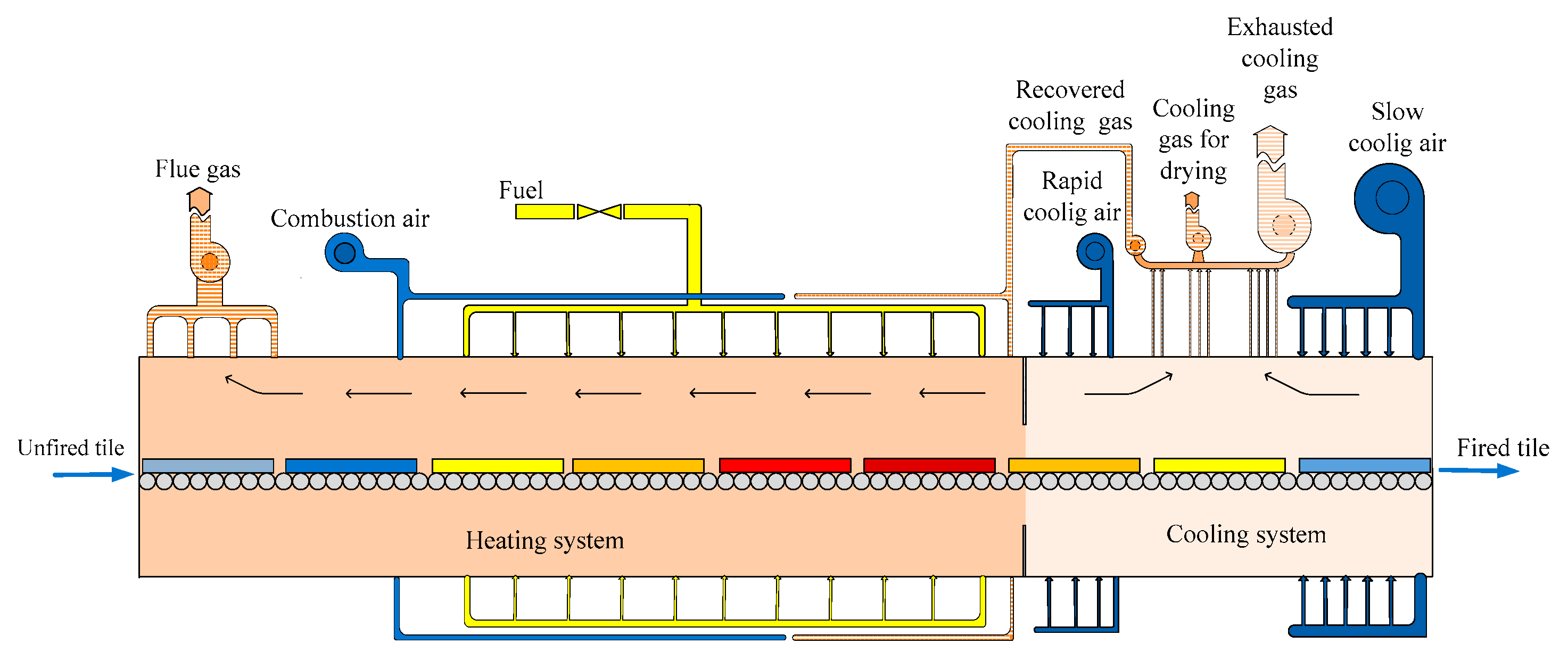



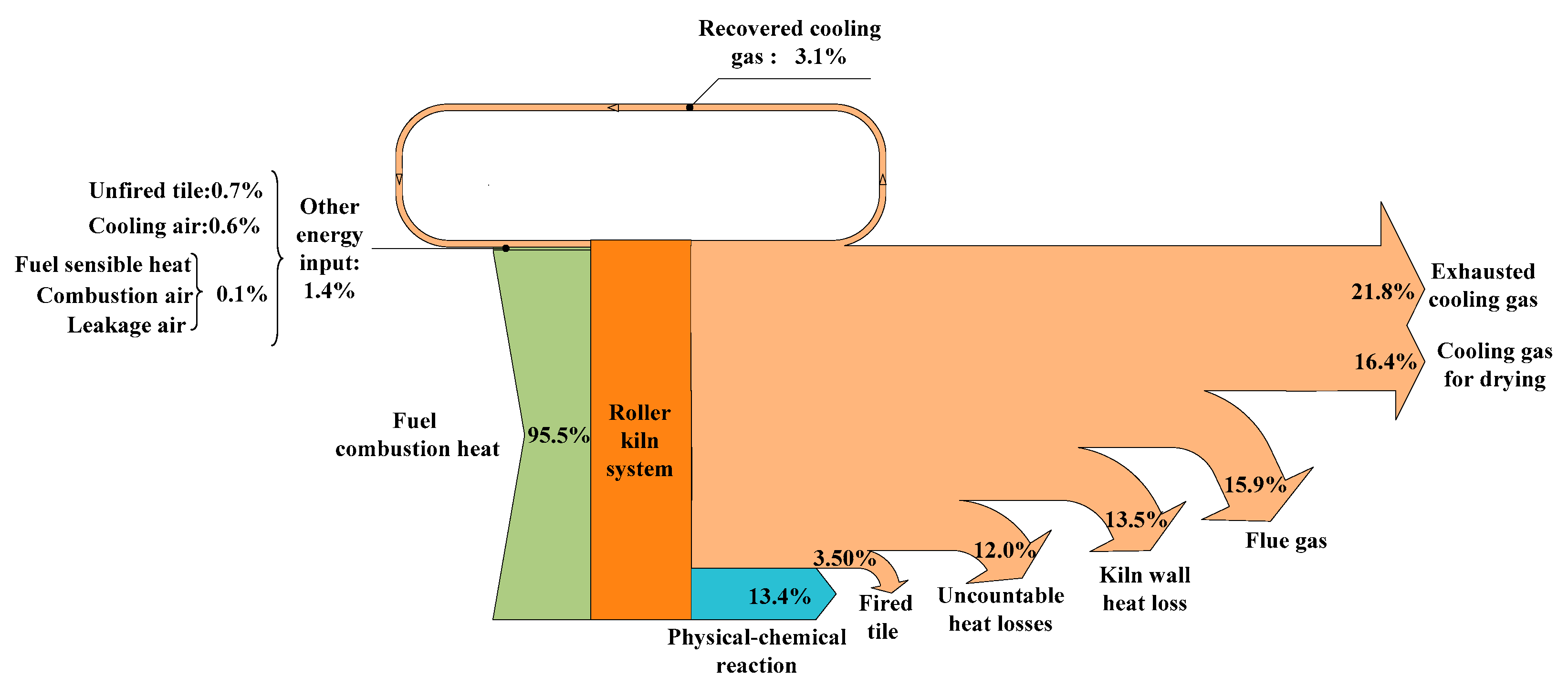
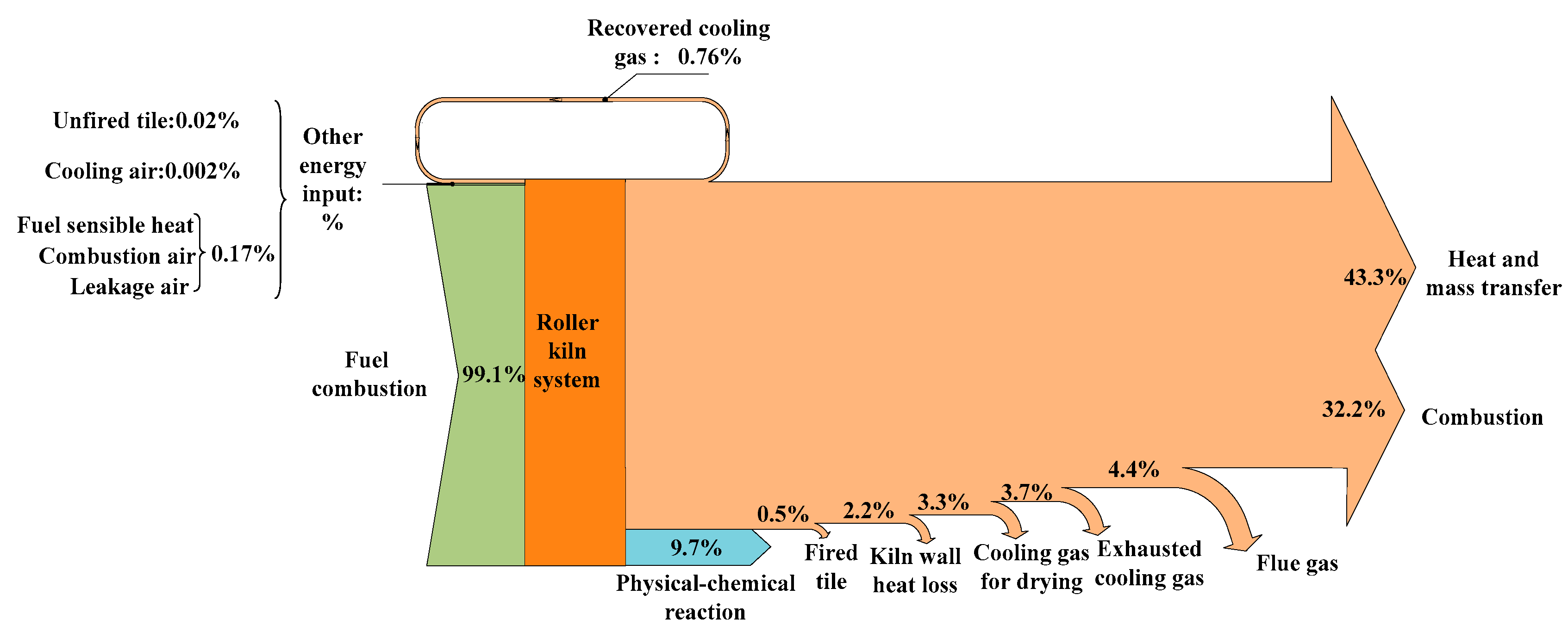
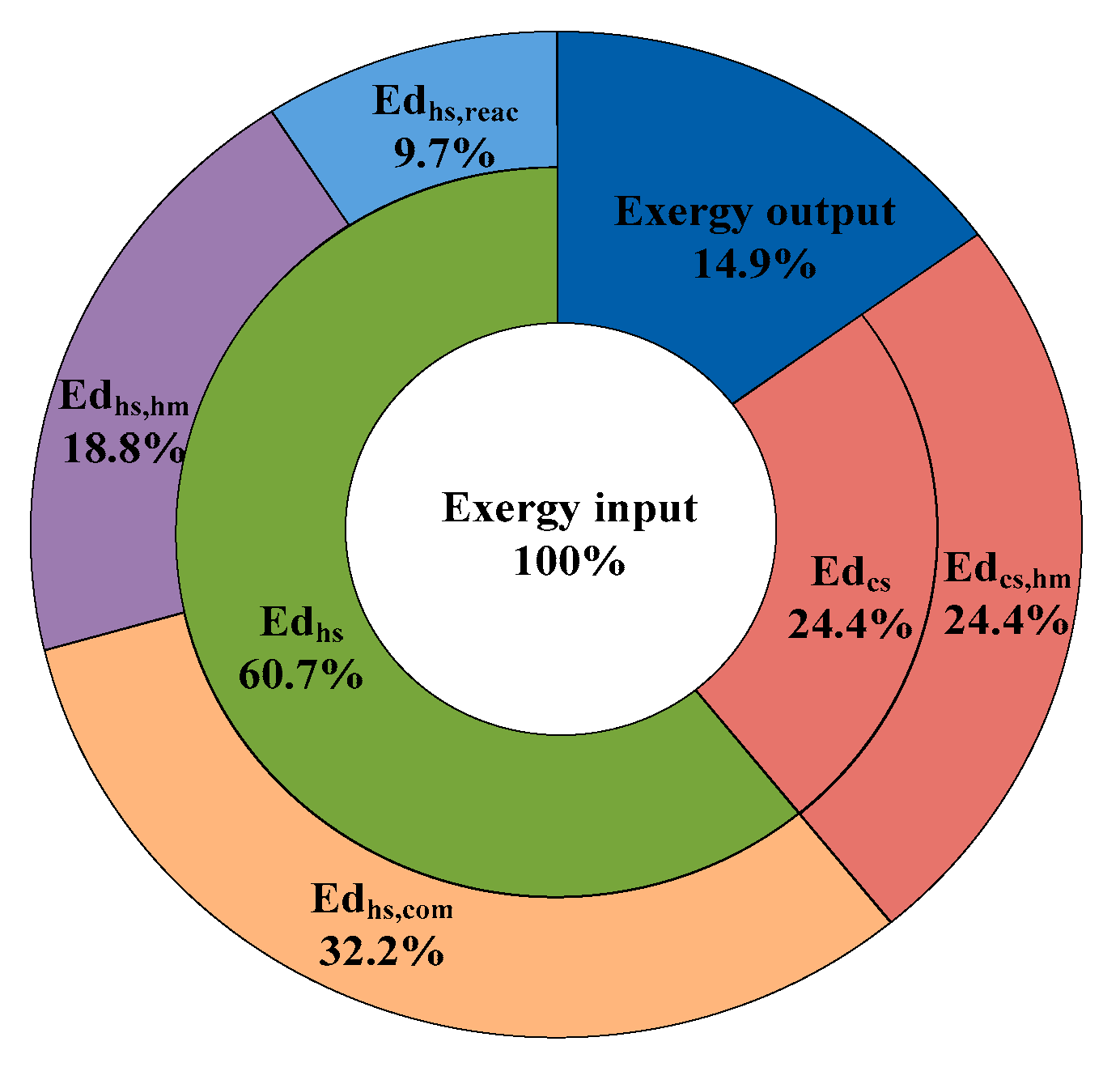
| Term | Equation | Explanation |
|---|---|---|
| General calculation equation | (1) | where is the specific heat, and is the temperature. |
| Combustion heat of the fuel | (2) | where is low heating value of the fuel. |
| Latent heat | (3) | where is latent heat of vaporization. |
| Chemical reaction | (4) | where is the reaction heat. |
| Kiln wall heat loss | (pls. see Equations (3) and (4) and equations in Table 2 |
| Equation | Explaination |
|---|---|
| (1) | where is convection coefficient. |
| (2) | where and are the thickness and conductivity coefficient of material layer of , respectively. |
| (3) | where is position coefficient, is the emissivity of the surface, and is Stefane-Boltzman constant as 5.67 ×10–8 W/m2 K4. |
| Term | Equation | Explanation |
|---|---|---|
| General calculation equation of physical exergy | (1) | where is the enthalpy and is the entropy, the subscript stands for the ambient environment. |
| Exergy of chemical reaction | (2) | where and are the Gibbs sum of products and reactants. |
| Combustion exergy of the fuel | (3) | where is the relation between the chemical exergy and . |
| Chemical exergy of a gas mixture | (4) | where is the Molar fraction, is the specific exergy and is the universal gas constant. |
| Latent heat of exergy | (5) | where is the phase-transition temperature. |
| Kiln wall exergy loss | (6) | where is the amount of exchanged heat through the kiln wall, is the average temperature in the kiln. |
| Exergy destruction | (7) | where represents for the entropy generation. |
| Instrument | Accuracy |
|---|---|
| Weighing machine, (kg) | ±2% |
| Gas velocity measuring device, (m/s) | ±2% |
| Thermocouple, (°C) | ±1.5% |
| Pressure Transducer, (kPa) | ±3.0 |
| Input Matter | Amount (kg/h) | Output Matter | Amount (kg/h) |
|---|---|---|---|
| Unfired tile | 9074 | Fired tile | 8711 |
| Fuel (water-gas) | 3591 | Flue gas | 11,492 |
| Combustion air | 4044 | Exhausted cooling gas | 30,099 |
| Recovered cooling gas | 3008 | Recovered cooling gas | 3008 |
| Cooling air | 51,151 | Cooling gas for drying | 18,043 |
| Leakage air | 486 | -- | -- |
| Total | 71,354 | Total | 71,354 |
| Material | a | b | c | d | Temperature Range (K) |
|---|---|---|---|---|---|
| CO | 28.16 | 0.1675 × 10–2 | 0.5372 × 10–5 | −2.222 × 10–9 | 273–1500 |
| H2 | 29.11 | −0.1916 × 10–2 | 0.4003 × 10–5 | −0.8704 × 10–9 | 273–1500 |
| CH4 | 19.89 | 5.024 × 10–2 | 1.269 × 10–5 | −11.01 × 10–9 | 273–1500 |
| O2 | 25.48 | 1.520 × 10–2 | −0.71559 × 10–5 | 1.312 × 10–9 | 273–1500 |
| N2 | 28.9 | −0.1571 × 10–2 | 0.8081 × 10–5 | −2.873 × 10–9 | 273–1500 |
| CO2 | 22.26 | 5.981 × 10–2 | −3.501 × 10–5 | 7.469 × 10–9 | 273–1500 |
| H2O | 32.24 | 0.1923 × 10–2 | 1.005 × 10–5 | −3.595 × 10–9 | 273–1500 |
| NO | 29.34 | −0.09395 × 10–2 | 0.9747 × 10–5 | −4.187 × 10–9 | 273–1500 |
| NO2 | 22.9 | 5.715 × 10–2 | −3.52 × 10–5 | 7.87 × 10–9 | 273–1500 |
| Mineral Formula | Chemical Composition | ||
|---|---|---|---|
| Term | Mass Percentage | Term | Mass Percentage |
| Kaolin | 21% | SiO2 | 69.30% |
| Illite | 15% | Al2O3 | 19.80% |
| Quartz | 26% | Fe2O3 | 0.28% |
| Potassium feldspar | 7% | CaO | 0.36% |
| Albite | 31% | MgO | 0.16% |
| Total | 100% | K2O | 1.12% |
| Na2O | 5.05% | ||
| IL | 3.93% | ||
| Total | 100% | ||
| T(K) | N(mol) | ‘S(kJ/mol·K) | △HF(kJ/mol) | T(K) | N(mol) | S(kJ/mol·K) | △HF(kJ/mol) | ||
|---|---|---|---|---|---|---|---|---|---|
| R-1 | R-4 | ||||||||
| Reactants | Reactants | ||||||||
| Al2O3·2SiO2·2H2O(s) | 823 | 1 | 0.511 | −4110.446 | Al2O3·2SiO2(s) | 1373 | 1 | 0.504 | −3466.882 |
| Products | Products | ||||||||
| Al2O3·2SiO2(s) | 823 | 1 | 0.374 | −3333.506 | 3Al2O3·2SiO2(s) | 1373 | 0.3 | 0.98 | −6848.779 |
| H2O(g) | 823 | 2 | 0.224 | −246.444 | SiO2(s) | 1373 | 1.3 | 0.142 | −899.91 |
| △S(kJ/mol·K) | 0.311 | △S(kJ/mol·K) | 0.012 | ||||||
| Qr(kJ/mol) | 284.052 | Qr(kJ/mol) | −15.924 | ||||||
| R-2 | R-5 | ||||||||
| Reactants | Reactants | ||||||||
| KAl2(AlSi3O10)(OH)2(s) | 873 | 1 | 0.778 | −5958.991 | K(AlSi3O8)(s) | 1393 | 1 | 0.669 | −4015.322 |
| Products | Products | ||||||||
| KAlSi3O8(s) | 873 | 1 | 0.507 | −3980.799 | K(AlSi3O8)(l) | 1393 | 1 | 0.697 | −3976.728 |
| Al2O3(s) | 873 | 1 | 0.167 | −1672.958 | △S(kJ/mol·K) | 0.028 | |||
| H2O(g) | 873 | 1 | 0.229 | −247.186 | Qr(kJ/mol) | 38.594 | |||
| △S(kJ/mol·K) | 0.125 | R-6 | |||||||
| Qr(kJ/mol) | 58.048 | Reactants | |||||||
| R-3 | Na(AlSi3O8)(s) | 1413 | 1 | 0.637 | −4019.502 | ||||
| α-SiO2(s) | 846 | 1 | 0.104 | −907.056 | Products | ||||
| Products | Na(AlSi3O8)(l) | 1413 | 1 | 0.666 | −3988.88 | ||||
| β-SiO2(s) | 846 | 1 | 0.105 | −906.328 | △S(kJ/mol·K) | 0.029 | |||
| △S(kJ/mol·K) | 0.001 | Qr(kJ/mol) | 30.622 | ||||||
| Qr(kJ/mol) | 0.728 |
| Term | T0 (K) | T (K) | CP (kJ/(kg•K)) | m (kg/h) | Amount (kJ/h) | |
|---|---|---|---|---|---|---|
| Input flow | Unfired tile | 298 | 315 | 0.851 | 9074 | 131,261.217 |
| Fuel (water-gas) sensible heat | 298 | 300 | 1.446 | 3591 | 103,86.102 | |
| Fuel (water-gas) combustion heat | -- | -- | -- | -- | 19,233,755.100 | |
| Combustion air | 298 | 300 | 1.143 | 4044 | 9242.054 | |
| Recovered cooling gas | 298 | 471 | 1.214 | 3008 | 631,799.484 | |
| Cooling air | 298 | 300 | 1.143 | 51,151 | 116,898.402 | |
| Leakage air | 298 | 300 | 1.143 | 486 | 1110.692 | |
| Total | -- | -- | -- | 71,354 | 20,134,453.051 | |
| Output flow | Fired tile | 298 | 392 | 0.871 | 8711 | 713,155.284 |
| Flue gas | 298 | 540 | 1.151 | 11,492 | 3,200,698.681 | |
| Exhausted cooling gas | 298 | 420 | 1.193 | 30,099 | 4,380,848.522 | |
| Cooling gas for drying | 298 | 450 | 1.206 | 18,043 | 3,307,497.612 | |
| Recovered cooling gas | 298 | 491 | 1.222 | 3008 | 709,484.631 | |
| Latent heat of the water | -- | -- | -- | -- | 74,063.875 | |
| Reaction enthalpy | -- | -- | -- | -- | 2,622,881.905 | |
| Kiln wall heat loss | -- | -- | -- | 2,718,276.253 | ||
| Uncountable energy losses | -- | -- | -- | -- | 2,407,546,288 | |
| Total | -- | -- | -- | 71,354 | 20,134,453.051 |
| T (K) | M (kg/mol) | Ni (kmol) | s (kJ/kmol•K) | xi | Rln(xi·P) | Ni·si (kJ/kg•K) | Total Entropy (kJ/kg•K) | |
|---|---|---|---|---|---|---|---|---|
| Fuel (water-gas) | 28,803.190 | |||||||
| CO | 300 | 0.028 | 47.188 | 197.841 | 0.32 | −9.473 | 9782.837 | |
| H2 | 300 | 0.002 | 22.334 | 130.658 | 0.15 | −15.773 | 3270.321 | |
| CH4 | 300 | 0.016 | 2.955 | 186.434 | 0.02 | −32.525 | 647.047 | |
| O2 | 300 | 0.032 | 0.295 | 205.329 | 0.002 | −51.668 | 75.771 | |
| N2 | 300 | 0.028 | 70.488 | 191.789 | 0.478 | −6.137 | 13,951.351 | |
| CO2 | 300 | 0.044 | 4.425 | 214 | 0.03 | −29.154 | 1075.863 | |
| Combustion air | 28,175.358 | |||||||
| N2 | 300 | 0.028 | 109.417 | 191.789 | 0.7748 | −2.121 | 21,217.067 | |
| O2 | 300 | 0.032 | 29.068 | 205.329 | 0.2059 | −13.139 | 6350.326 | |
| CO2 | 300 | 0.044 | 0.042 | 214 | 0.0003 | −67.441 | 11.925 | |
| H2O(g) | 300 | 0.018 | 2.683 | 189.167 | 0.019 | −32.951 | 596.039 | |
| Recovered cooling gas | 22,543.714 | |||||||
| N2 | 471 | 0.03 | 81.393 | 206.74 | 0.77 | −2.121 | 16,999.795 | |
| O2 | 471 | 0.03 | 21.623 | 220.69 | 0.21 | −13.139 | 5056.098 | |
| CO2 | 471 | 0.04 | 0.032 | 234.86 | 0.00 | −67.441 | 9.529 | |
| H2O(g) | 471 | 0.02 | 1.996 | 206.66 | 0.02 | −32.951 | 478.293 | |
| Combustion gas | 100,918.179 | |||||||
| CO2 | 1413 | 0.04 | 30.88 | 292.18 | 0.08 | −21.220 | 9678.425 | |
| CO | 1413 | 0.03 | 0.0001 | 246.05 | 0.00 | −131.449 | 0.020 | |
| NO | 1413 | 0.03 | 0.0001 | 262.707 | 0.000000547 | −119.878 | 0.049 | |
| N O2 | 1413 | 0.046 | 0.0001 | 315.82 | 0.000000145 | −130.917 | 0.025 | |
| N2 | 1413 | 0.028 | 305.898 | 241.88 | 0.7715 | −2.157 | 74,650.284 | |
| O2 | 1413 | 0.032 | 34.836 | 258.067 | 0.0879 | −20.216 | 9694.323 | |
| H2O | 1413 | 0.018 | 25.197 | 250.742 | 0.0636 | −22.906 | 6895.053 | |
| Over total entropy | 21,395.918 |
| Term | To (K) | T (K) | CP (kJ/(kg•K)) | m (kg/h) | Enthalpy (kJ/h) | Entropy (kJ/kg•K) | Physical Exergy (kJ/h) | Chemical Exergy (kJ/h) | Total Exergy (kJ/h) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Input flow | Unfired tile | 298 | 315 | 0.851 | 9074 | 131,261.217 | 428.368 | 3607.464 | -- | 3607.464 |
| Fuel (water-gas) | 298 | 300 | 1.446 | 3591 | 10,386.102 | 34.736 | 34.698 | 19,233,755.100 | 19,233,789.798 | |
| Combustion air | 298 | 300 | 1.143 | 4044 | 9242.054 | 30.910 | 30.876 | -- | 30.876 | |
| Recovered cooling gas | 298 | 471 | 1.214 | 3008 | 631,799.484 | 1671.766 | 133,613.345 | -- | 133,613.345 | |
| Cooling air | 298 | 300 | 1.143 | 51,151 | 116,898.402 | 390.966 | 390.530 | -- | 390.530 | |
| Leakage air | 298 | 300 | 1.143 | 486 | 1110.692 | 3.715 | 3.711 | -- | 3.711 | |
| Total | -- | -- | -- | 71,354 | -- | -- | -- | -- | 19,371,435.724 | |
| Output flow | Fired tile | 298 | 392 | 0.871 | 8711 | 713,155.284 | 2080.049 | 93,300.670 | -- | 93,300.670 |
| Flue gas | 298 | 540 | 1.151 | 11,492 | 3,200,698.681 | 7862.551 | 857,658.364 | -- | 857,658.364 | |
| Exhausted cooling gas | 298 | 420 | 1.193 | 30,099 | 4,380,848.522 | 12,322.437 | 708,762.222 | -- | 708,762.222 | |
| Cooling gas for drying | 298 | 450 | 1.206 | 18,043 | 3,307,497.612 | 8968.412 | 634,910.708 | -- | 634,910.708 | |
| Recovered cooling gas | 298 | 491 | 1.222 | 3008 | 709,484.631 | 1835.656 | 162,459.147 | -- | 162,459.147 | |
| Latent heat of the water | -- | -- | -- | -- | -- | -- | -- | -- | 14,223.512 | |
| Kiln wall heat loss | -- | -- | -- | -- | -- | -- | -- | -- | 423,867.0236 | |
| Total | -- | -- | -- | 71,354 | -- | -- | -- | -- | 2,895,181.646 | |
| Exergy destruction | Combustion | -- | -- | -- | -- | -- | -- | -- | -- | 6,236,292.406 |
| Calcination reaction | -- | -- | -- | -- | -- | -- | -- | -- | 1,860,965 | |
| Heat and mass transfer | -- | -- | -- | -- | -- | -- | -- | -- | 8,378,996.672 | |
| Total | -- | -- | -- | -- | -- | -- | -- | -- | 16,476,254.08 |
| Operating Parameters | Cost Analysis | Environmental Analysis | ||
|---|---|---|---|---|
| Average Cost-saving (RMB/h) | Contribution (%) | Average Carbon Dioxide Emission Reduction (kg/h) | Contribution (%) | |
| Cooling air mass | 2.836 | 18.774 | 10.597 | 18.774% |
| Cooling air temperature | 4.679 | 30.976 | 17.484 | 30.976% |
| Cooling air residence time | 7.590 | 50.250 | 28.363 | 50.250% |
| Total | 15.104 | 100.000 | 56.444 | 100.000 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Yang, H.; Xu, K. Thermal Performance Combined with Cooling System Parameters Study for a Roller Kiln Based on Energy-Exergy Analysis. Energies 2020, 13, 3922. https://doi.org/10.3390/en13153922
Wang Y, Yang H, Xu K. Thermal Performance Combined with Cooling System Parameters Study for a Roller Kiln Based on Energy-Exergy Analysis. Energies. 2020; 13(15):3922. https://doi.org/10.3390/en13153922
Chicago/Turabian StyleWang, Yali, Haidong Yang, and Kangkang Xu. 2020. "Thermal Performance Combined with Cooling System Parameters Study for a Roller Kiln Based on Energy-Exergy Analysis" Energies 13, no. 15: 3922. https://doi.org/10.3390/en13153922
APA StyleWang, Y., Yang, H., & Xu, K. (2020). Thermal Performance Combined with Cooling System Parameters Study for a Roller Kiln Based on Energy-Exergy Analysis. Energies, 13(15), 3922. https://doi.org/10.3390/en13153922






