Assessment of Multiple Anaerobic Co-Digestions and Related Microbial Community of Molasses with Rice-Alcohol Wastewater
Abstract
1. Introduction
2. Materials and Methods
2.1. Sludge and Wastewater
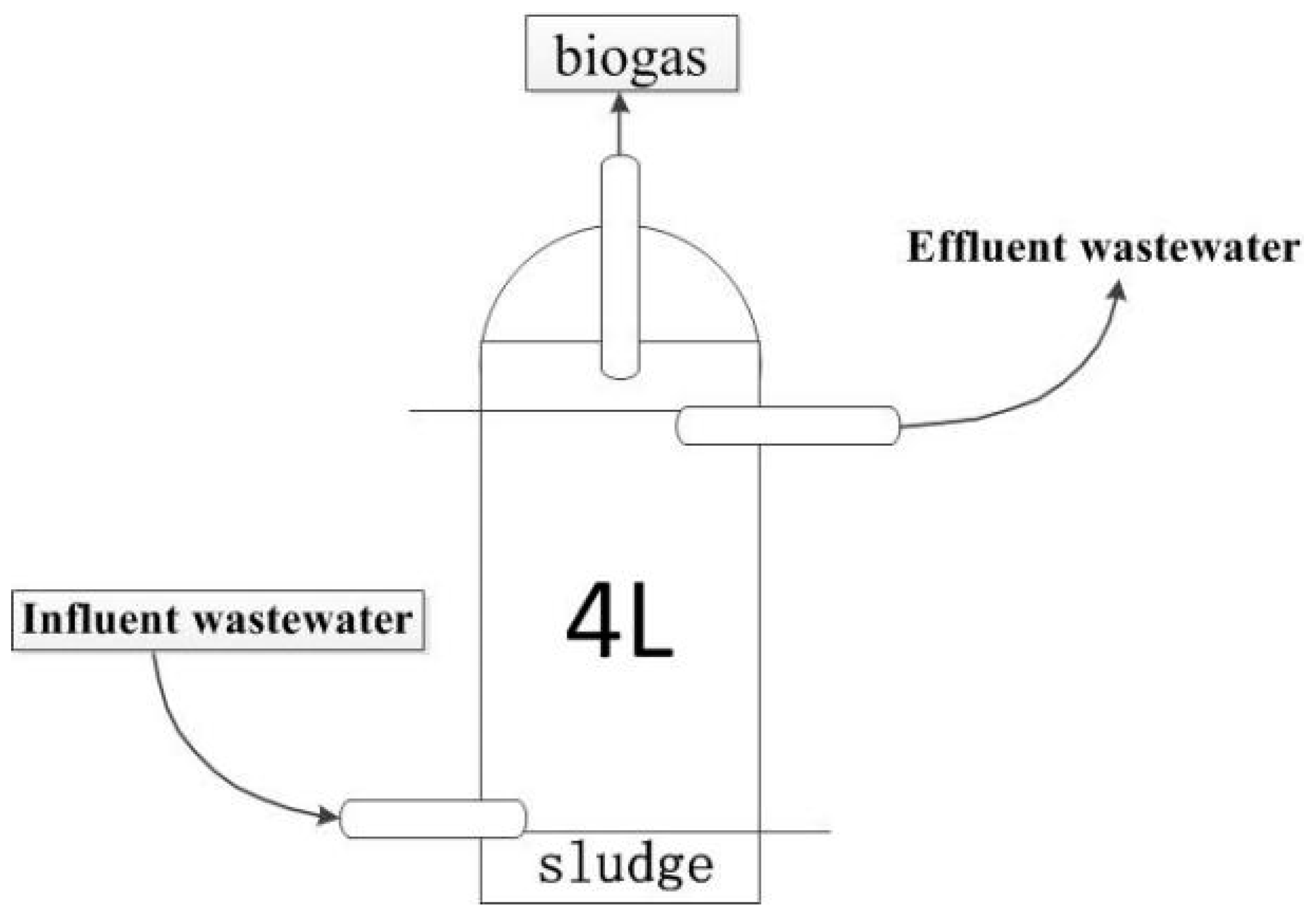
2.2. Digesters Setup
2.3. Operating Conditions for Co-Digestion and Mono-Digestion Experiment
2.4. Analytical Methods
2.5. High-Throughput Sequencing for Microbial Community
Extraction of DNA, PCR Amplification and Sequencing
3. Results and Discussion
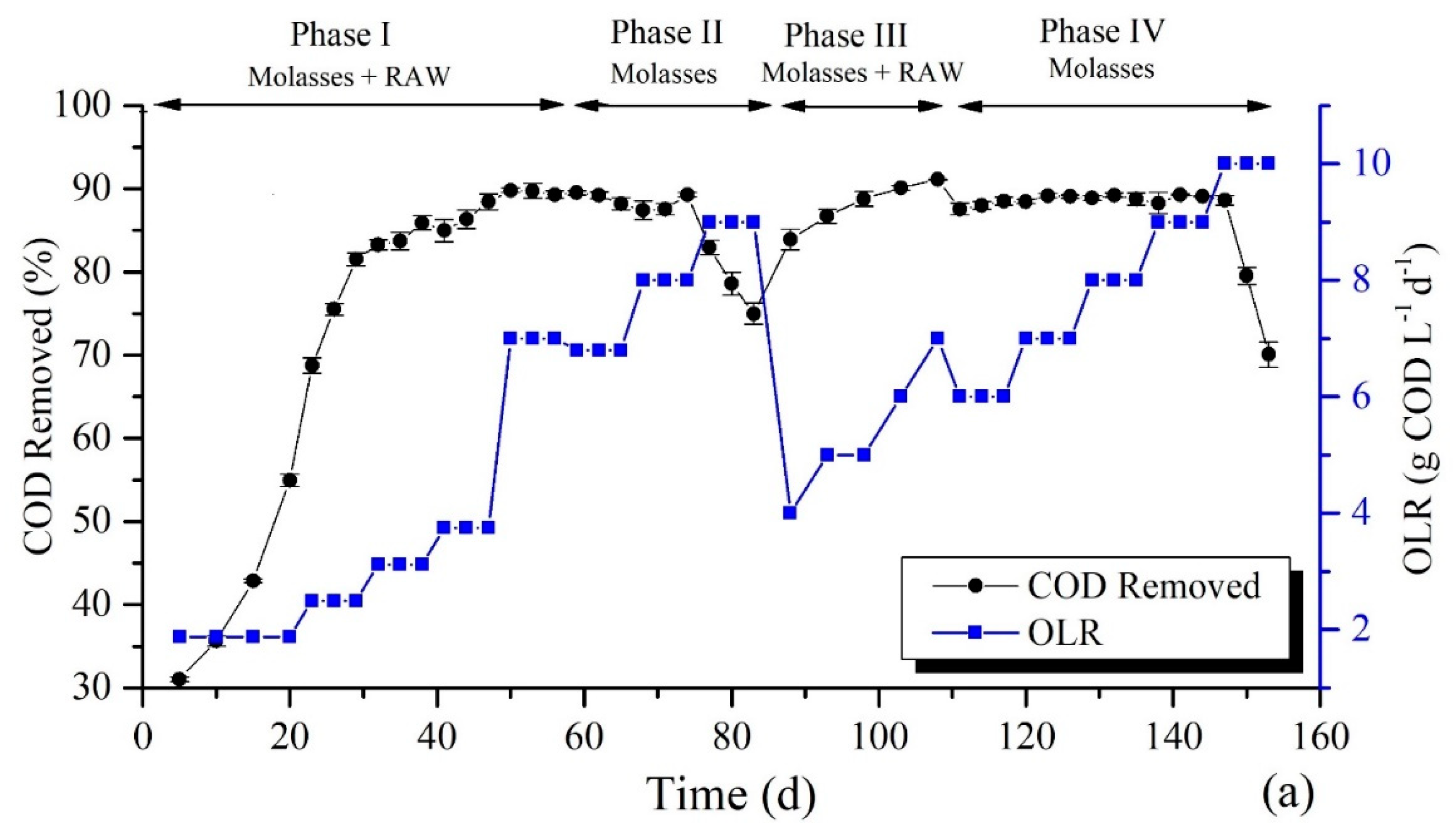
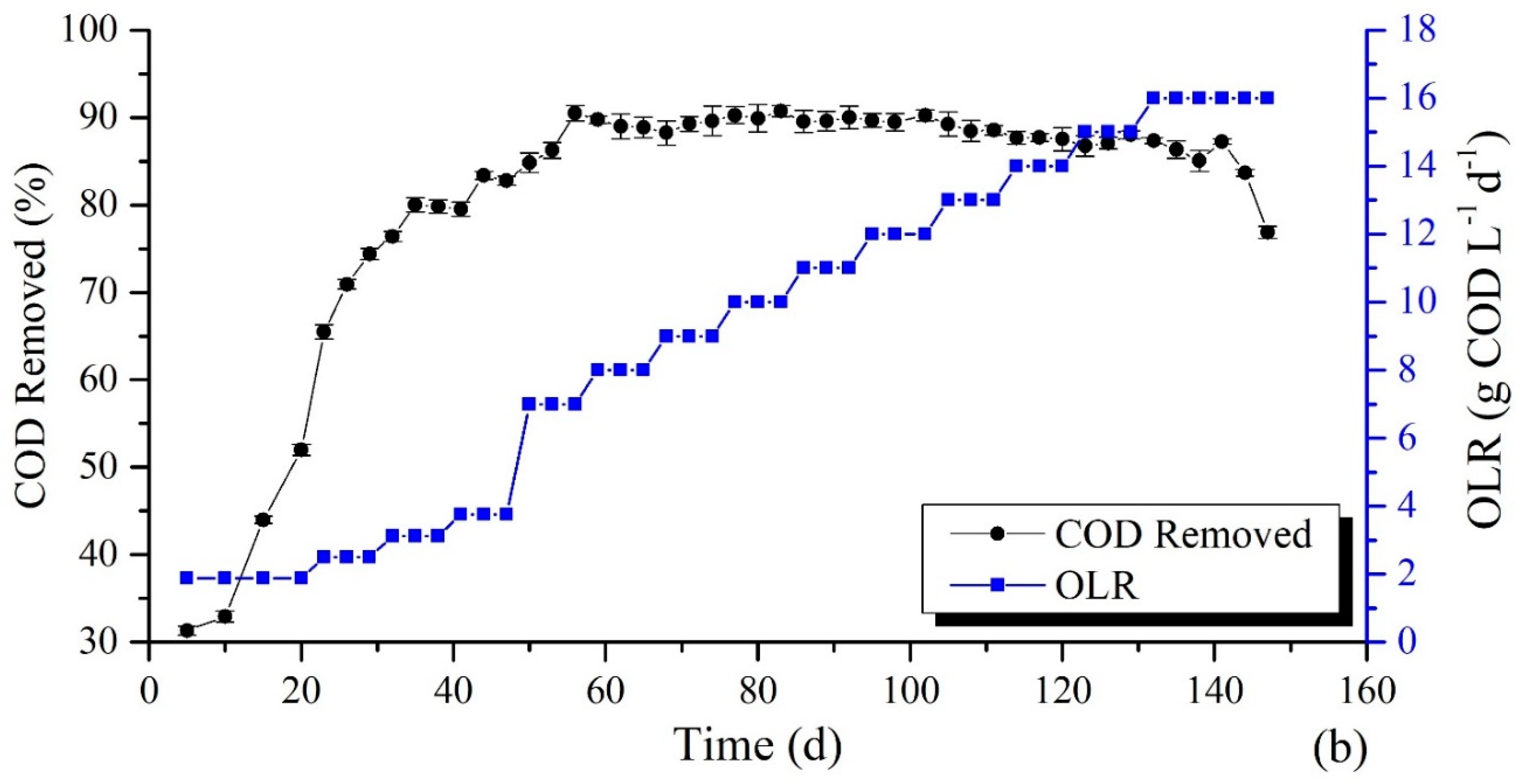
3.1. Impact of Operating Conditions on COD Removal Rate
Mono-Digestion and Co-Digestion Process
3.2. Methane Contents in Biogas under Different OLR Conditions
3.3. Impact of Increasing OLR on pH
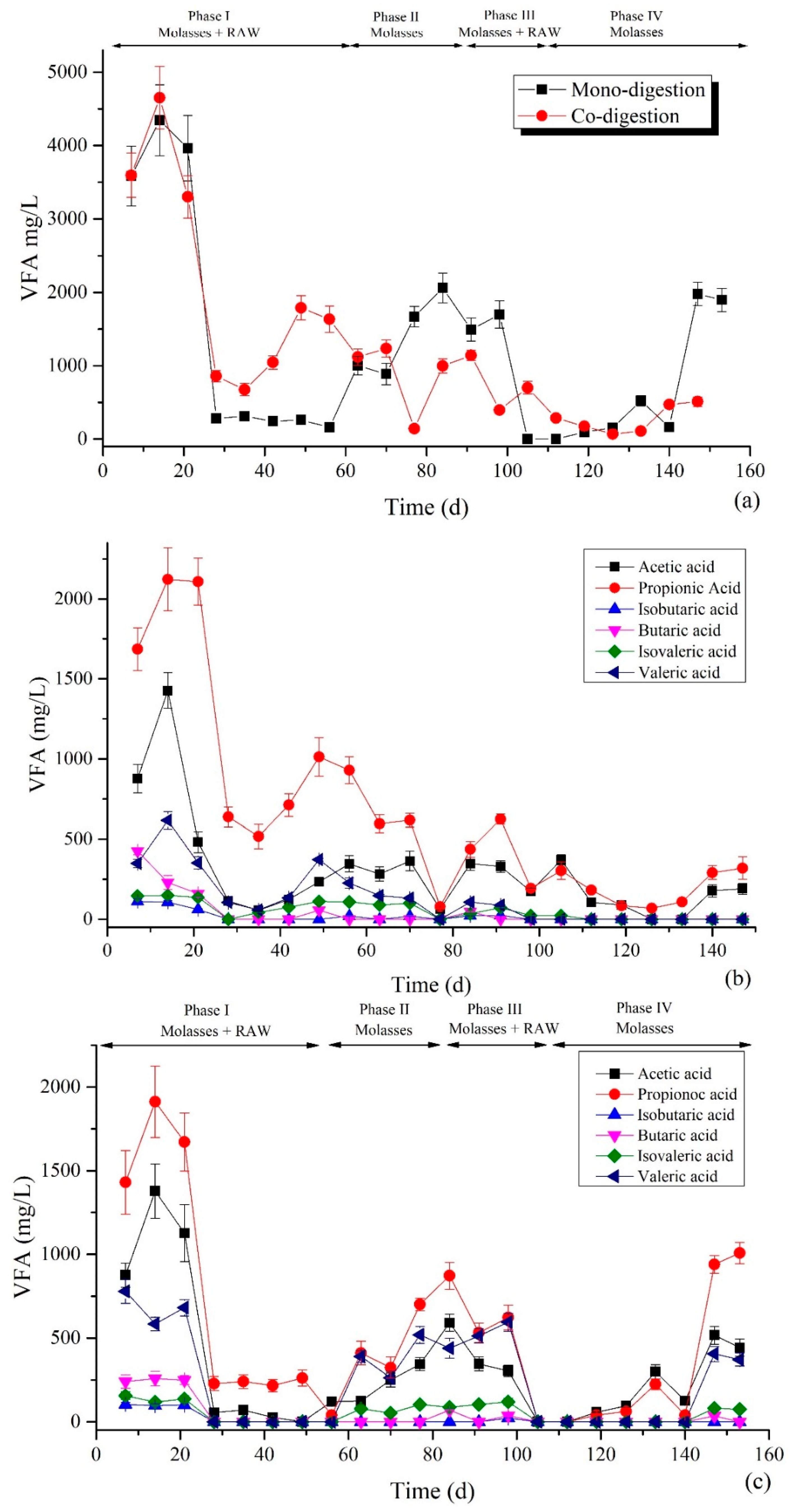
3.4. Detection of VFAs Concentration
3.5. Microbial Community
3.5.1. Sequencing and Microbial Community in Response to Increasing OLRs
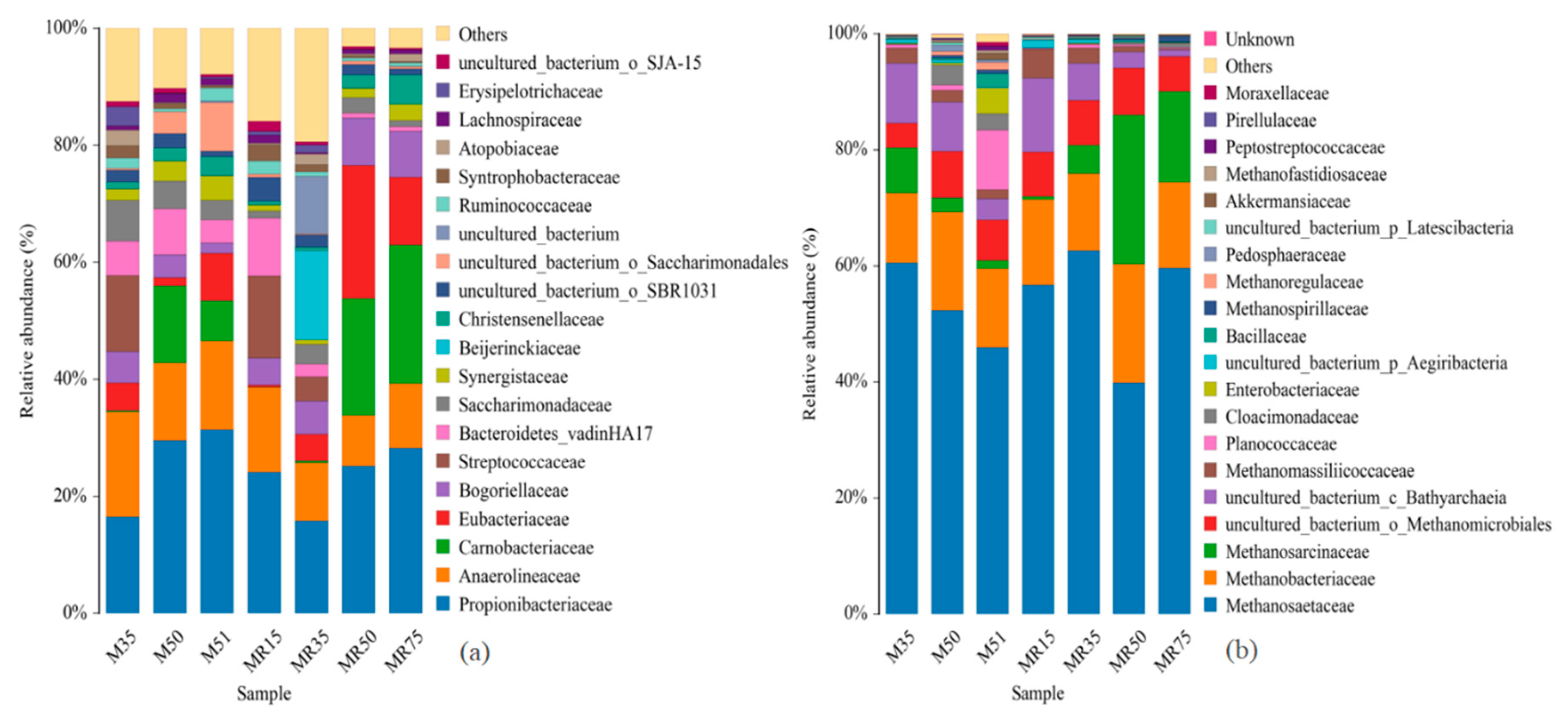
Composition of Bacterial Community
Composition of Archaeal Community
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Scoma, A.; Coma, M.; Kerckhof, F.M.; Boon, N.; Rabaey, K. Efficient molasses fermentation under high salinity by inocula of marine and terrestrial origin. Biotechnol. Biofuels 2017, 10, 23. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.A.; Fuess, L.T.; Soares, L.A.; Damianovic, M.H.R.Z. Thermophilic biohydrogen production from sugarcane molasses under low pH: Metabolic and microbial aspects. Int. J. Hydrogen Energy 2020, 7, 4182–4192. [Google Scholar] [CrossRef]
- Poggi-Varaldo, H.M.; Munoz-Paez, K.M.; Escamilla-Alvarado, C.; Robledo-Narváez, P.N.; Ponce-Noyola, M.T.; Calva-Calva, G.; Rinderknecht-Seijas, N.F. Biohydrogen, biomethane and bioelectricity as crucial components of biorefinery of organic wastes: A review. Waste Manag. Res. 2014, 32, 353–365. [Google Scholar] [CrossRef] [PubMed]
- Kongjan, P.; Sompong, O.; Angelidaki, I. Performance and microbial community analysis of two-stage process with extreme thermophilic hydrogen and thermophilic methane production from hydrolysate in UASB reactors. Bioresour. Technol. 2011, 102, 4028–4035. [Google Scholar] [CrossRef] [PubMed]
- Keskin, T.; Hallenbeck, P.C. Hydrogen production from sugar industry wastes using single-stage photofermentation. Bioresour. Technol. 2012, 112, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Scoma, A.; Rebecchi, S.; Bertin, L.; Fava, F. High impact biowastes from South European agro-industries as feedstock for second-generation biorefineries. Crit. Rev. Biotechnol. 2016, 36, 175–189. [Google Scholar] [CrossRef]
- Detman, A.; Chojnacka, A.; Błaszczyk, M.; Kaźmierczak, W.; Piotrowski, J.; Sikora, A. Biohydrogen and biomethane (Biogas) production in the consecutive stages of anaerobic digestion of molasses. Pol. J. Environ. Stud. 2017, 26, 1023–1029. [Google Scholar] [CrossRef]
- Shen, P.; Han, F.; Su, S.; Zhang, J.; Chen, Z.; Li, J.; Wu, B. Using pig manure to promote fermentation of sugarcane molasses alcohol wastewater and its effects on microbial community structure. Bioresour. Technol. 2014, 155, 323–329. [Google Scholar] [CrossRef]
- Dareioti, M.A.; Dokianakis, S.N.; Stamatelatou, K.; Zafiri, C.; Kornaros, M. Biogas production from anaerobic co-digestion of agroindustrial wastewaters under mesophilic conditions in a two-stage process. Desalination 2009, 248, 891–906. [Google Scholar] [CrossRef]
- Calabrò, P.S.; Gori, M.; Lubello, C. European trends in greenhouse gases emissions from integrated solid waste management. Environ. Technol. 2015, 36, 2125–2137. [Google Scholar] [CrossRef]
- Calabrò, P.S.; Panzera, M.F. Biomethane production tests on ensiled orange peel waste. Int. J. Heat Technol. 2017, 35, S130–S136. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, R.; Chen, C.; Liu, G.; He, Y.; Liu, X. Biogas production from co-digestion of corn stover and chicken manure under anaerobic wet, hemi-solid, and solid state conditions. Bioresour. Technol. 2013, 149, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Yu, Z.; Zhao, J.; Zhang, H. Do microbial communities in an anaerobic bioreactor change with continuous feeding sludge into a full-scale anaerobic digestion system? Bioresour. Technol. 2018, 249, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Jiang, Q.; Qian, F.; Zhou, Q.; Jiang, C.; Shen, P. Semi-continuous feeding combined with traditional domestication improved anaerobic performance during treatment of cassava stillage. Bioresour. Technol. 2019, 291, 121807. [Google Scholar] [CrossRef] [PubMed]
- Shah, F.A.; Mahmood, Q.; Rashid, N.; Pervez, A.; Raja, I.A.; Shah, M.M. Co-digestion, pretreatment and digester design for enhanced methanogenesis. Renew. Sustain. Energy Rev. 2015, 42, 627–642. [Google Scholar] [CrossRef]
- Vanwonterghem, I.; Jensen, P.D.; Ho, D.P.; Batstone, D.J.; Tyson, G.W. Linking microbial community structure, interactions and function in anaerobic digesters using new molecular techniques. Curr. Opin. Biotechnol. 2014, 27, 55–64. [Google Scholar] [CrossRef]
- Wang, P.; Wang, H.; Qiu, Y.; Ren, L.; Jiang, B. Microbial characteristics in anaerobic digestion process of food waste for methane production–A review. Bioresour. Technol. 2018, 248, 29–36. [Google Scholar] [CrossRef]
- Werner, T. Next generation sequencing in functional genomics. Brief. Bioinform. 2010, 11, 499–511. [Google Scholar] [CrossRef]
- De Vrieze, J.; Verstraete, W.; Boon, N. Repeated pulse feeding induces functional stability in anaerobic digestion. Microb. Biotechnol. 2013, 6, 414–424. [Google Scholar] [CrossRef]
- Yu, H.; Zhu, Z.; Hu, W.; Zhang, H. Hydrogen production from rice winery wastewater in an upflow anaerobic reactor by using mixed anaerobic cultures. Int. J. Hydrogen Energy 2002, 27, 1359–1365. [Google Scholar] [CrossRef]
- Yu, H.; Gu, G. Biomethanation of brewery wastewater using an anaerobic upflow blanket filter. J. Clean. Prod. 1996, 4, 219–223. [Google Scholar] [CrossRef]
- Lukitawesa; Patinvoh, R.J.; Millati, R.; Sárvári-Horváth, I.; Taherzadeh, M.J. Factors influencing volatile fatty acids production from food wastes via anaerobic digestion. Bioengineered 2020, 11, 39–52. [Google Scholar] [CrossRef] [PubMed]
- De Vrieze, J.; Hennebel, T.; Van den Brande, J.; Ro’il, M.B.; Bruton, T.A.; Vankelecom, I.F.; Boon, N. Anaerobic digestion of molasses by means of a vibrating and non-vibrating submerged anaerobic membrane bioreactor. Biomass Bioenergy 2014, 68, 95–105. [Google Scholar] [CrossRef]
- Fang, C.; Boe, K.; Angelidaki, I. Anaerobic co-digestion of desugared molasses with cow manure; focusing on sodium and potassium inhibition. Bioresour. Technol. 2011, 102, 1005–1011. [Google Scholar] [CrossRef]
- Labatut, R.A.; Angenent, L.T.; Scott, N.R. Biochemical methane potential and biodegradability of complex organic substrates. Bioresour. Technol. 2011, 102, 2255–2264. [Google Scholar] [CrossRef]
- Pellera, F.M.; Gidarakos, E. Anaerobic digestion of solid agroindustrial waste in semi-continuous mode: Evaluation of mono-digestion and co-digestion systems. J. Waste Manag. 2017, 68, 103–119. [Google Scholar] [CrossRef]
- Lateef, S.A.; Beneragama, N.; Yamashiro, T.; Iwasaki, M.; Ying, C.; Umetsu, K. Biohydrogen production from co-digestion of cow manure and waste milk under thermophilic temperature. Bioresour. Technol. 2012, 110, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Neshat, S.A.; Mohammadi, M.; Najafpour, G.D.; Lahijani, P. Anaerobic co-digestion of animal manures and lignocellulosic residues as a potent approach for sustainable biogas production. Renew. Sustain. Energy Rev. 2017, 79, 308–322. [Google Scholar] [CrossRef]
- Eslami, H.; Hashemi, H.; Fallahzadeh, R.A.; Khosravi, R.; Fard, R.F.; Ebrahimi, A.A. Effect of organic loading rates on biogas production and anaerobic biodegradation of composting leachate in the anaerobic series bioreactors. Ecol. Eng. 2018, 110, 165–171. [Google Scholar] [CrossRef]
- De Vrieze, J.; Plovie, K.; Verstraete, W.; Boon, N. Co-digestion of molasses or kitchen waste with high-rate activated sludge results in a diverse microbial community with stable methane production. J. Environ. Manag. 2015, 152, 75–82. [Google Scholar] [CrossRef]
- Mu, L.; Zhang, L.; Zhu, K.; Ma, J.; Ifran, M.; Li, A. Anaerobic co-digestion of sewage sludge, food waste and yard waste: Synergistic enhancement on process stability and biogas production. Sci. Total Environ. 2020, 704, 135429. [Google Scholar] [CrossRef]
- Rodríguez-Abalde, Á.; Guivernau, M.; Prenafeta-Boldú, F.X.; Flotats, X.; Fernández, B. Characterization of microbial community dynamics during the anaerobic co-digestion of thermally pre-treated slaughterhouse wastes with glycerin addition. Bioprocess Biosyst. Eng. 2019, 42, 1175–1184. [Google Scholar] [CrossRef]
- Meng, X.; Yuan, X.; Ren, J.; Wang, X.; Zhu, W.; Cui, Z. Methane production and characteristics of the microbial community in a two-stage fixed-bed anaerobic reactor using molasses. Bioresour. Technol. 2017, 241, 1050–1059. [Google Scholar] [CrossRef]
- Capson-Tojo, G.; Rouez, M.; Crest, M.; Steyer, J.P.; Delgenès, J.P.; Escudié, R. Food waste valorization via anaerobic processes: A review. Rev. Environ. Sci. Bio/Technol. 2016, 15, 499–547. [Google Scholar] [CrossRef]
- Balussou, D.; Kleyböcker, A.; McKenna, R.; Möst, D.; Fichtner, W. An economic analysis of three operational co-digestion biogas plants in Germany. Waste Biomass Valoriz. 2012, 3, 23–41. [Google Scholar] [CrossRef]
- Yang, F.; Li, W.; Sun, M.; Li, Q.; Wang, M.; Sun, Y. Improved buffering capacity and methane production by anaerobic co-digestion of corn stalk and straw depolymerization wastewater. Energies 2018, 11, 1751. [Google Scholar] [CrossRef]
- Lee, D.J.; Lee, S.Y.; Bae, J.S.; Kang, J.G.; Kim, K.H.; Rhee, S.S.; Seo, D.C. Effect of volatile fatty acid concentration on anaerobic degradation rate from field anaerobic digestion facilities treating food waste leachate in South Korea. J. Chem. 2015, 2015, 9. [Google Scholar] [CrossRef]
- Björnsson, L.; Murto, M.; Mattiasson, B. Evaluation of parameters for monitoring an anaerobic co-digestion process. Appl. Microbiol. Biotechnol. 2000, 54, 844–849. [Google Scholar] [CrossRef]
- Hameed, S.A.; Riffat, R.; Li, B.; Naz, I.; Badshah, M.; Ahmed, S.; Ali, N. Microbial population dynamics in temperature-phased anaerobic digestion of municipal wastewater sludge. J. Chem. Technol. Biotechnol. 2019, 94, 1816–1831. [Google Scholar] [CrossRef]
- Zhang, X.; Qu, Y.; Ma, Q.; Zhang, Z.; Li, D.; Wang, J.; Zhou, J. Illumina MiSeq sequencing reveals diverse microbial communities of activated sludge systems stimulated by different aromatics for indigo biosynthesis from indole. PLoS ONE 2015, 10, e0125732. [Google Scholar] [CrossRef]
- Cui, H.; Su, X.; Wei, S.; Zhu, Y.; Lu, Z.; Wang, Y.; Pang, S. Comparative Analyses of Methanogenic and Methanotrophic Communities between Two Different Water Regimes in Controlled Wetlands on the Qinghai-Tibetan Plateau, China. Curr. Microbiol. 2018, 75, 484–491. [Google Scholar] [CrossRef]
- Gagliano, M.C.; Ismail, S.B.; Stams, A.J.M.; Plugge, C.M.; Temmink, H.; Van Lier, J.B. Biofilm formation and granule properties in anaerobic digestion at high salinity. Water Res. 2017, 121, 61–71. [Google Scholar] [CrossRef]



| COD g/L | BOD g/L | TN | pH | |
|---|---|---|---|---|
| Molasses | 862.842–935.62 | 486.35–618.46 | 1.42–2.2 | 5.7–6.2 |
| RAW | 35–40 | 18.4–21.67 | 0.08–0.16 | 4.6–5.2 |
| S.No. | Run Time (Days) | Influent COD(g/L) | OLR (g COD/L.d) | HRT (Days) | Flow Rate (L/d) | M:RAW |
|---|---|---|---|---|---|---|
| 1 | 0–20 | 15 | 1.875 | 8 | 0.5 | 1:1 |
| 2 | 21–29 | 20 | 2.5 | 8 | 0.5 | 1:1 |
| 3 | 30–38 | 25 | 3.12 | 8 | 0.5 | 2:1 |
| 4 | 39–47 | 30 | 6 | 5 | 0.8 | 3:1 |
| 5 | 48–56 | 35 | 7 | 5 | 0.8 | 4:1 |
| 6 | 57–65 | 40 | 8 | 5 | 0.8 | 5:1 |
| 7 | 66–74 | 45 | 9 | 5 | 0.8 | 5:1 |
| 8 | 75–83 | 50 | 10 | 5 | 0.8 | 5:1 |
| 9 | 84–92 | 55 | 11 | 5 | 0.8 | 5:1 |
| 10 | 93–101 | 60 | 12 | 5 | 0.8 | 5:1 |
| 11 | 102–111 | 65 | 13 | 5 | 0.8 | 6:1 |
| 12 | 112–120 | 70 | 14 | 5 | 0.8 | 6:1 |
| 13 | 121–129 | 75 | 15 | 5 | 0.8 | 7:1 |
| 14 | 130–147 | 80 | 16 | 5 | 0.8 | 8:1 |
| S.No | Run Time (Days) | Influent COD (g/L) | OLR (g COD/L.d) | HRT (Days) | Flow Rate (L/d) | M:RAW |
|---|---|---|---|---|---|---|
| 1 | 0–20 | 15 | 1.875 | 8 | 0.5 | 1:1 |
| 2 | 21–29 | 20 | 2.5 | 8 | 0.5 | 1:1 |
| 3 | 30–38 | 25 | 3.12 | 8 | 0.5 | 2:1 |
| 4 | 39–47 | 30 | 6 | 5 | 0.8 | 3:1 |
| 5 | 48–56 | 35 | 7 | 5 | 0.8 | 4:1 |
| Remove RAW and feed only molasses | ||||||
| 6 | 57–65 | 35 | 7 | 5 | 0.8 | 1:00 |
| 7 | 66–74 | 40 | 8 | 5 | 0.8 | 1:00 |
| 8 | 75–83 | 45 | 9 | 5 | 0.8 | 1:00 |
| Acclimatization of reactors with co-digestion of molasses and RAW | ||||||
| 9 | 84–88 | 20 | 4 | 5 | 0.8 | 3:1 |
| 10 | 89–98 | 25 | 5 | 5 | 0.8 | 4:1 |
| 11 | 99–103 | 30 | 6 | 5 | 0.8 | 4:1 |
| 12 | 104–108 | 35 | 7 | 5 | 0.8 | 4:1 |
| Remove RAW and feed only molasses | ||||||
| 13 | 109–117 | 30 | 6 | 5 | 0.8 | 1:00 |
| 14 | 118–126 | 35 | 7 | 5 | 0.8 | 1:00 |
| 15 | 127–135 | 40 | 8 | 5 | 0.8 | 1:00 |
| 16 | 136–144 | 45 | 9 | 5 | 0.8 | 1:00 |
| 17 | 145–153 | 50 | 10 | 5 | 0.8 | 1:00 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, S.; Lu, F.; Jiang, Q.; Jiang, C.; Kashif, M.; Shen, P. Assessment of Multiple Anaerobic Co-Digestions and Related Microbial Community of Molasses with Rice-Alcohol Wastewater. Energies 2020, 13, 4866. https://doi.org/10.3390/en13184866
Khan S, Lu F, Jiang Q, Jiang C, Kashif M, Shen P. Assessment of Multiple Anaerobic Co-Digestions and Related Microbial Community of Molasses with Rice-Alcohol Wastewater. Energies. 2020; 13(18):4866. https://doi.org/10.3390/en13184866
Chicago/Turabian StyleKhan, Sohail, Fuzhi Lu, Qiong Jiang, Chengjian Jiang, Muhammad Kashif, and Peihong Shen. 2020. "Assessment of Multiple Anaerobic Co-Digestions and Related Microbial Community of Molasses with Rice-Alcohol Wastewater" Energies 13, no. 18: 4866. https://doi.org/10.3390/en13184866
APA StyleKhan, S., Lu, F., Jiang, Q., Jiang, C., Kashif, M., & Shen, P. (2020). Assessment of Multiple Anaerobic Co-Digestions and Related Microbial Community of Molasses with Rice-Alcohol Wastewater. Energies, 13(18), 4866. https://doi.org/10.3390/en13184866





