Highly Efficient Production of DMF from Biomass-Derived HMF on Recyclable Ni-Fe/TiO2 Catalysts
Abstract
:1. Introduction
2. Materials and Methods
2.1. TiO2 Supports and Catalysts
2.2. Characterization Techniques
2.3. Catalytic Tests
3. Results
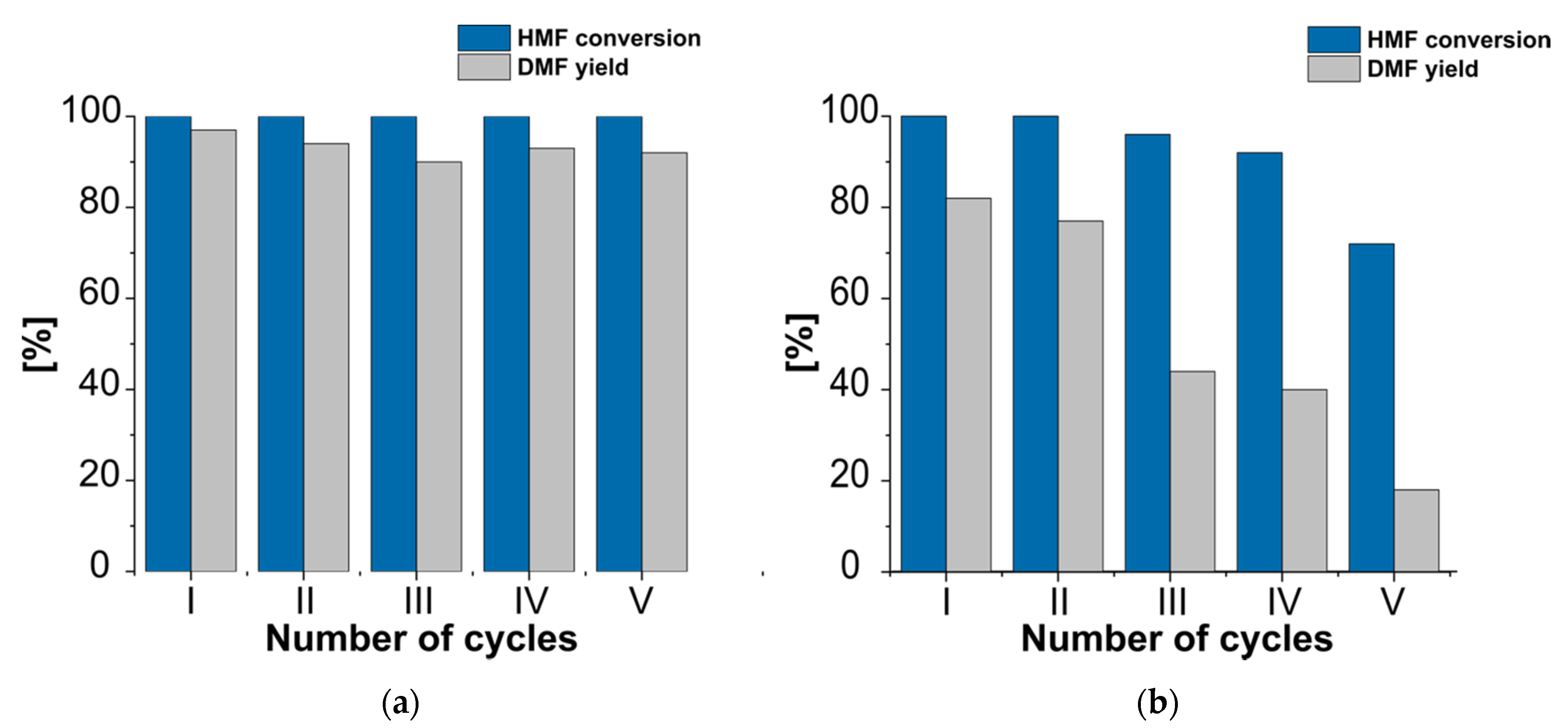
3.1. Catalytic Activity
3.2. Catalyst Characterization
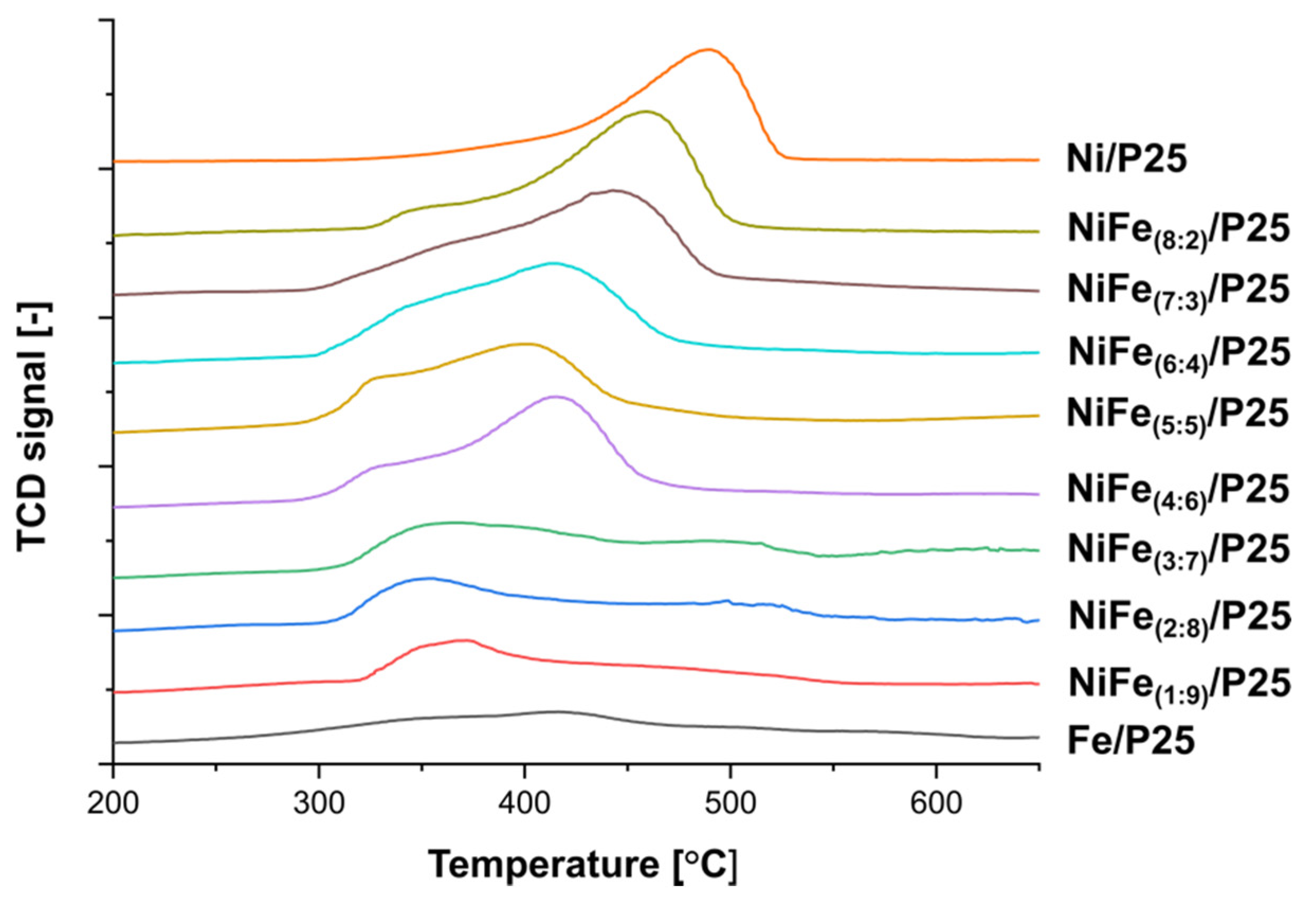
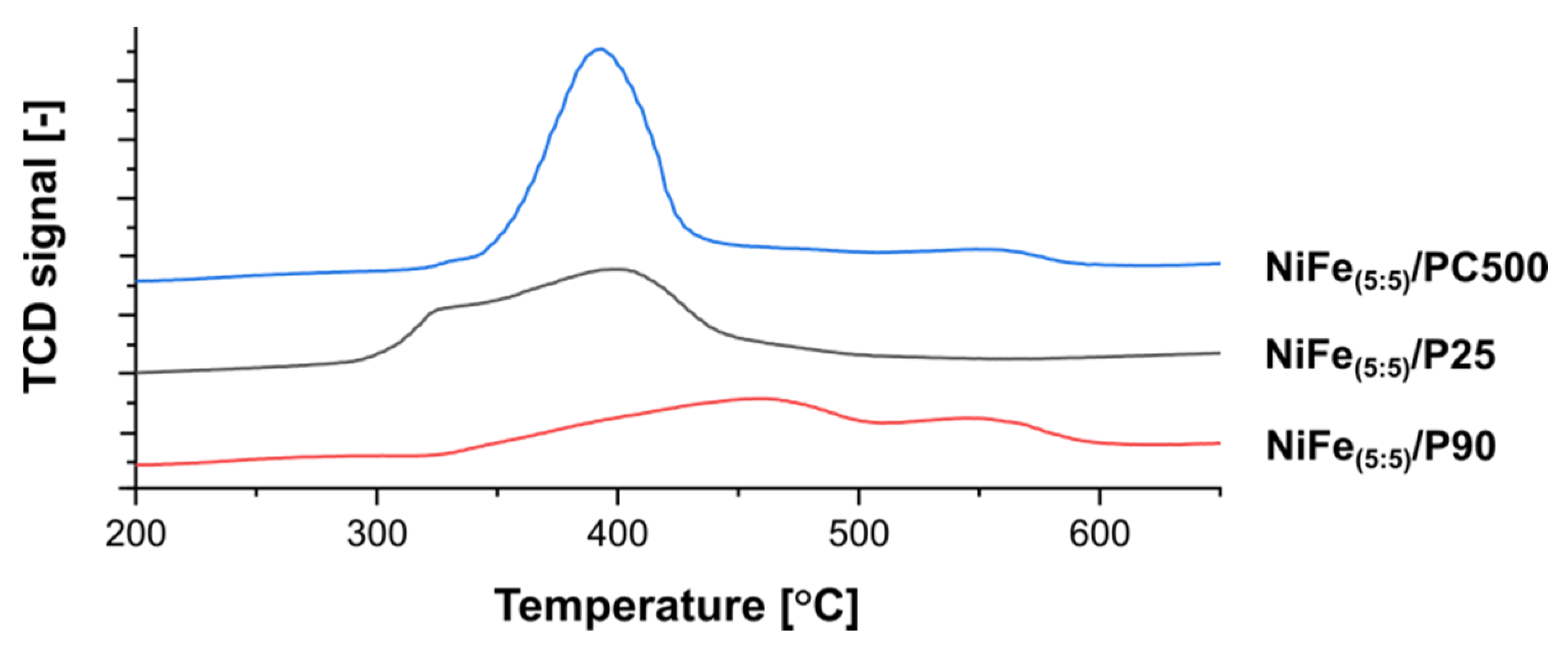
3.2.1. Temperature-Programmed Reduction Analysis
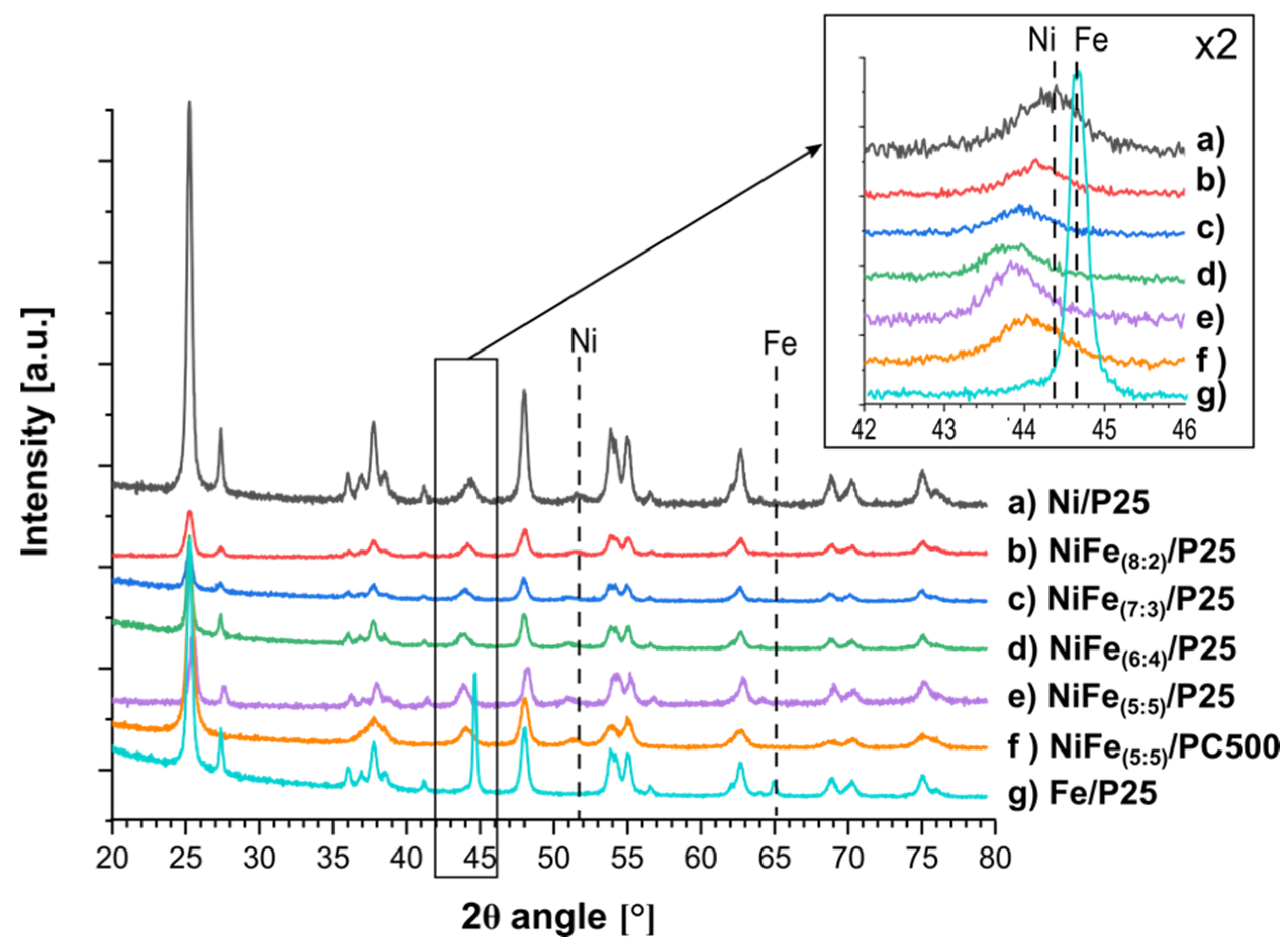
3.2.2. X-ray Diffraction Measurements
3.2.3. Acidity
3.2.4. FTIR Analysis
3.2.5. ToF-SIMS
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chen, S.; Wojcieszak, R.; Dumeignil, F.; Marceau, E.; Royer, S. How catalysts and experimental conditions determine the selective hydroconversion of furfural and 5-hydroxymethylfurfural. Chem. Rev. 2018, 118, 11023–11117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menegazzo, F.; Ghedini, E.; Signoretto, M. 5-hydroxymethylfurfural (HMF) production from real biomasses. Molecules 2018, 23, 2201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Putten, R.; Van Der Waal, J.C.; De Jong, E.; Rasrendra, C.B.; Heeres, H.J.; De Vries, J.G. Hydroxymethylfurfural, a versatile platform chemical made from renewable resources. Chem. Rev. 2013, 113, 1499–1597. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; He, L.; Chen, J.; Zheng, J.; Ye, L.; Lin, H.; Yuan, Y. Robust and recyclable nonprecious bimetallic nanoparticles on carbon nanotubes for the hydrogenation and hydrogenolysis of 5-hydroxymethylfurfural. ChemCatChem 2015, 7, 1701–1707. [Google Scholar] [CrossRef]
- Hu, L.; Lin, L.; Liu, S. Chemoselective hydrogenation of biomass-derived 5-hydroxymethylfurfural into the liquid biofuel 2,5-dimethylfuran. Ind. Eng. Chem. Res. 2014, 53, 9969–9978. [Google Scholar] [CrossRef]
- Nishimura, S.; Ebitani, K. Catalytic conversions of biomass-derived furaldehydes toward biofuels. Green Chem. Process. Synth. 2017, 51–68. [Google Scholar] [CrossRef] [Green Version]
- Przydacz, M.; Jędrzejczyk, M.; Brzezińska, M.; Rogowski, J.; Keller, N.; Ruppert, A.M. Solvothermal hydrodeoxygenation of hydroxymethylfurfural derived from biomass towards added value chemicals on Ni/TiO2 catalysts. J. Supercrit. Fluids 2020, 163, 104827. [Google Scholar] [CrossRef]
- Wang, J.; Wei, Q.; Ma, Q.; Guo, Z.; Qin, F.; Ismagilov, Z.R.; Shen, W. Constructing Co@N-doped graphene shell catalyst via Mott-Schottky effect for selective hydrogenation of 5-hydroxylmethylfurfural. Appl. Catal. B Environ. 2020, 263, 118339. [Google Scholar] [CrossRef]
- Wang, X.; Liang, X.; Li, J.; Li, Q. Catalytic hydrogenolysis of biomass-derived 5-hydroxymethylfurfural to biofuel 2, 5-dimethylfuran. Appl. Catal. A Gen. 2019, 576, 85–95. [Google Scholar] [CrossRef]
- Kohli, K.; Prajapati, R.; Sharma, B.K. Bio-based chemicals from renewable biomass for integrated biorefineries. Energies 2019, 12, 233. [Google Scholar] [CrossRef] [Green Version]
- Seemala, B.; Cai, C.M.; Wyman, C.E.; Christopher, P. Support induced control of surface composition in Cu-Ni/TiO2 catalysts enables high yield co-conversion of HMF and furfural to methylated furans. ACS Catal. 2017, 7, 4070–4082. [Google Scholar] [CrossRef] [Green Version]
- Hu, S.; Xue, M.; Chen, H.; Shen, J. The effect of surface acidic and basic properties on the hydrogenation of aromatic rings over the supported nickel catalysts. Chem. Eng. J. 2010, 162, 371–379. [Google Scholar] [CrossRef]
- Sitthisa, S.; Resasco, D.E. Hydrodeoxygenation of furfural over supported metal catalysts: A comparative study of Cu, Pd and Ni. Catal. Lett. 2011, 141, 784–791. [Google Scholar] [CrossRef]
- Rodiansono; Khairi, S.; Hara, T.; Ichikuni, N.; Shimazu, S. Highly efficient and selective hydrogenation of unsaturated carbonyl compounds using Ni-Sn alloy catalysts. Catal. Sci. Technol. 2012, 2, 2139–2145. [Google Scholar] [CrossRef]
- Alamillo, R.; Tucker, M.; Chia, M.; Pagán-Torres, Y.; Dumesic, J. The selective hydrogenation of biomass-derived 5-hydroxymethylfurfural using heterogeneous catalysts. Green Chem. 2012, 14, 1413–1419. [Google Scholar] [CrossRef]
- Davda, R.R.; Shabaker, J.W.; Huber, G.W.; Cortright, R.D.; Dumesic, J.A. Aqueous-phase reforming of ethylene glycol on silica-supported metal catalysts. Appl. Catal. B Environ. 2003, 43, 13–26. [Google Scholar] [CrossRef]
- Gyngazova, M.S.; Negahdar, L.; Blumenthal, L.C.; Palkovits, R. Experimental and kinetic analysis of the liquid phase hydrodeoxygenation of 5-hydroxymethylfurfural to 2,5-dimethylfuran over carbon-supported nickel catalysts. Chem. Eng. Sci. 2017, 173, 455–464. [Google Scholar] [CrossRef]
- Sun, K.; Shao, Y.; Li, Q.; Liu, Q.; Wu, W.; Wang, Y.; Hu, S.; Xiang, J.; Liu, Q.; Hu, X. Cu-based catalysts for hydrogenation of 5-hydroxymethylfurfural: Understanding of the coordination between copper and alkali/alkaline earth additives. Mol. Catal. 2019, 474, 110407. [Google Scholar] [CrossRef]
- Xiong, J.; Chen, J.; Zhang, J. Liquid-phase hydrogenation of o-chloronitrobenzene over supported nickel catalysts. Catal. Commun. 2007, 8, 345–350. [Google Scholar] [CrossRef]
- Huo, W.; Zhang, C.; Yuan, H.; Jia, M.; Ning, C.; Tang, Y. Vapor-phase selective hydrogenation of maleic anhydride to succinic anhydride over Ni/TiO2 catalysts. J. Ind. Eng. Chem. 2014, 20, 4140–4145. [Google Scholar] [CrossRef]
- Ho, S.W.; Chu, C.Y.; Chen, S.G. Effect of thermal treatment on the nickel state and CO hydrogenation activity of titania-supported nickel catalysts. J. Catal. 1998, 178, 34–48. [Google Scholar] [CrossRef]
- Raj, K.J.A.; Prakash, M.G.; Mahalakshmy, R.; Elangovan, T.; Viswanathan, B. Selective hydrogenation of acetophenone over nickel supported on titania. Catal. Sci. Technol. 2012, 2, 1429–1436. [Google Scholar] [CrossRef]
- Cheng, C.; Shen, D.; Xiao, R.; Wu, C. Methanation of syngas (H2/CO) over the different Ni-based catalysts. Fuel 2017, 189, 419–427. [Google Scholar] [CrossRef]
- Hammedi, T.; Triki, M.; Ksibi, Z.; Ghorbel, A.; Medina, F. Catalytic wet hydrogen peroxide oxidation of p-hydroxybenzoic acid over Fe/TiO2 and 0.5Ru–3Fe/TiO2. J. Sol-Gel Sci. Technol. 2015, 76, 679–685. [Google Scholar] [CrossRef]
- Valinejad-Moghaddam, S.; Rezaei, M.; Meshkani, F.; Daroughegi, R. Carbon dioxide methanation over Ni-M/Al2O3 (M: Fe, CO, Zr, La and Cu) catalysts synthesized using the one-pot sol-gel synthesis method. Int. J. Hydrogen Energy 2018, 43, 16522–16533. [Google Scholar] [CrossRef]
- Wu, H.; Zou, M.; Guo, L.; Ma, F.; Mo, W.; Yu, Y.; Mian, I.; Liu, J.; Yin, S.; Tsubaki, N. Effects of calcination temperatures on the structure-activity relationship of Ni-La/Al2O3 catalysts for syngas methanation. RSC Adv. 2020, 10, 3166–3174. [Google Scholar] [CrossRef] [Green Version]
- Ali, S.; Khader, M.M.; Almarri, M.J.; Abdelmoneim, A.G. Ni-based nano-catalysts for the dry reforming of methane. Catal. Today 2020, 343, 26–37. [Google Scholar] [CrossRef]
- Song, Y.; Lin, W.; Guo, X.; Dong, L.; Mu, X.; Tian, H.; Wang, L. Aromatization and isomerization of methylcyclohexane over Ni catalysts supported on different supports. Green Energy Environ. 2019, 4, 75–82. [Google Scholar] [CrossRef]
- Mebrahtu, C.; Krebs, F.; Perathoner, S.; Abate, S.; Centi, G.; Palkovits, R. Hydrotalcite based Ni-Fe/(Mg, Al)O:X catalysts for CO2 methanation-tailoring Fe content for improved CO dissociation, basicity, and particle size. Catal. Sci. Technol. 2018, 8, 1016–1027. [Google Scholar] [CrossRef]
- Sreekanth, P.M.; Smirniotis, P.G. Selective reduction of NO with CO over titania supported transition metal oxide catalysts. Catal. Lett. 2008, 122, 37–42. [Google Scholar] [CrossRef]
- Lin, Y.; Zhu, Y.; Pan, X.; Bao, X. Modulating the methanation activity of Ni by the crystal phase of TiO2. Catal. Sci. Technol. 2017, 7, 2813–2818. [Google Scholar] [CrossRef]
- Ebrahimi, F.; Li, H.Q. Structure and properties of electrodeposited nanocrystalline FCC Ni-Fe alloys. Rev. Adv. Mater. Sci. 2003, 5, 134–138. [Google Scholar]
- Yousefi, E.; Sharafi, S.; Irannejad, A. The structural, magnetic, and tribological properties of nanocrystalline Fe-Ni permalloy and Fe-Ni-TiO2 composite coatings produced by pulse electro co-deposition. J. Alloys Compd. 2018, 753, 308–319. [Google Scholar] [CrossRef]
- Jia, P.; Lan, X.; Li, X.; Wang, T. Highly selective hydrogenation of furfural to cyclopentanone over a NiFe bimetallic catalyst in a methanol/water solution with a solvent effect. ACS Sustain. Chem. Eng. 2019, 7, 15221–15229. [Google Scholar] [CrossRef]
- Cherifi, O.; Bettahar, M.M.; Auroux, A. Microcalorimetric study of the acidity and basicity of Ni/SiO2 catalysts modified by metallic additives Fe, Co, Zr and Ce. Thermochim. Acta 1997, 306, 131–134. [Google Scholar] [CrossRef]
- Tu, M.; Shen, J.; Chen, Y. Preparation, characterization and microcalorimetric studies of nickel-iron hydrotalcites and their decompositions. Thermochim. Acta 1997, 302, 117–124. [Google Scholar] [CrossRef]
- Scaranto, J.; Giorgianni, S. A quantum-mechanical study of CO adsorbed on TiO2: A comparison of the Lewis acidity of the rutile (1 1 0) and the anatase (1 0 1) surfaces. J. Mol. Struct. 2008, 858, 72–76. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, Y.P.; Liu, C.J. CO adsorbed infrared spectroscopy study of Ni/Al2O3 catalyst for CO2 reforming of methane. Catal. Lett. 2007, 118, 306–312. [Google Scholar] [CrossRef]
- Zhu, X.; Huo, P.; Zhang, Y.-P.; Cheng, D.-G.; Liu, C.-J. Structure and reactivity of plasma treated Ni/Al2O3 catalyst for CO2 reforming of methane. Appl. Catal. B Environ. 2008, 81, 132–140. [Google Scholar]
- Mihaylov, M.; Hadjiivanov, K.; Knözinger, H. Formation of Ni(CO)4 during the interaction between CO and silica-supported nickel catalyst: An FTIR spectroscopic study. Catal. Lett. 2001, 76, 59–63. [Google Scholar] [CrossRef]
- Bartholomew, C.H.; Pannell, R.B. The stoichiometry of hydrogen and carbon monoxide chemisorption on alumina-and silica-supported nickel. J. Catal. 1980, 65, 390–401. [Google Scholar] [CrossRef]
- Ruppert, A.M.; Jędrzejczyk, M.; Potrzebowska, N.; Kaźmierczak, K.; Brzezińska, M.; Sneka-Płatek, O.; Sautet, P.; Keller, N.; Michel, C.; Grams, J. Supported gold-nickel nano-alloy as a highly efficient catalyst in levulinic acid hydrogenation with formic acid as an internal hydrogen source. Catal. Sci. Technol. 2018, 8, 4318–4331. [Google Scholar] [CrossRef] [Green Version]
- Soszka, E.; Reijneveld, H.M.; Jędrzejczyk, M.; Rzeźnicka, I.; Grams, J.; Ruppert, A.M. Chlorine influence on palladium doped nickel catalysts in levulinic acid hydrogenation with formic acid as hydrogen source. ACS Sustain. Chem. Eng. 2018, 6, 14607–14613. [Google Scholar] [CrossRef]
- Ye, R.P.; Gong, W.; Sun, Z.; Sheng, Q.; Shi, X.; Wang, T.; Yao, Y.; Razink, J.J.; Lin, L.; Zhou, Z.; et al. Enhanced stability of Ni/SiO2 catalyst for CO2 methanation: Derived from nickel phyllosilicate with strong metal-support interactions. Energy 2019, 188, 116059. [Google Scholar] [CrossRef]
- Sitthisa, S.; An, W.; Resasco, D.E. Selective conversion of furfural to methylfuran over silica-supported NiFe bimetallic catalysts. J. Catal. 2011, 284, 90–101. [Google Scholar] [CrossRef]
- Putro, W.S.; Kojima, T.; Hara, T.; Ichikuni, N.; Shimazu, S. Selective hydrogenation of unsaturated carbonyls by Ni-Fe-based alloy catalysts. Catal. Sci. Technol. 2017, 7, 3637–3646. [Google Scholar] [CrossRef]
- Li, C.; Xu, G.; Zhai, Y.; Liu, X.; Ma, Y.; Zhang, Y. Hydrogenation of biomass-derived ethyl levulinate into gamma-valerolactone by activated carbon supported bimetallic Ni and Fe catalysts. Fuel 2017, 203, 23–31. [Google Scholar] [CrossRef]

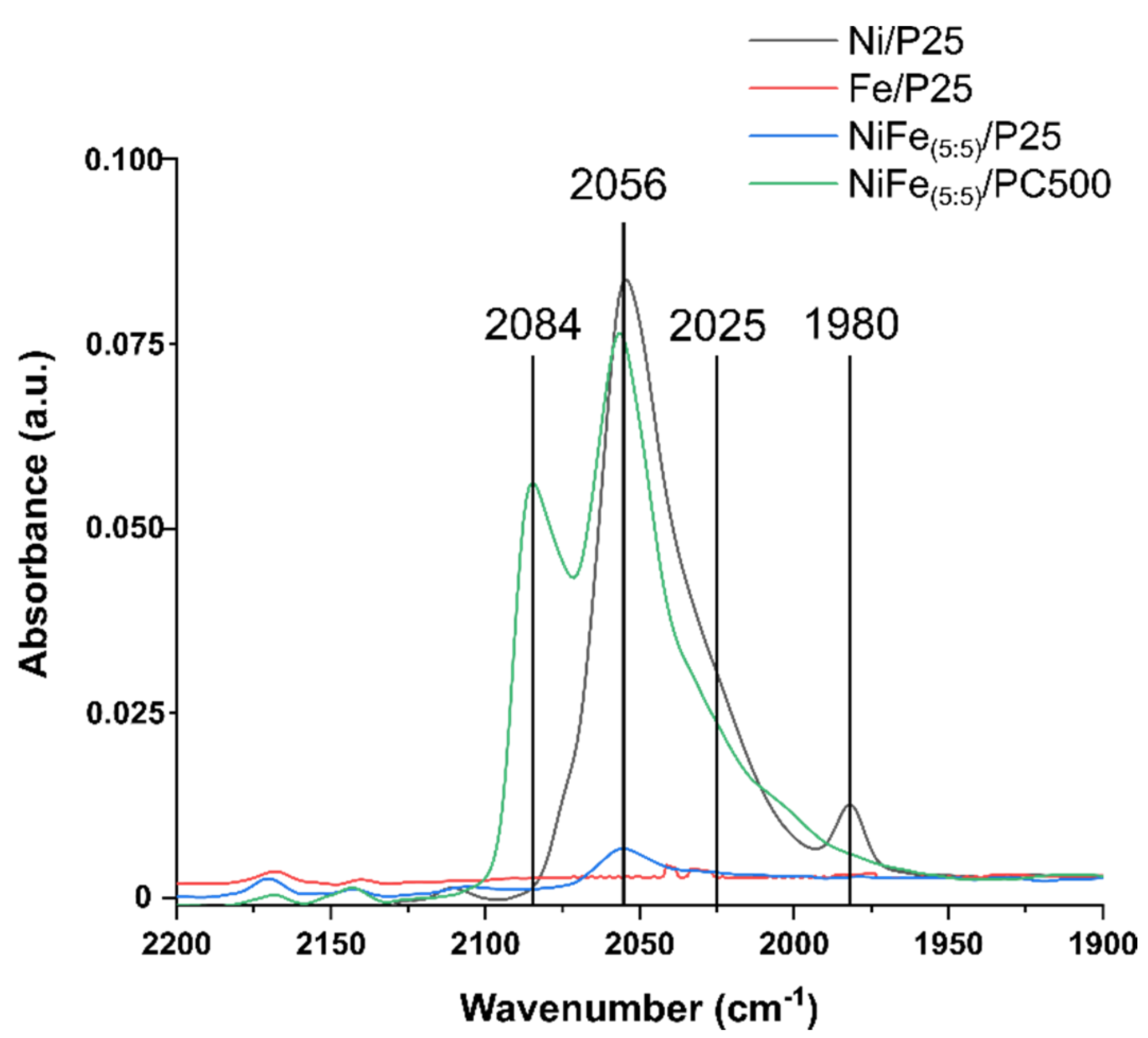
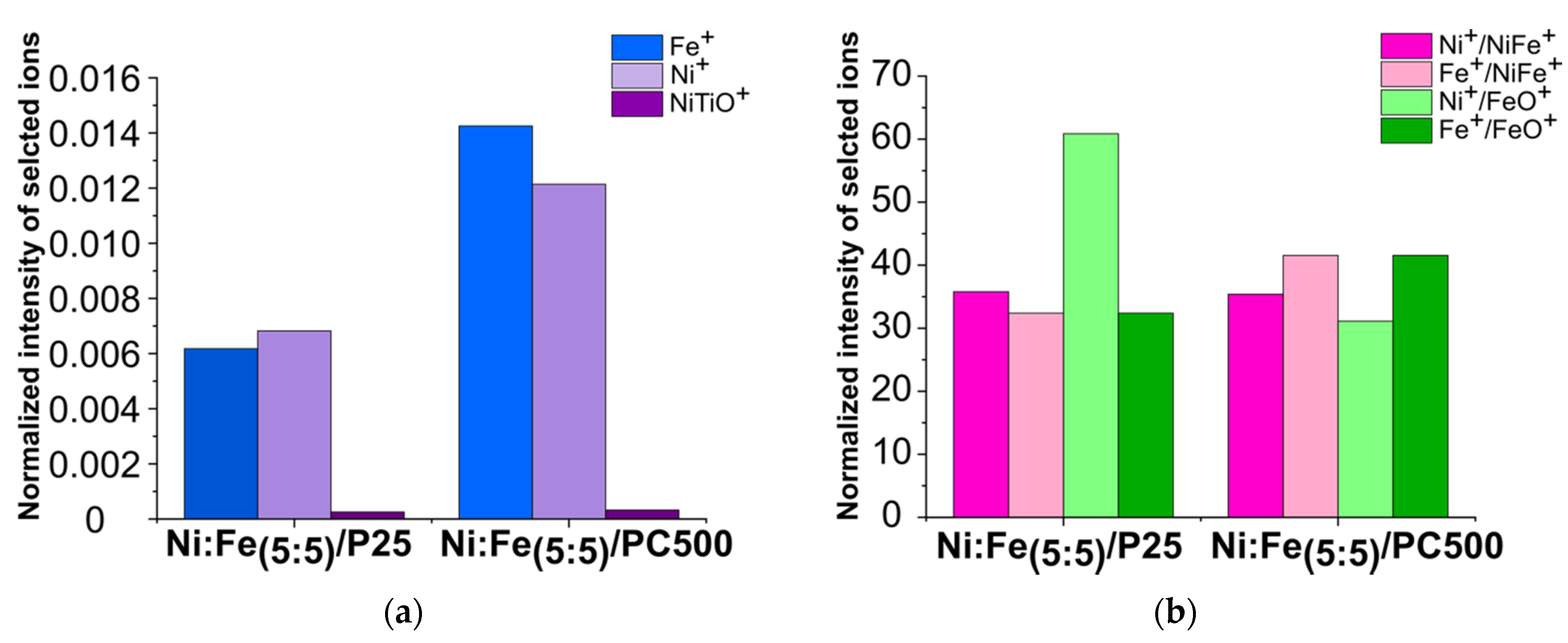
| Catalyst | HMF Conversion (%) | Product Yield (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 5-MF | BHMF | BHMTHF | 5-MFA | DMF | MTHFA | DMTHF | OTHERS | ||
| Ni/P25 | 100 | 0 | 0 | 6 | 0 | 69 | 3 | 22 | 0 |
| NiFe(8:2)/P25 | 100 | 0 | 0 | 28 | 8 | 43 | 14 | 2 | 5 |
| NiFe(7:3)/P25 | 100 | 0 | 0 | 16 | 0 | 57 | 8 | 10 | 9 |
| NiFe(6:4)/P25 | 100 | 0 | 0 | 10 | 0 | 70 | 5 | 4 | 11 |
| NiFe(5:5)/P25 | 100 | 0 | 0 | 7 | 0 | 75 | 4 | 2 | 12 |
| NiFe(4:6)/P25 | 100 | 0 | 1 | 10 | 1 | 65 | 9 | 3 | 11 |
| Fe/P25 | 16 | 6 | 4 | 1 | 0 | 0 | 0 | 0 | 5 |
| Catalyst | HMF Conversion (%) | Product Yield (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 5-MF | BHMF | BHMTHF | 5-MFA | DMF | MTHFA | DMTHF | OTHERS | ||
| NiFe(5:5)/P25 | 100 | 0 | 0 | 4 | 0 | 74 | 6 | 6 | 10 |
| NiFe(5:5)/P90 | 100 | 0 | 2 | 23 | 0 | 40 | 14 | 10 | 11 |
| NiFe(5:5)/PC500 | 100 | 1 | 0 | 0 | 3 | 90 | 0 | 0 | 6 |
| NiFe(5:5)/PC500(1) | 100 | 0 | 0 | 0 | 0 | 97 | 0 | 0 | 5 |
| Entry | Catalyst | Acidity (μmol/m2) |
|---|---|---|
| 1 | Fe(10)/P25 | 8.7 |
| 2 | NiFe(1:9)/P25 | 8.3 |
| 3 | NiFe(2:8)/P25 | 8.1 |
| 4 | NiFe(3:7)/P25 | 7.6 |
| 5 | NiFe(4:6)/P25 | 9.8 |
| 6 | NiFe(5:5)/P25 | 9.7 |
| 7 | NiFe(6:4)/P25 | 11.1 |
| 8 | NiFe(7:3)/P25 | 10.9 |
| 9 | NiFe(8:2)/P25 | 9.2 |
| 10 | Ni(10)/P25 | 9.6 |
| 11 | P25 | 7.5 |
| 12 | NiFe (5:5)/P90 | 6.0 |
| 13 | NiFe (5:5)/PC500 | 1.8 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Przydacz, M.; Jędrzejczyk, M.; Rogowski, J.; Szynkowska-Jóźwik, M.; Ruppert, A.M. Highly Efficient Production of DMF from Biomass-Derived HMF on Recyclable Ni-Fe/TiO2 Catalysts. Energies 2020, 13, 4660. https://doi.org/10.3390/en13184660
Przydacz M, Jędrzejczyk M, Rogowski J, Szynkowska-Jóźwik M, Ruppert AM. Highly Efficient Production of DMF from Biomass-Derived HMF on Recyclable Ni-Fe/TiO2 Catalysts. Energies. 2020; 13(18):4660. https://doi.org/10.3390/en13184660
Chicago/Turabian StylePrzydacz, Martyna, Marcin Jędrzejczyk, Jacek Rogowski, Małgorzata Szynkowska-Jóźwik, and Agnieszka M. Ruppert. 2020. "Highly Efficient Production of DMF from Biomass-Derived HMF on Recyclable Ni-Fe/TiO2 Catalysts" Energies 13, no. 18: 4660. https://doi.org/10.3390/en13184660





