Fluorine-Doped LiNi0.8Mn0.1Co0.1O2 Cathode for High-Performance Lithium-Ion Batteries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of NCM and NCMF
2.2. Material Characterization
2.3. Cell Assembly and Electrochemical Characterization
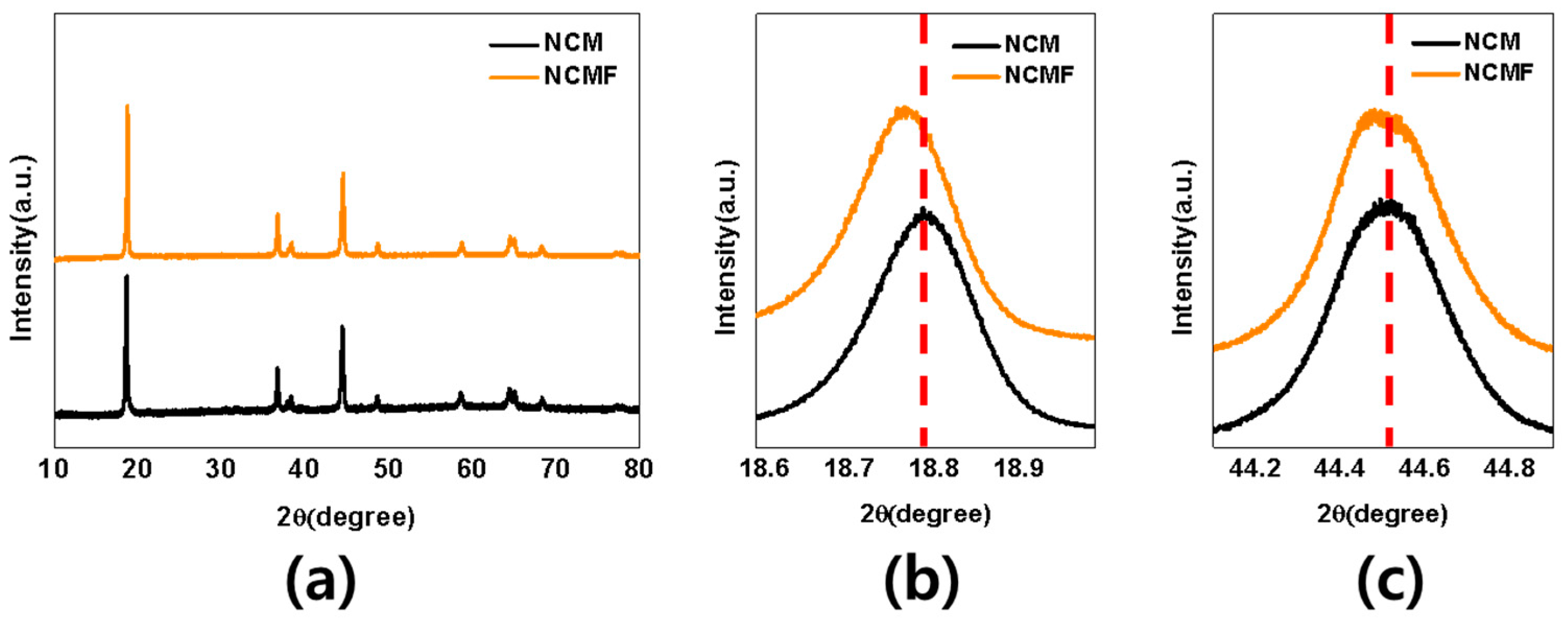
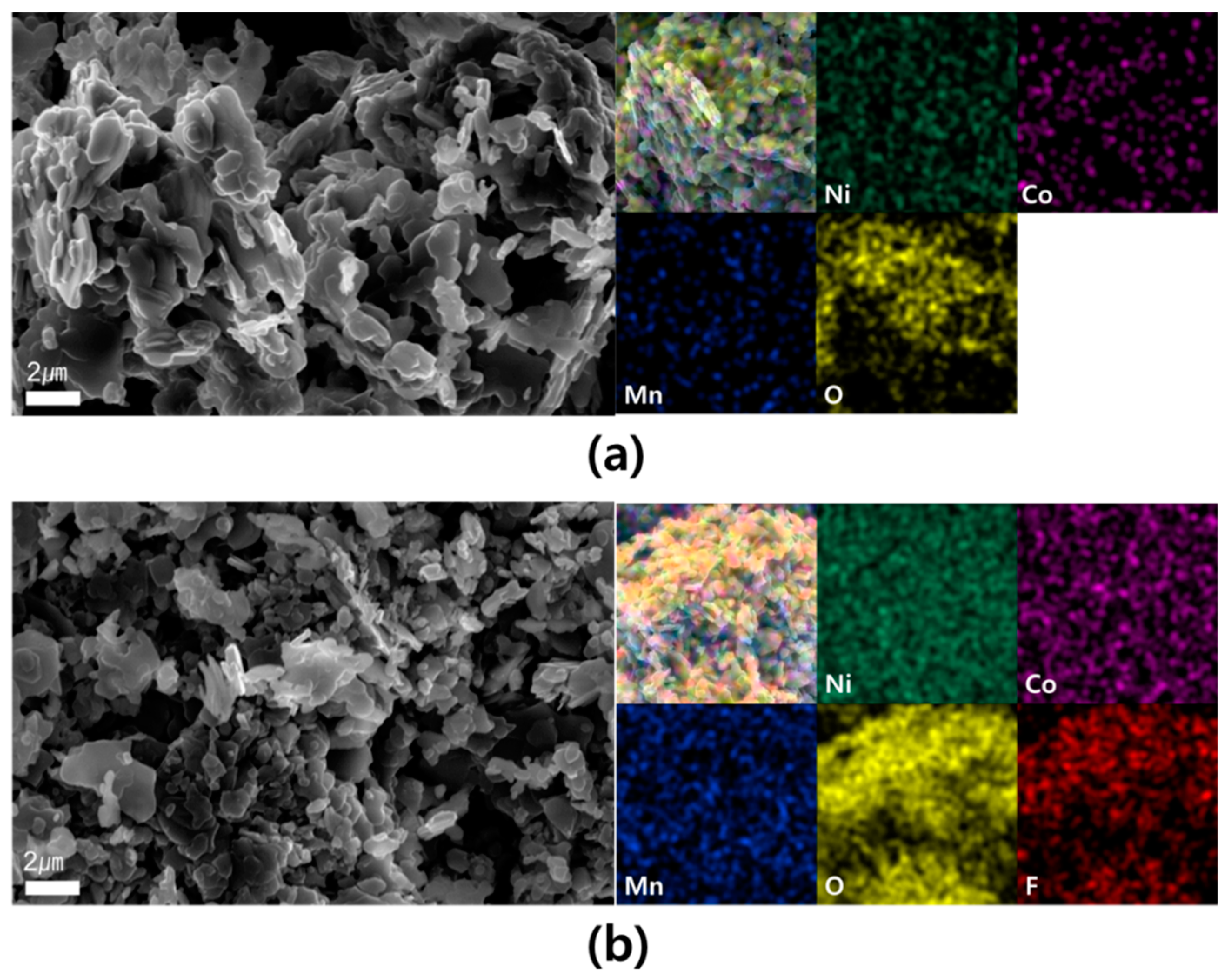
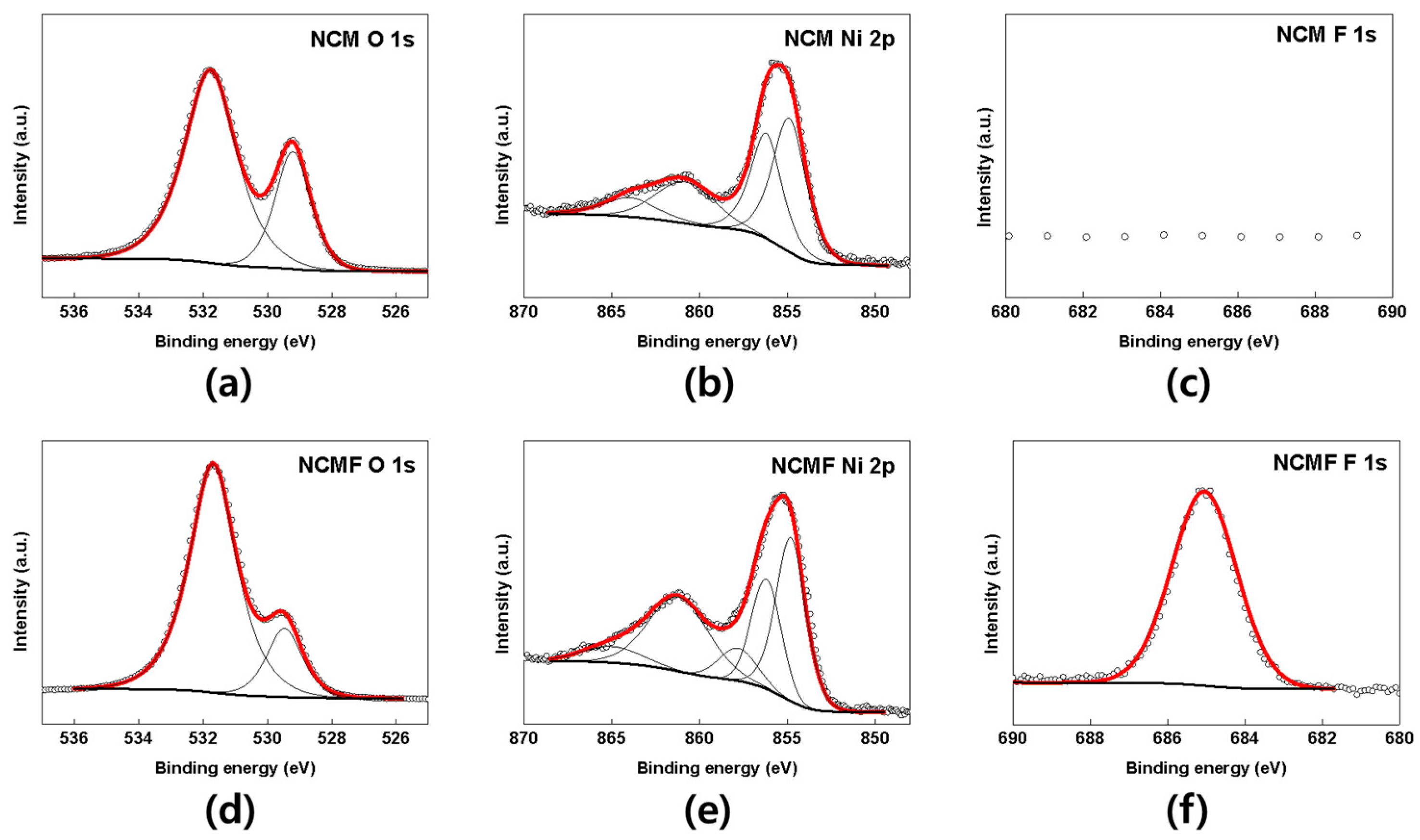
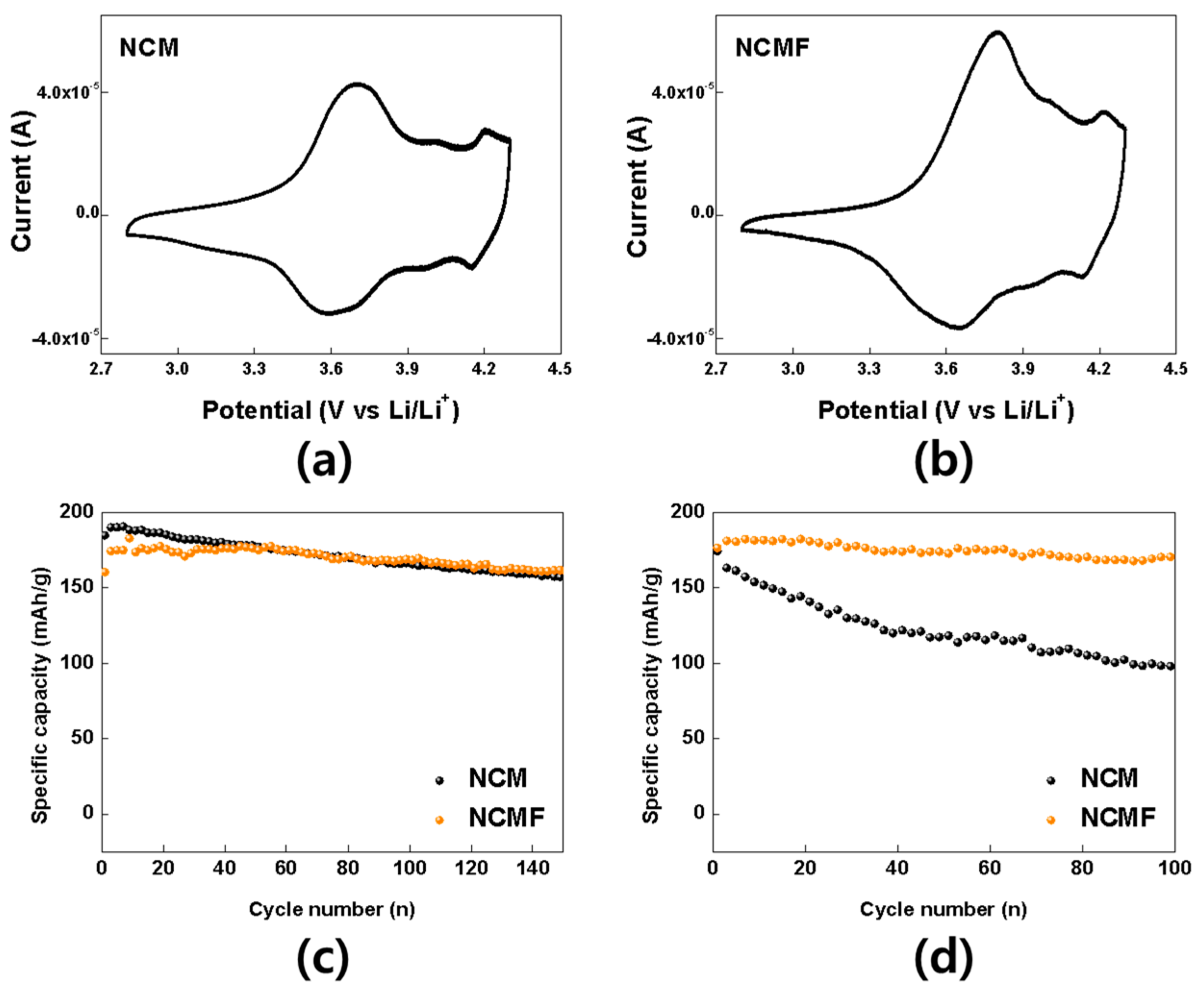
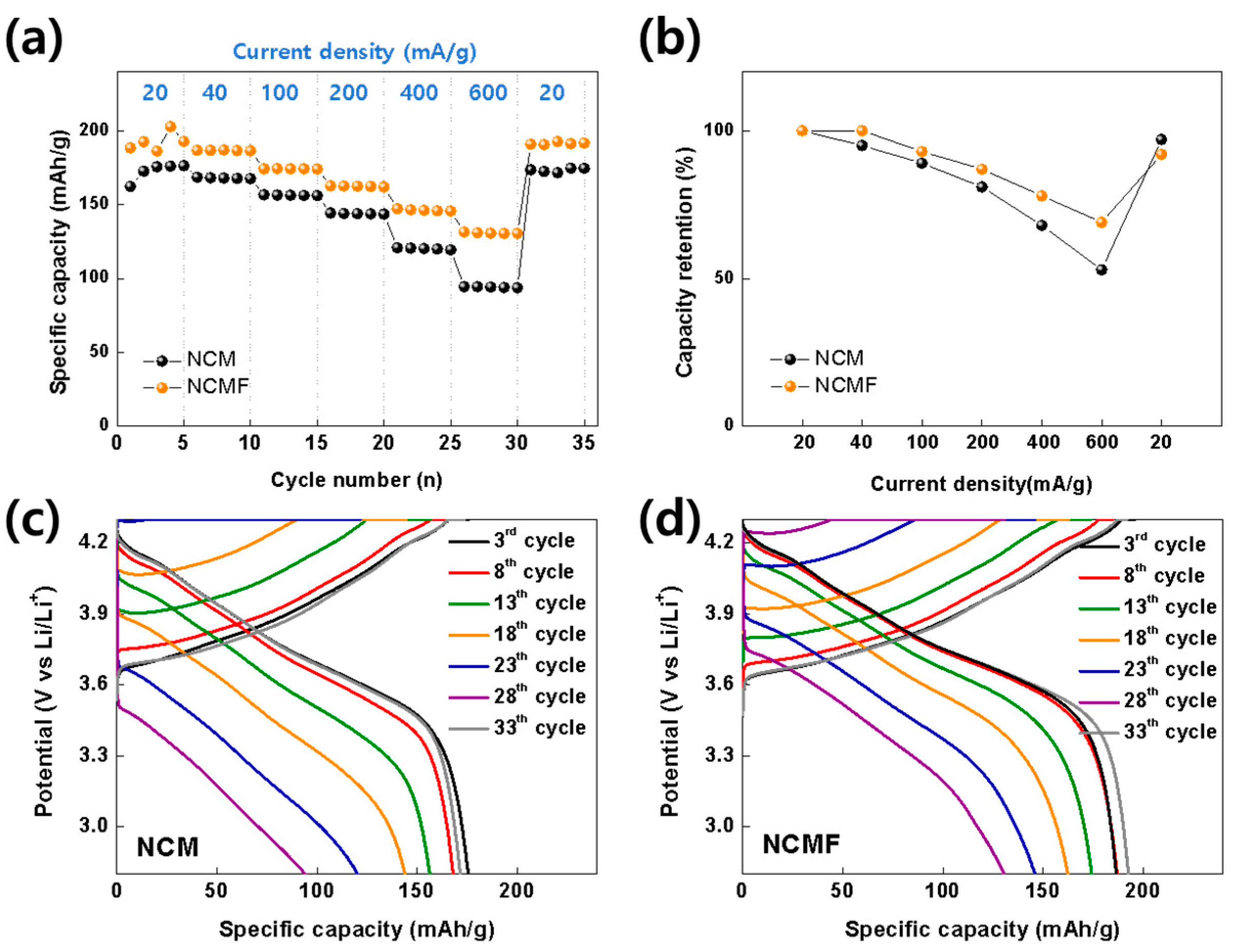
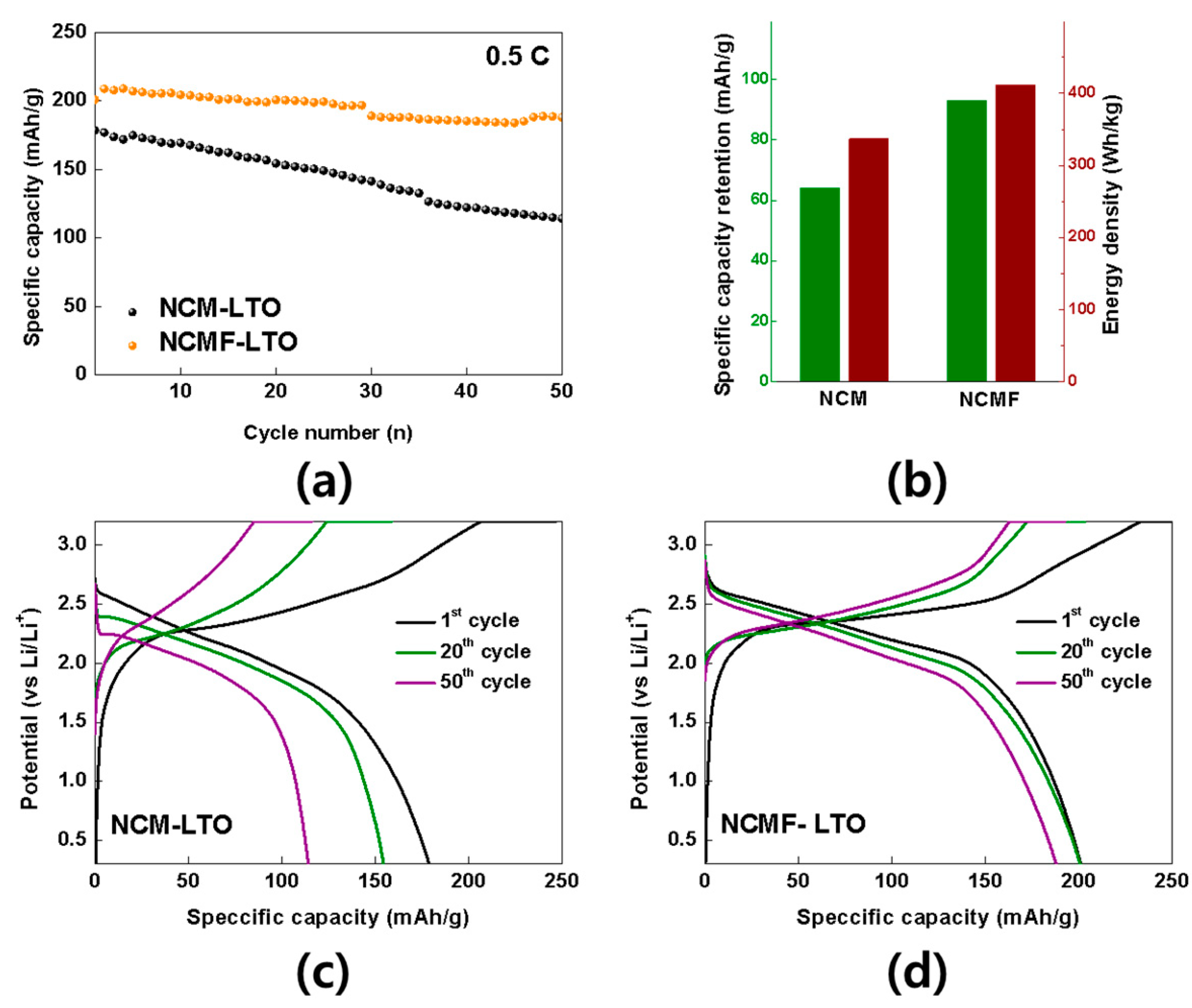
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schipper, F.; Nayak, P.K.; Erickson, E.M.; Amalraj, S.F.; Srur-Lavi, O.; Penki, T.R.; Talianker, M.; Grinblat, J.; Sclar, H.; Breuer, O.; et al. Study of Cathode Materials for Lithium-Ion Batteries: Recent Progress and New Challenges. Inorganics 2017, 5, 32. [Google Scholar] [CrossRef]
- Xia, Y.; Zheng, J.; Wang, C.; Gu, M. Designing principle for Ni-rich cathode materials with high energy density for practical applications. Nano Energy 2018, 49, 434–452. [Google Scholar] [CrossRef]
- Ali, M.U.; Zafar, A.; Nengroo, S.H.; Hussain, S.; Alvi, M.J.; Kim, H.-J. Towards a Smarter Battery Management System for Electric Vehicle Applications: A Critical Review of Lithium-Ion Battery State of Charge Estimation. Energies 2019, 12, 446. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Li, M.; Chu, L.; Jiang, B.; Ruoxu, L.; Xiaopei, Z.; Cao, G. Layered ternary metal oxides: Performance degradation mechanisms as cathodes, and design strategies for high-performance batteries. Prog. Mater. Sci. 2020, 111, 100655. [Google Scholar] [CrossRef]
- Manthiram, A. A reflection on lithium-ion battery cathode chemistry. Nature Comm. 2020, 11, 1550. [Google Scholar] [CrossRef]
- Kwon, J.-S.; Lee, S.-E.; Lim, J.-H.; Choi, J.; Kim, J. Performance and Life Degradation Characteristics Analysis of NCM LIB for BESS. Electronics 2018, 7, 406. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Zhou, F.; Liu, B.; Zhou, H.; Zhang, Q.; Kong, J.; Wang, Q. Progress in Preparation and Modification of LiNi0.6Mn0.2Co0.2O2 Cathode Material for High Energy Density Li-Ion Batteries. Int. J. Electrochem. 2018. [Google Scholar] [CrossRef] [Green Version]
- Sun, G.; Yin, X.; Yang, W.; Zhang, J.; Du, Q.; Ma, Z.; Shao, G.; Wang, Z.-B. Synergistic effects of ion doping and surface-modifying for lithium transition-metal oxide: Synthesis and characterization of La2O3-modified LiNi1/3Co1/3Mn1/3O2. Electrochim. Acta 2018, 272, 11–21. [Google Scholar] [CrossRef]
- Park, S.; Kim, D.; Ku, H.; Jo, M.; Kim, S.; Song, J.; Yu, J.; Kwon, K. The effect of Fe as an impurity element for sustainable resynthesis of Li[Ni1/3Co1/3Mn1/3]O2 cathode material from spent lithium-ion batteries. Electrochim. Acta 2019, 296, 814–822. [Google Scholar] [CrossRef]
- Liang, L.; Zhang, W.; Zhao, F.; Denis, D.K.; Zaman, F.U.; Hou, L.; Yuan, C. Surface/Interface Structure Degradation of Ni-Rich Layered Oxide Cathodes toward Lithium-Ion Batteries: Fundamental Mechanisms and Remedying Strategies. Adv. Mater. Interfaces 2020, 7, 1901749. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.S. Problems and their origins of Ni-rich layered oxide cathode materials. Energy Storage Mater. 2020, 24, 247–254. [Google Scholar] [CrossRef]
- Li, T.; Yuan, X.-Z.; Zhang, L.; Song, D.; Shi, K.; Bock, C. Degradation Mechanisms and Mitigation Strategies of Nickel-Rich, NMC-Based Lithium-Ion Batteries. Electrochem. Energy Rev. 2020, 3, 43–80. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Qi, L.; Xiao, P.; Yu, Y.; Chen, X.; Yang, W. Effect of precursor structures on the electrochemical performance of Ni-rich LiNi0.88Co0.12O2 cathode materials. Electrochim. Acta 2018, 270, 319–329. [Google Scholar] [CrossRef]
- Schipper, F.; Dixit, M.; Kovacheva, D.; Talianker, M.; Haik, O.; Grinblat, J.; Erickson, E.M.; Ghanty, C.; Major, D.T.; Markovsky, B.; et al. Stabilizing nickel-rich layered cathode materials by a high-charge cation doping strategy: Zirconium-doped LiNi0.6Co0.2Mn0.2O2. J. Mater. Chem. A 2016, 4, 16073–16084. [Google Scholar] [CrossRef]
- Xue, L.; Zhang, Q.; Zhu, X.; Gu, L.; Yue, J.; Xia, Q.; Xing, T.; Chen, T.; Yao, Y.; Xia, H. 3D LiCoO2 nanosheets assembled nanorod arrays via confined dissolution-recrystallization for advanced aqueous lithium-ion batteries. Nano Energy 2019, 56, 463–472. [Google Scholar] [CrossRef]
- Jang, B.-C.; Son, J.-T. Structural Characterization of New Composition Core–Shell Li[(Ni0.88Co0.07Al0.05)x(Ni0.55Co0.2Mn0.2Al0.05)y]O2 Spherical Particles as a Cathode Material for Lithium-Ion Batteries. J. Nanosci. Nanotechnol. 2016, 16, 10681. [Google Scholar] [CrossRef]
- Do, S.J.; Santhoshkumar, P.; Kang, S.H.; Prasanna, K.; Jo, Y.N.; Lee, C.W. Al-Doped Li[Ni0.78Co0.1Mn0.1Al0.02]O2 for High Performance of Lithium Ion Batteries. Ceram. Int. 2019, 45, 6972–6977. [Google Scholar] [CrossRef]
- Breddemann, U.; Erickson, E.M.; Davis, V.; Schipper, F.; Ellwanger, M.; Daub, M.; Hoffmann, A.; Erk, C.; Markovsky, B.; Aurbach, D.; et al. Fluorination of Li-Rich Lithium-Ion-Battery Cathode Materials by Fluorine Gas: Chemistry, Characterization, and Electrochemical Performance in Half Cells. ChemElectroChem 2019, 6, 3337–3349. [Google Scholar] [CrossRef]
- Wei, Z.; Zhao, D.; He, H.; Cao, W.; Dong, G. A noise-tolerant model parameterization method for lithium-ion battery management system. Appl. Energy 2020, 268, 114932. [Google Scholar] [CrossRef]
- Wei, Z.; Zhao, J.; Ji, D.; Tseng, K.J. A multi-timescale estimator for battery state of charge and capacity dual estimation based on an online identified model. Appl. Energy 2017, 204, 1264–1274. [Google Scholar] [CrossRef]
- Xiao, X.; Liu, X.; Qiao, L.; Li, S. A Li-ion Battery Management System Based on MCU and OZ8920. Procedia Eng. 2012, 29, 738–743. [Google Scholar] [CrossRef] [Green Version]
- Kong, F.; Liang, C.; Longo, R.C.; Yeon, D.-H.; Zheng, Y.; Park, J.-H.; Doo, S.-G.; Cho, K. Conflicting Roles of Anion Doping on the Electrochemical Performance of Li-Ion Battery Cathode Materials. Chem. Mater. 2016, 28, 6942–6952. [Google Scholar] [CrossRef]
- Binder, J.O.; Culver, S.P.; Pinedo, R.; Weber, D.A.; Friedrich, M.S.; Gries, K.I.; Volz, K.; Zeier, W.G.; Janek, J. Investigation of Fluorine and Nitrogen as Anionic Dopants in Nickel-Rich Cathode Materials for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2018, 10, 44452–44462. [Google Scholar] [CrossRef] [PubMed]
- Yue, P.; Wang, Z.; Guo, H.; Xiong, X.; Li, X. A low temperature fluorine substitution on the electrochemical performance of layered LiNi0.8Co0.1Mn0.1O2−zFz cathode materials. Electrochim. Acta 2013, 92, 1–8. [Google Scholar] [CrossRef]
- Vanaphuti, P.; Chen, J.; Cao, J.; Bigham, K.; Chen, B.; Yang, L.; Chen, H.; Wang, Y. Enhanced Electrochemical Performance of the Lithium-Manganese-Rich Cathode for Li-Ion Batteries with Na and F CoDoping. ACS Appl. Mater. Interfaces 2019, 11, 37842–37849. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, L.; Zhao, P.; Du, Z. Facile Synthesis of a High-Capacity LiNi0.8Co0.15Al0.05O2 Nanoplate Cathode with a {010} Orientation for Lithium-ion Batteries Naval Research. Int. J. Electrochem. Sci. 2018, 13, 10382–10389. [Google Scholar] [CrossRef]
- Kim, J.-H.; Park, K.-J.; Kim, S.J.; Yoon, C.S.; Sun, Y.-K. A method of increasing the energy density of layered Ni-rich Li[Ni1-2xCoxMnx]O2 cathodes(x = 0.05, 0.1, 0.2). J. Mater. Chem. A 2019, 7, 2694–2701. [Google Scholar] [CrossRef]
- Park, K.-J.; Choi, M.-J.; Maglia, F.; Kim, S.-J.; Kim, K.-H.; Yoon, C.S.; Sun, Y.-K. High-Capacity Concentration Gradient Li[Ni0.865Co0.120Al0.015]O2 Cathode for Lithium-Ion Batteries. Adv. Energy Mater. 2018, 8, 1703612. [Google Scholar] [CrossRef]
- Tang, W.; Chen, Z.; Xiong, F.; Chen, F.; Huang, C.; Gao, Q.; Wang, T.; Yang, Z.; Zhang, W. An effective etching-induced coating strategy to shield LiNi0.8Co0.1Mn0.1O2 electrode materials by LiAlO2. J. Power Sources 2019, 412, 246–254. [Google Scholar] [CrossRef]
- Li, H.; Zhou, P.; Liu, F.; Li, H.; Cheng, F.; Chen, J. Stabilizing nickel-rich layered oxide cathodes by magnesium doping for rechargeable lithium-ion batteries. Chem. Sci. 2019, 10, 1374. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Yang, H.; Li, T.; Liu, C.; Tong, H.; Chen, J.; Liu, Z.; Xia, L.; Chen, Z.; Duan, J.; et al. The Effects of Reversibility of H2-H3 Phase Transition on Ni-Rich Layered Oxide Cathode for High-Energy Lithium-Ion Batteries. Front. Chem. 2019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Q.; Chen, Q.; Zhang, Q.; Xiao, Y.; Zhong, X.; Dong, G.; Delplancke-Ogletree, M.-P.; Terryn, H.; Baert, K.; Reniers, F.; et al. In situ electrochromic efficiency of a nickel oxide thin film: Origin of electrochemical process and electrochromic degradation. J. Mater. Chem. C 2018, 6, 646. [Google Scholar] [CrossRef]
- Pei, C.; Gu, Y.; Liu, Z.; Yu, X.; Feng, L. Iron-nickel layered double hydroxide boosted by simple fluoridation with enhanced performance for electrochemical oxygen evolution reaction. ChemSusChem 2019, 12. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.K.; Ghosh, S.; Ghosal, P.; Martha, S.K. Synergistic effect of 3D electrode architecture and fluorine doping of Li1.2Ni0.15Mn0.55Co0.1O2 for high energy density lithium-ion batteries. J. Power Sources 2017, 356, 115–123. [Google Scholar] [CrossRef]
- Kim, D.; Shiiba, H.; Zettsu, N.; Yamada, T.; Kimijima, T.; Sánchez-Santolino, G.; Ishikawa, R.; Ikuhara, Y.; Teshima, K. Full picture discovery for mixed-fluorine anion effects on high-voltage spinel lithium nickel manganese oxide cathodes. NPG Asia Mater. 2017, 9. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Chen, D.; Chang, C. Improved electrochemical performance of LiNi0.8Co0.1Mn0.1O2 cathode materials via incorporation of rubidium cations into the original Li sites. RSC Adv. 2017, 7, 51721. [Google Scholar] [CrossRef] [Green Version]
- Schipper, F.; Bouzaglo, H.; Dixit, M.; Erickson, E.M.; Weigel, T.; Talianker, M.; Grinblat, J.; Burstein, L.; Schmidt, M.; Lampert, J.; et al. From Surface ZrO2 Coating to Bulk Zr Doping by High Temperature Annealing of Nickel-Rich Lithiated Oxides and Their Enhanced Electrochemical Performance in Lithium Ion Batteries. Adv. Energy Mater. 2017, 8, 1701682. [Google Scholar] [CrossRef]
- Subramaniyam, C.M.; Celio, H.; Shiva, K.; Gao, H.; Goodneough, J.B.; Liu, H.K.; Dou, S.X. Long stable cycling of fluorine-doped nickel-rich layered cathodes for lithium batteries. Sustain. Energy Fuels 2017, 1, 1292. [Google Scholar] [CrossRef]
- Choi, S.; Kim, M.-C.; Moon, S.-H.; Kim, H.; Park, K.-W. F-doped Li1.15Ni0.275Ru0.575O2 cathode materials with long cycle life and improved rate performance. Electrochim. Acta 2019, 326, 135015. [Google Scholar] [CrossRef]
- Kim, H.; Kim, M.-C.; Choi, S.; Moon, S.-H.; Kim, Y.-S.; Park, K.-W. Facile one-pot synthesis of Ge/TiO2 nanocomposite structures with improved electrochemical performance. Nanoscale 2019, 11, 17415. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, H.; Tan, M.; Liu, J.; Hu, Y.; Liu, S.; Shu, X.; Li, H.; Ran, Q.; Cai, J.; et al. Yttrium modified Ni-rich LiNi0.8Co0.1Mn0.1O2 with enhanced electrochemical performance as high energy density cathode material at 4.5 V high voltage. J. Alloys Compd. 2019, 774, 82–92. [Google Scholar] [CrossRef]
- Yang, J.; Xia, Y. Suppressing the Phase Transition of the Layered Ni-Rich Oxide Cathode during High-Voltage Cycling by Introducing Low-Content Li2MnO3. ACS Appl. Mater. Interfaces 2016, 8, 1297–1308. [Google Scholar] [CrossRef] [PubMed]
- Jung, R.; Metzger, M.; Maglia, F.; Stinner, C.; Gasteiger, H.A. Oxygen Release and Its Effect on the Cycling Stability of LiNixMnyCozO2 (NMC) Cathode Materials for Li-Ion Batteries. J. Electrochem. Soc. 2017, 164, A1361–A1377. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.; Kim, S.-B.; Park, D.-H.; Park, K.-W. Fluorine-Doped LiNi0.8Mn0.1Co0.1O2 Cathode for High-Performance Lithium-Ion Batteries. Energies 2020, 13, 4808. https://doi.org/10.3390/en13184808
Kim H, Kim S-B, Park D-H, Park K-W. Fluorine-Doped LiNi0.8Mn0.1Co0.1O2 Cathode for High-Performance Lithium-Ion Batteries. Energies. 2020; 13(18):4808. https://doi.org/10.3390/en13184808
Chicago/Turabian StyleKim, Hyeona, Sung-Beom Kim, Deok-Hye Park, and Kyung-Won Park. 2020. "Fluorine-Doped LiNi0.8Mn0.1Co0.1O2 Cathode for High-Performance Lithium-Ion Batteries" Energies 13, no. 18: 4808. https://doi.org/10.3390/en13184808




