Acid-Assisted Organosolv Pre-Treatment and Enzymatic Hydrolysis of Cynara cardunculus L. for Glucose Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Characterization of Raw Material and Pre-Treated Material
2.2. Design of Experiment
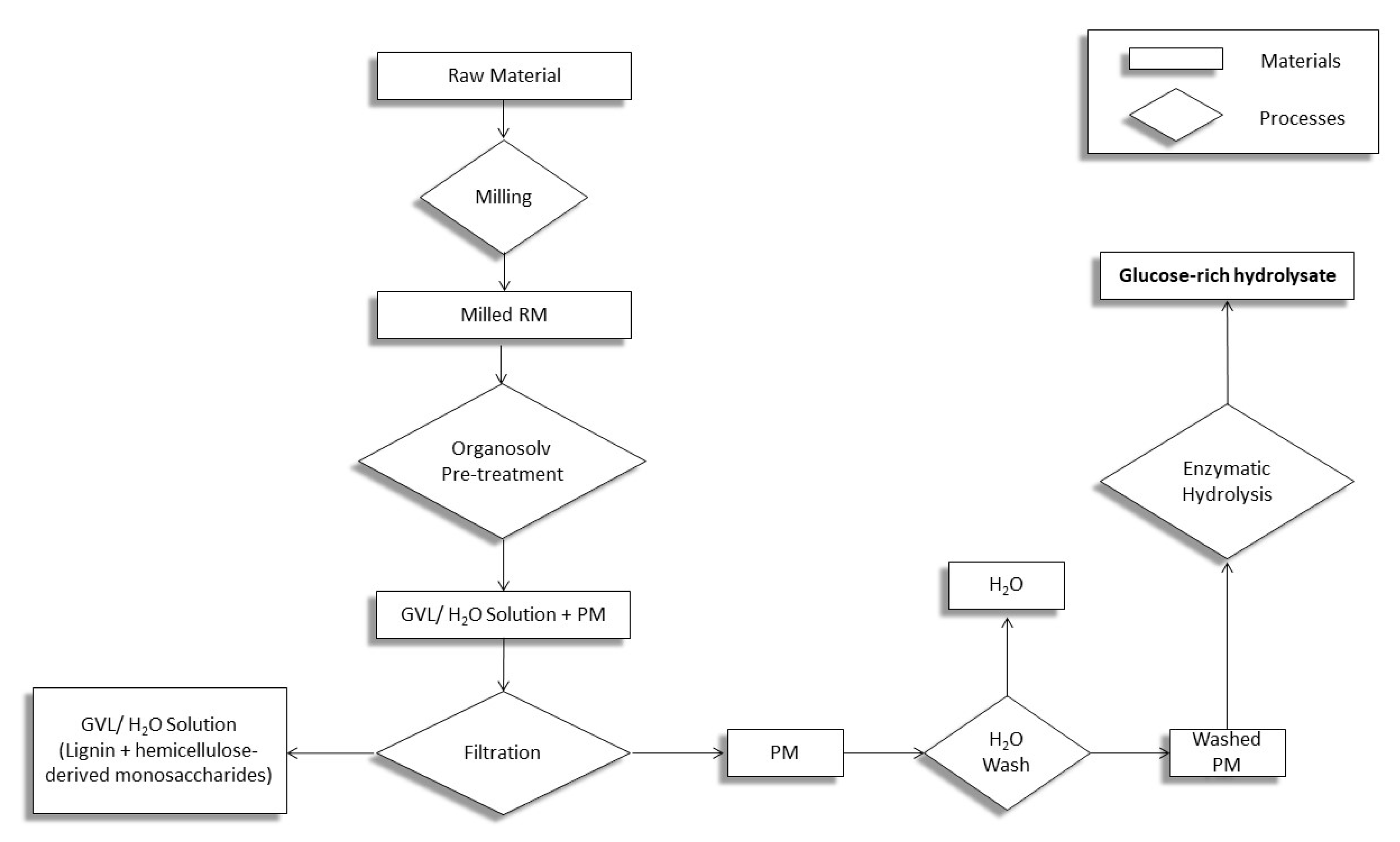
2.3. Process Flowchart
2.4. Enzymatic Hydrolysis
3. Results and Discussion
3.1. Raw Material Characterization
3.2. Cellulose Recovery and Digestibility
3.3. Design of Experiment
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Friend, A.D. Terrestrial plant production and climate change. J. Exp. Bot. 2010, 61, 1293–1309. [Google Scholar] [CrossRef] [PubMed]
- Agbor, V.B.; Cicek, N.; Sparling, R.; Berlin, A.; Levin, D.B. Biomass pretreatment: Fundamentals toward application. Biotechnol. Adv. 2011, 29, 675–685. [Google Scholar] [CrossRef] [PubMed]
- Wyman, C.E. Biomass ethanol: Technical progress, opportunities, and commercial challenges. Annu. Rev. Energy Environ. 1999, 24, 189–226. [Google Scholar] [CrossRef]
- Gomes, F.J.; Santos, F.A.; Colodette, J.L.; Demuner, I.F.; Batalha, L.A. Literature review on biorefinery processes integrated to the pulp industry. Nat. Resour. 2014, 5, 419–432. [Google Scholar] [CrossRef]
- Rodríguez, F.; Sanchez, A.; Parra, C. Role of Steam Explosion on Enzymatic Digestibility, Xylan Extraction, and Lignin Release of Lignocellulosic Biomass. ACS Sustain. Chem. Eng. 2017, 5, 5234–5240. [Google Scholar] [CrossRef]
- Xu, J. Chapter 9-Microwave Pretreatment. In Pretreatment of Biomass; Pandey, A., Negi, S., Binod, P., Larroche, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 157–172. [Google Scholar] [CrossRef]
- Calvo-Flores, F.G.; Dobado, J.A. Lignin as renewable raw material. ChemSusChem 2010, 3, 1227–1235. [Google Scholar] [CrossRef]
- Meng, X.; Bhagia, S.; Wang, Y.; Zhou, Y.; Pu, Y.; Dunlap, J.R.; Shuai, L.; Ragauskas, A.J.; Yoo, C.G. Effects of the advanced organosolv pretreatment strategies on structural properties of woody biomass. Ind. Crop. Prod. 2020, 146, 112–144. [Google Scholar] [CrossRef]
- Grangeiro, L.C.; de Almeida, S.G.C.; de Mello, B.S.; Fuess, L.T.; Sarti, A.; Dussán, K.J. New trends in biogas production and utilization. In Sustain. Bioenergy; Elsevier: Amsterdam, The Netherlands, 2019; pp. 199–223. [Google Scholar]
- Zhang, Z.; Harrison, M.D.; Rackemann, D.W.; Doherty, W.O.; O’Hara, I.M. Organosolv pretreatment of plant biomass for enhanced enzymatic saccharification. Green Chem. 2016, 18, 360–381. [Google Scholar] [CrossRef]
- Gelosia, M.; Ingles, D.; Pompili, E.; D’Antonio, S.; Cavalaglio, G.; Petrozzi, A.; Coccia, V. Fractionation of lignocellulosic residues coupling steam explosion and organosolv treatments using green solvent γ-valerolactone. Energies 2017, 10, 1264. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, H.; Guo, H.; Huang, C.; Xiong, L.; Chen, X. Kinetic study on the liquefaction of wood and its three cell wall component in polyhydric alcohols. Appl. Energy 2014, 113, 1596–1600. [Google Scholar] [CrossRef]
- Mellmer, M.A.; Alonso, D.M.; Luterbacher, J.S.; Gallo, J.M.R.; Dumesic, J.A. Effects of γ-valerolactone in hydrolysis of lignocellulosic biomass to monosaccharides. Green Chem. 2014, 16, 4659–4662. [Google Scholar] [CrossRef]
- Shuai, L.; Questell-Santiago, Y.M.; Luterbacher, J.S. A mild biomass pretreatment using γ-valerolactone for concentrated sugar production. Green Chem. 2016, 18, 937–943. [Google Scholar] [CrossRef]
- Lê, H.Q.; Pokki, J.-P.; Borrega, M.; Uusi-Kyyny, P.; Alopaeus, V.; Sixta, H. Chemical Recovery of γ-Valerolactone/Water Biorefinery. Ind. Eng. Chem. Res. 2018, 57, 15147–15158. [Google Scholar] [CrossRef] [PubMed]
- Angelini, L.G.; Ceccarini, L.; o Di Nasso, N.N.; Bonari, E. Long-term evaluation of biomass production and quality of two cardoon (Cynara cardunculus L.) cultivars for energy use. Biomass Bioenergy 2009, 33, 810–816. [Google Scholar] [CrossRef]
- Ballesteros, M.; Negro, M.J.; Manzanares, P.; Ballesteros, I.; Sáez, F.; Oliva, J.M. Fractionation of cynara cardunculus (cardoon) biomass by dilute-acid pretreatment. In Applied Biochemistry and Biotecnology; Springer: Berlin, Germany, 2007; pp. 239–252. [Google Scholar]
- Bertini, A.; Gelosia, M.; Cavalaglio, G.; Barbanera, M.; Giannoni, T.; Tasselli, G.; Nicolini, A.; Cotana, F. Production of Carbohydrates from Cardoon Pre-Treated by Acid-Catalyzed Steam Explosion and Enzymatic Hydrolysis. Energies 2019, 12, 4288. [Google Scholar] [CrossRef]
- Ligero, P.; Villaverde, J.; Vega, A.; Bao, M. Acetosolv delignification of depithed cardoon (Cynara cardunculus) stalks. Ind. Crop. Prod. 2007, 25, 294–300. [Google Scholar] [CrossRef]
- Shatalov, A.A.; Pereira, H. Dissolving grade eco-clean cellulose pulps by integrated fractionation of cardoon (Cynara cardunculus L.) stalk biomass. Chem. Eng. Res. Des. 2014, 92, 2640–2648. [Google Scholar] [CrossRef]
- Sluiter, J.; Sluiter, A. Summative Mass Closure. In NREL, NREL/TP-510-48087; 2010; pp. 1–10. Available online: https://www.nrel.gov/docs/gen/fy11/48087.pdf (accessed on 13 August 2020).
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of structural carbohydrates and lignin in biomass. Lab. Anal. Proced. 2008, 1617, 1–16. [Google Scholar]
- Resch, M.; Baker, J.; Decker, S. Low Solids Enzymatic Saccharification of Lignocellulosic Biomass. Lab. Anal. Proced. (LAP) 2015. Available online: https://www.nrel.gov/docs/fy15osti/63351.pdf (accessed on 13 August 2020).
- Pan, X. Role of functional groups in lignin inhibition of enzymatic hydrolysis of cellulose to glucose. J. Biobased Mater. Bioenergy 2008, 2, 25–32. [Google Scholar] [CrossRef]
- Hambardzumyan, A.; Foulon, L.; Bercu, N.; Pernes, M.; Maigret, J.-E.; Molinari, M.; Chabbert, B.; Aguié-Beghin, V. Organosolv lignin as natural grafting additive to improve the water resistance of films using cellulose nanocrystals. Chem. Eng. J. 2015, 264, 780–788. [Google Scholar] [CrossRef]
- Zhao, X.; Cheng, K.; Liu, D. Organosolv pretreatment of lignocellulosic biomass for enzymatic hydrolysis. Appl. Microbiol. Biotechnol. 2009, 82, 815–827. [Google Scholar] [CrossRef] [PubMed]
- Luterbacher, J.S.; Azarpira, A.; Motagamwala, A.H.; Lu, F.; Ralph, J.; Dumesic, J.A. Lignin monomer production integrated into the γ-valerolactone sugar platform. Energy Environ. Sci. 2015, 8, 2657–2663. [Google Scholar] [CrossRef]
- Angelini, S.; Ingles, D.; Gelosia, M.; Cerruti, P.; Pompili, E.; Scarinzi, G.; Cavalaglio, G.; Cotana, F.; Malinconico, M. One-pot lignin extraction and modification in γ-valerolactone from steam explosion pre-treated lignocellulosic biomass. J. Clean. Prod. 2017, 151, 152–162. [Google Scholar] [CrossRef]
- Fang, W.; Sixta, H. Advanced Biorefinery based on the Fractionation of Biomass in γ-Valerolactone and Water. ChemSusChem 2015, 8, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Lê, H.Q.; Zaitseva, A.; Pokki, J.P.; Ståhl, M.; Alopaeus, V.; Sixta, H. Solubility of organosolv lignin in γ-valerolactone/water binary mixtures. ChemSusChem 2016, 9, 2939–2947. [Google Scholar] [CrossRef] [PubMed]
- Ballesteros, I.; Ballesteros, M.; Manzanares, P.; Negro, M.J.; Oliva, J.M.; Sáez, F. Dilute sulfuric acid pretreatment of cardoon for ethanol production. Biochem. Eng. J. 2008, 42, 84–91. [Google Scholar] [CrossRef]
- Zhang, K.; Pei, Z.; Wang, D. Organic solvent pretreatment of lignocellulosic biomass for biofuels and biochemicals: A review. Bioresour. Technol. 2016, 199, 21–33. [Google Scholar] [CrossRef]
- Wu, M.; Yan, Z.Y.; Zhang, X.M.; Xu, F.; Sun, R.C. Integration of mild acid hydrolysis in γ-valerolactone/water system for enhancement of enzymatic saccharification from cotton stalk. Bioresour. Technol. 2016, 200, 23–28. [Google Scholar] [CrossRef]



| Run | T (°C) | Acid (% w/w) | GVL/Water |
|---|---|---|---|
| 1 | 180 | 0.98 | 0.6 |
| 2 | 160 | 0.98 | 0.6 |
| 3 | 180 | 1.96 | 0.6 |
| 4 | 180 | 1.96 | 0.4 |
| 5 | 160 | 0.98 | 0.4 |
| 6 | 160 | 1.96 | 0.6 |
| 7 | 180 | 0.98 | 0.4 |
| 8 | 160 | 1.96 | 0.4 |
| Cellulose | Hemicellulose | Pectin | Acetyls | Lignin | Extractives |
|---|---|---|---|---|---|
| 30.52% | 17.17% | 4.67% | 5.02% | 14.21% | 7.64% |
| Run | Cellulose | Hemicellulose | Lignin | CD | CR | OY (g/100 g) |
|---|---|---|---|---|---|---|
| 1 | 92.47% | 0.00% | 7.33% | 76.06% | 69.33% | 17.87 |
| 2 | 95.71% | 0.03% | 4.26% | 47.17% | 89.60% | 14.32 |
| 3 | 9.88% | 0.00% | 9.42% | 0.00% | 1.27% | 0.00 |
| 4 | 78.05% | 0.00% | 21.95% | 94.18% | 38.92% | 12.42 |
| 5 | 77.82% | 2.74% | 19.44% | 60.67% | 92.87% | 19.09 |
| 6 | 95.93% | 0.00% | 4.07% | 100% | 81.11% | 27.47 |
| 7 | 91.09% | 0.08% | 8.83% | 55.58% | 67.60% | 12.73 |
| 8 | 87.06% | 0.06% | 12.88% | 85.39% | 85.29% | 24.67 |
| Run | T (°C) | Acid (% w/w) | CR (%) | CD (%) | OY (g/100 g) |
|---|---|---|---|---|---|
| 9 | 140 | 1.96 | 95.36 | 81.65 | 26.38 |
| 10 | 150 | 1.96 | 92.80 | 95.97 | 30.17 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gelosia, M.; Bertini, A.; Barbanera, M.; Giannoni, T.; Nicolini, A.; Cotana, F.; Cavalaglio, G. Acid-Assisted Organosolv Pre-Treatment and Enzymatic Hydrolysis of Cynara cardunculus L. for Glucose Production. Energies 2020, 13, 4195. https://doi.org/10.3390/en13164195
Gelosia M, Bertini A, Barbanera M, Giannoni T, Nicolini A, Cotana F, Cavalaglio G. Acid-Assisted Organosolv Pre-Treatment and Enzymatic Hydrolysis of Cynara cardunculus L. for Glucose Production. Energies. 2020; 13(16):4195. https://doi.org/10.3390/en13164195
Chicago/Turabian StyleGelosia, Mattia, Alessandro Bertini, Marco Barbanera, Tommaso Giannoni, Andrea Nicolini, Franco Cotana, and Gianluca Cavalaglio. 2020. "Acid-Assisted Organosolv Pre-Treatment and Enzymatic Hydrolysis of Cynara cardunculus L. for Glucose Production" Energies 13, no. 16: 4195. https://doi.org/10.3390/en13164195
APA StyleGelosia, M., Bertini, A., Barbanera, M., Giannoni, T., Nicolini, A., Cotana, F., & Cavalaglio, G. (2020). Acid-Assisted Organosolv Pre-Treatment and Enzymatic Hydrolysis of Cynara cardunculus L. for Glucose Production. Energies, 13(16), 4195. https://doi.org/10.3390/en13164195








