Solar-hybrid Thermochemical Gasification of Wood Particles and Solid Recovered Fuel in a Continuously-Fed Prototype Reactor
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Beechwood Gasification
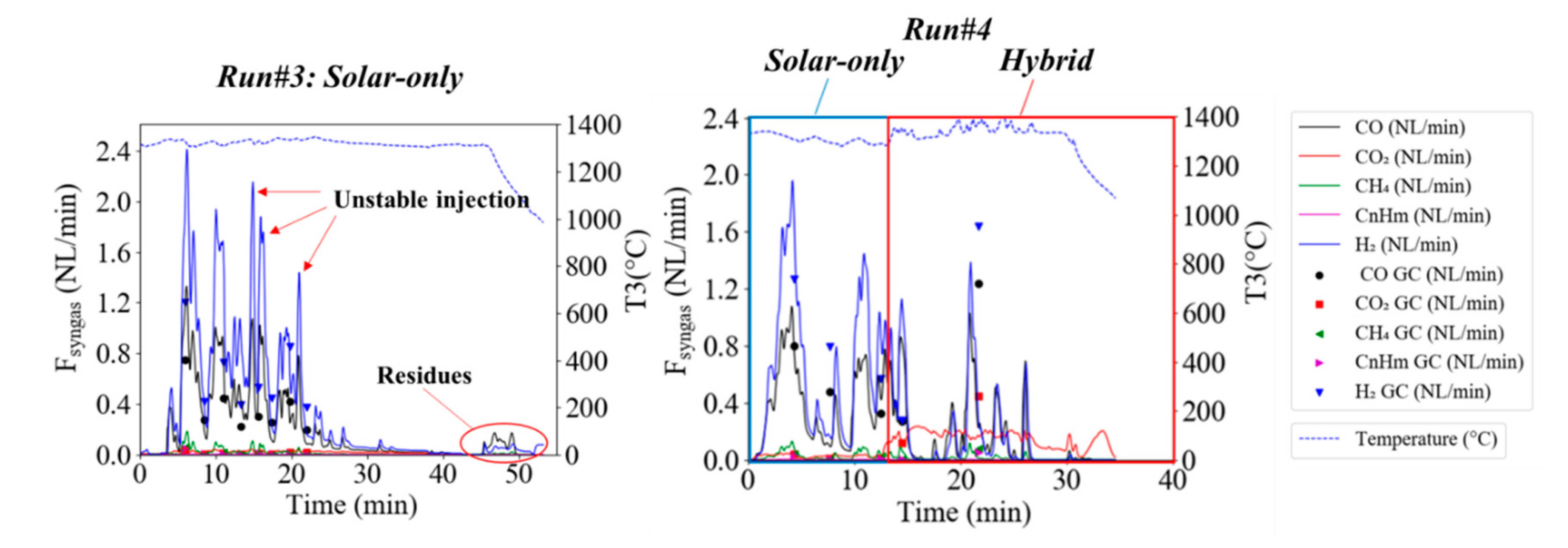
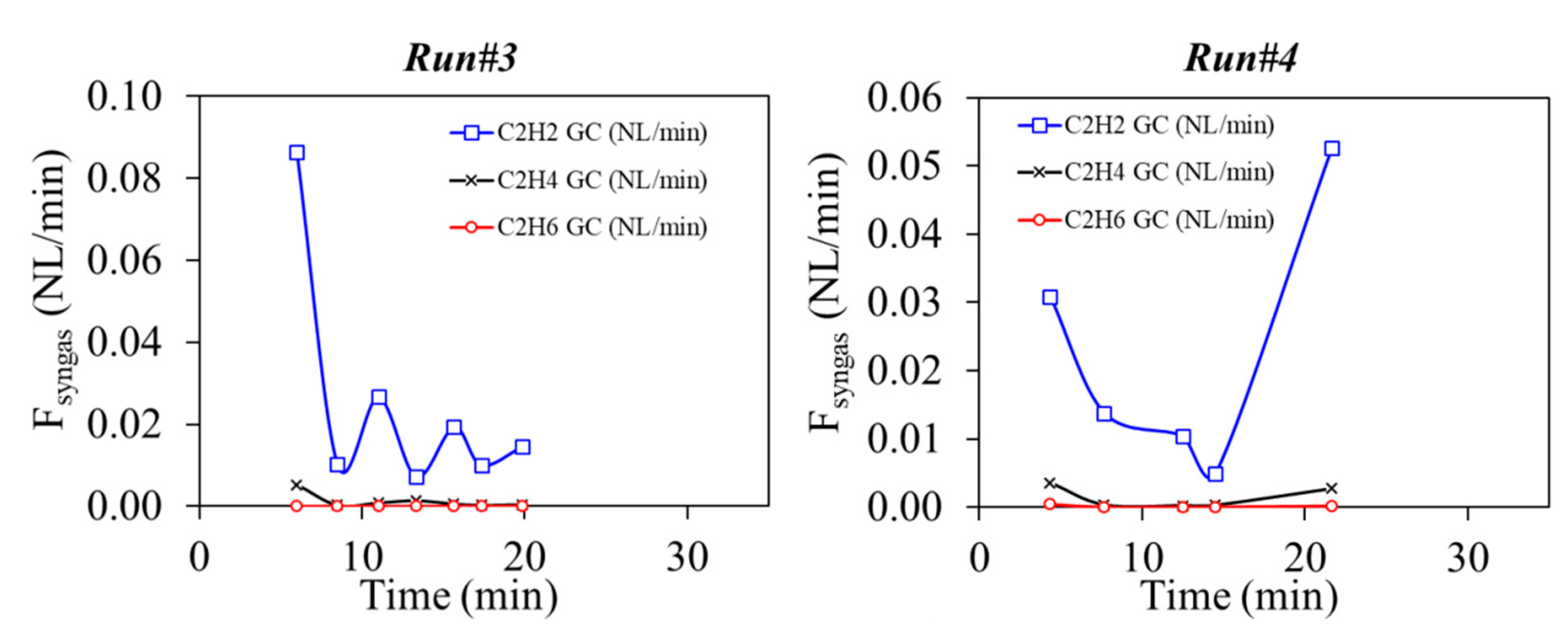
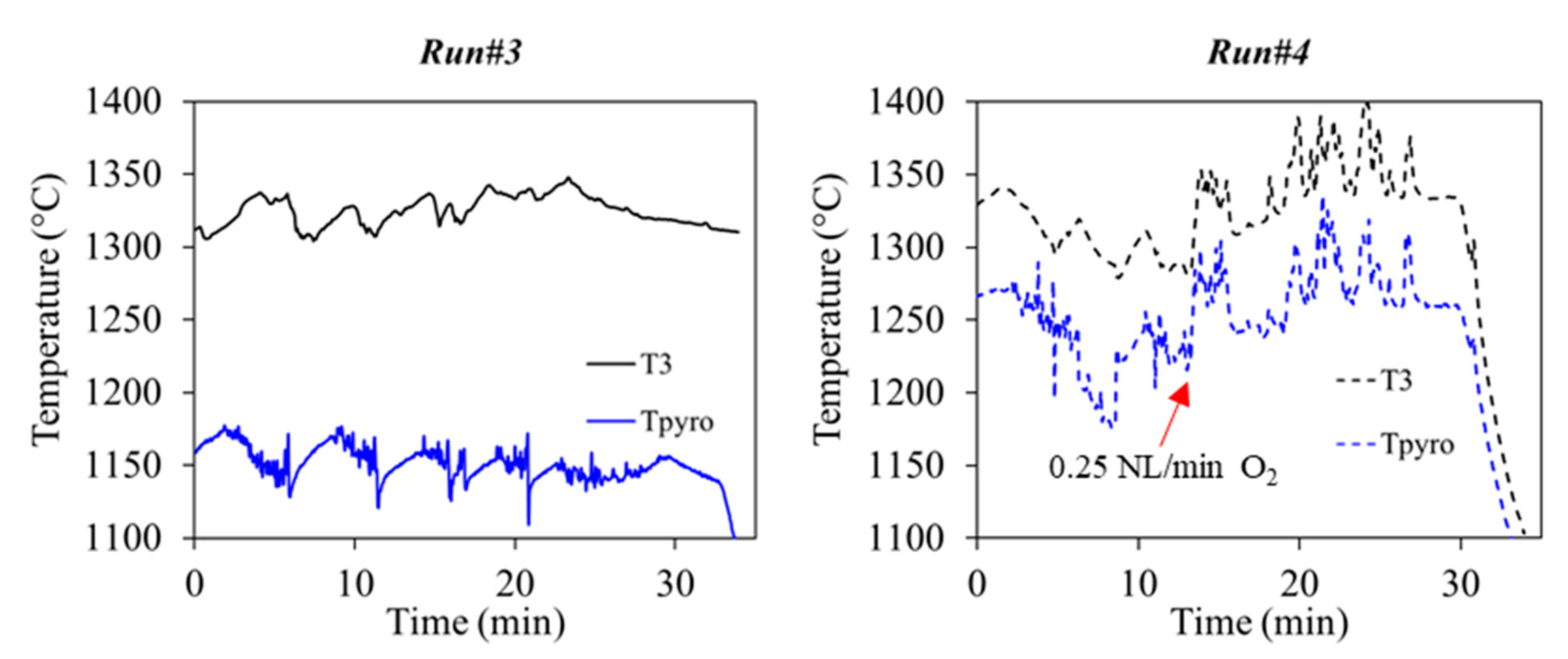
3.2. Solid Recovered Fuels Gasification
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Probstein, R.F.; Hicks, E.H. Synthetic Fuels; Dover Publications Inc.: Mineola, NY, USA, 2006; p. 11501. [Google Scholar]
- Breault, R.W. Gasification processes old and new: A basic review of the major technologies. Energies 2010, 3, 216–240. [Google Scholar] [CrossRef] [Green Version]
- Jafri, Y.; Furusjö, E.; Kirtania, K.; Gebart, B.R. Performance of a pilot-scale entrained-flow black liquor gasifier. Energy Fuels 2016, 30, 3175–3185. [Google Scholar] [CrossRef]
- Larsson, A.; Seemann, M.; Neves, D.; Thunman, H. Evaluation of performance of industrial-scale dual fluidized bed gasifiers using the chalmers 2–4-MWth gasifier. Energy Fuels 2013, 27, 6665–6680. [Google Scholar] [CrossRef]
- Pfeifer, C.; Koppatz, S.; Hofbauer, H. Steam gasification of various feedstocks at a dual fluidised bed gasifier: Impacts of operation conditions and bed materials. Biomass Convers. Biorefinery 2011, 1, 39–53. [Google Scholar] [CrossRef]
- Valin, S.; Ravel, S.; De Vincent, P.P.; Thiery, S.; Miller, H. Fluidized bed air gasification of solid recovered fuel and woody biomass: Influence of experimental conditions on product gas and pollutant release. Fuel 2019, 242, 664–672. [Google Scholar] [CrossRef]
- Wu, H.; Liu, Q.; Bai, Z.; Xie, G.; Zheng, J.; Su, B. Thermodynamics analysis of a novel steam/air biomass gasification combined cooling, heating and power system with solar energy. Appl. Therm. Eng. 2020, 164, 114494. [Google Scholar] [CrossRef]
- Guo, P.; Saw, W.L.; Van Eyk, P.; Ashman, P.; Nathan, G.; Stechel, E. Fischer-tropschliquid fuel production by co-gasification of coal and biomass in a solar hybrid dual fluidized bed gasifier. Energy Procedia 2015, 69, 1770–1779. [Google Scholar] [CrossRef] [Green Version]
- Bruckner, A.P. Continuous duty solar coal gasification system using molten slag and direct-contact heat exchange. Sol. Energy 1985, 34, 239–247. [Google Scholar] [CrossRef]
- Hathaway, B.J.; Davidson, J.H. Demonstration of a prototype molten salt solar gasification reactor. Sol. Energy 2017, 142, 224–230. [Google Scholar] [CrossRef]
- Loutzenhiser, P.G.; Muroyama, A.P. A review of the state-of-the-art in solar-driven gasification processes with carbonaceous materials. Sol. Energy 2017, 156, 93–100. [Google Scholar] [CrossRef]
- Arnavat, M.P.; Tora, E.; Bruno, J.C.; Coronas, A. State of the art on reactor designs for solar gasification of carbonaceous feedstock. Sol. Energy 2013, 97, 67–84. [Google Scholar] [CrossRef]
- Yadav, D.; Banerjee, R. A review of solar thermochemical processes. Renew. Sustain. Energy Rev. 2016, 54, 497–532. [Google Scholar] [CrossRef]
- Chuayboon, S.; Abanades, S. An overview of solar decarbonization processes, reacting oxide materials, and thermochemical reactors for hydrogen and syngas production. Int. J. Hydrog. Energy 2020, 45, 25783–25810. [Google Scholar] [CrossRef]
- Bellouard, Q.; Rodat, S.; Abanades, S.; Ravel, S.; Frayssines, P.-É. Design, simulation and experimental study of a directly-irradiated solar chemical reactor for hydrogen and syngas production from continuous solar-driven wood biomass gasification. Int. J. Hydrog. Energy 2019, 44, 19193–19205. [Google Scholar] [CrossRef]
- Bellouard, Q.; Rodat, S.; Dupassieux, N.; Abanades, S. A high temperature drop-tube and packed-bed solar reactor for continuous biomass gasification. AIP Conf. Proc. 2017, 1850, 100001. [Google Scholar] [CrossRef]
- Kodama, T.; Kondoh, Y.; Tamagawa, T.; Funatoh, A.; Shimizu, K.-I.; Kitayama, Y. Fluidized bed coal gasification with CO2 under direct irradiation with concentrated visible light. Energy Fuels 2002, 16, 1264–1270. [Google Scholar] [CrossRef]
- Gokon, N.; Ono, R.; Hatamachi, T.; Liuyun, L.; Kim, H.-J.; Kodama, T. CO2 gasification of coal cokes using internally circulating fluidized bed reactor by concentrated Xe-light irradiation for solar gasification. Int. J. Hydrog. Energy 2012, 37, 12128–12137. [Google Scholar] [CrossRef]
- Zgraggen, A.; Haueter, P.; Trommer, D.; Romero, M.; DeJesus, J.; Steinfeld, A. Hydrogen production by steam-gasification of petroleum coke using concentrated solar power—II Reactor design, testing, and modeling. Int. J. Hydrog. Energy 2006, 31, 797–811. [Google Scholar] [CrossRef]
- Piatkowski, N.; Wieckert, C.; Steinfeld, A. Experimental investigation of a packed-bed solar reactor for the steam-gasification of carbonaceous feedstocks. Fuel Process. Technol. 2009, 90, 360–366. [Google Scholar] [CrossRef]
- Müller, F.; Poživil, P.; Van Eyk, P.; Villarrazo, A.; Haueter, P.; Wieckert, C.; Nathan, G.; Steinfeld, A. A pressurized high-flux solar reactor for the efficient thermochemical gasification of carbonaceous feedstock. Fuel 2017, 193, 432–443. [Google Scholar] [CrossRef] [Green Version]
- Lichty, P.; Perkins, C.; Woodruff, B.; Bingham, C.; Weimer, A. Rapid high temperature solar thermal biomass gasification in a prototype cavity reactor. J. Sol. Energy Eng. 2010, 132, 011012. [Google Scholar] [CrossRef]
- Kruesi, M.; Jovanovic, Z.R.; Santos, E.C.D.; Yoon, H.C.; Steinfeld, A. Solar-driven steam-based gasification of sugarcane bagasse in a combined drop-tube and fixed-bed reactor—Thermodynamic, kinetic, and experimental analyses. Biomass Bioenergy 2013, 52, 173–183. [Google Scholar] [CrossRef]
- Gregg, D.; Taylor, R.; Campbell, J.; Taylor, J.; Cotton, A. Solar gasification of coal, activated carbon, coke and coal and biomass mixtures. Sol. Energy 1980, 25, 353–364. [Google Scholar] [CrossRef]
- Piatkowski, N.; Steinfeld, A. Solar-driven coal gasification in a thermally irradiated packed-bed reactor. Energy Fuels 2008, 22, 2043–2052. [Google Scholar] [CrossRef]
- Abe, T.; Gokon, N.; Izawa, T.; Kodama, T. Internally-circulating fluidized bed reactor using thermal storage material for solar coal coke gasification. Energy Procedia 2015, 69, 1722–1730. [Google Scholar] [CrossRef] [Green Version]
- Boujjat, H.; Rodat, S.; Chuayboon, S.; Abanades, S. Experimental and CFD investigation of inert bed materials effects in a high-temperature conical cavity-type reactor for continuous solar-driven steam gasification of biomass. Chem. Eng. Sci. 2020, 228, 115970. [Google Scholar] [CrossRef]
- Z’Graggen, A.; Steinfeld, A. A two-phase reactor model for the steam-gasification of carbonaceous materials under concentrated thermal radiation. Chem. Eng. Process. Process. Intensif. 2008, 47, 655–662. [Google Scholar] [CrossRef]
- Bellouard, Q.; Abanades, S.; Rodat, S.; Dupassieux, N. Solar thermochemical gasification of wood biomass for syngas production in a high-temperature continuously-fed tubular reactor. Int. J. Hydrog. Energy 2017, 42, 13486–13497. [Google Scholar] [CrossRef]
- Hathaway, B.J.; Kittelson, D.; Davidson, J.H. Development of a molten salt reactor for solar gasification of biomass. Energy Procedia 2014, 49, 1950–1959. [Google Scholar] [CrossRef] [Green Version]
- Muroyama, A.P.; Guscetti, I.; Schieber, G.L.; Haussener, S.; Loutzenhiser, P.G. Design and demonstration of a prototype 1.5 kWth hybrid solar/autothermal steam gasifier. Fuel 2018, 211, 331–340. [Google Scholar] [CrossRef] [Green Version]
- Boujjat, H.; Rodat, S.; Chuayboon, S.; Abanades, S. Experimental and numerical study of a directly irradiated hybrid solar/combustion spouted bed reactor for continuous steam gasification of biomass. Energy 2019, 189, 116118. [Google Scholar] [CrossRef]
- Boujjat, H.; Junior, G.M.Y.; Rodat, S.; Abanades, S. Dynamic simulation and control of solar biomass gasification for hydrogen-rich syngas production during allothermal and hybrid solar/autothermal operation. Int. J. Hydrog. Energy 2020, 45, 25827–25837. [Google Scholar] [CrossRef]
- Rodat, S.; Abanades, S.; Boujjat, H.; Chuayboon, S. On the path toward day and night continuous solar high temperature thermochemical processes: A review. Renew. Sustain. Energy Rev. 2020, 132, 110061. [Google Scholar] [CrossRef]
- Ischia, G.; Castello, D.; Orlandi, M.; Miotello, A.; Rosendahl, L.A.; Fiori, L. Waste to biofuels through zero-energy hydrothermal solar plants: Process design. Chem. Eng. Trans. 2020, 80, 7–12. [Google Scholar]
- Ischia, G.; Orlandi, M.; Fendrich, M.; Bettonte, M.; Merzari, F.; Miotello, A.; Fiori, L. Realization of a solar hydrothermal carbonization reactor: A zero-energy technology for waste biomass valorization. J. Environ. Manag. 2020, 259, 110067. [Google Scholar] [CrossRef]
- Arena, U.; Di Gregorio, F. Gasification of a solid recovered fuel in a pilot scale fluidized bed reactor. Fuel 2014, 117, 528–536. [Google Scholar] [CrossRef]
- Fryda, L.; Panopoulos, K.; Kakaras, E. Agglomeration in fluidised bed gasification of biomass. Powder Technol. 2008, 181, 307–320. [Google Scholar] [CrossRef]
- Kirnbauer, F.; Hofbauer, H. Investigations on bed material changes in a dual fluidized bed steam gasification plant in Gussing, Austria. Energy Fuels 2011, 25, 3793–3798. [Google Scholar] [CrossRef]
- ADEME. État de l’art de la Production et de l’utilisation de Combustibles Solides de. 2012. Available online: https://www.ademe.fr/etat-lart-production-lutilisation-combustibles-solides-recuperation (accessed on 20 September 2020).
- Bellouard, Q.; Abanades, S.; Rodat, S. Biomass gasification in an innovative spouted-bed solar reactor: Experimental proof of concept and parametric study. Energy Fuels 2017, 31, 10933–10945. [Google Scholar] [CrossRef]
- Boujjat, H.; Rodat, S.; Chuayboon, S.; Abanades, S. Numerical simulation of reactive gas-particle flow in a solar jet spouted bed reactor for continuous biomass gasification. Int. J. Heat Mass Transf. 2019, 144, 118572. [Google Scholar] [CrossRef]
- Speight, J.G. Combustion of Hydrocarbons, in Handbook of Industrial Hydrocarbon Processes; Elsevier: Amsterdam, The Nederland, 2011. [Google Scholar]
- Chuayboon, S.; Abanades, S.; Rodat, S. Insights into the influence of biomass feedstock type, particle size and feeding rate on thermochemical performances of a continuous solar gasification reactor. Renew. Energy 2019, 130, 360–370. [Google Scholar] [CrossRef]
- Chuayboon, S.; Abanades, S.; Rodat, S. Experimental analysis of continuous steam gasification of wood biomass for syngas production in a high-temperature particle-fed solar reactor. Chem. Eng. Process. Process. Intensif. 2018, 125, 253–265. [Google Scholar] [CrossRef]
- Chuayboon, S.; Abanades, S.; Rodat, S. Comprehensive performance assessment of a continuous solar-driven biomass gasifier. Fuel Process. Technol. 2018, 182, 1–14. [Google Scholar] [CrossRef]
- Glassman, I. Combustion, 3rd ed.; Academic Press: New York, NY, USA, 1996; p. 631. [Google Scholar]
- Goodwin, D.G.; Speth, R.L.; Moffat, H.K.; Weber, B.W. Cantera: An Object-Oriented Software Toolkit for Chemical Kinetics, Thermodynamics, and Transport Processes. 2017. Available online: https://zenodo.org/record/1174508#.X3KE1-0RVEY (accessed on 24 August 2018).
- Kwon, E.; Westby, K.J.; Castaldi, M.J. Transforming Municipal Solid Waste (MSW) into fuel via the gasification/pyrolysis process. In North American Waste-to-Energy Conference, Proceedings of the 18th Annual North American Waste-to-Energy Conference, Orlando, FL, USA, 11–13 May 2010; ASME International: New York, NY, USA, 2010; pp. 53–60. [Google Scholar]
- Khosasaeng, T.; Suntivarakorn, R. Effect of equivalence ratio on an efficiency of single throat downdraft gasifier using RDF from municipal solid waste. Energy Procedia 2017, 138, 784–788. [Google Scholar] [CrossRef]
- Materazzi, M.; Lettieri, P.; Taylor, R.; Chapman, C. Performance analysis of RDF gasification in a two stage fluidized bed–plasma process. Waste Manag. 2016, 47, 256–266. [Google Scholar] [CrossRef] [Green Version]








| C (wt.%) | H (wt.%) | O (wt.%) | N (wt.%) | S (wt.%) | Ash (wt.%) | Cl (wt.%) | Moisture (wt.%) | LHV (MJ/kg) | |
|---|---|---|---|---|---|---|---|---|---|
| SRF | 48.6 | 5.7 | 25.8 | 2.9 | 0.9 | 15.0 | 1.1 | 8.9 | 20.6 |
| wood | 48.3 | 6.7 | 44.4 | 0.1 | <0.1% | 0.4 | <0.1% | 8.9 | 16.8 |
| Beechwood Particles | Solid Recovered Fuels | ||||
|---|---|---|---|---|---|
| Runs | #1 | #2 | #3 | #4 | |
| Temperature, Toperating (°C) | 1300 | 1300 | 1300 | 1300–1350 | |
| Feedstock mass, mfeedstock (g) | 30.00 | 27.90 | 20.00 | 20.00 | |
| Voltage to feeder motor, Umotor (V) | 9.5 | 10.5 | 12.0 | 12.0 | |
| Operating mode | Allothermal | Hybrid | Allothermal | Allothermal | Hybrid |
| Qsolar (kWthermal) | 1.2 | 0.8 | 1.0 | 1.0 | 1.0 |
| Ffeedstock (g.min−1) | 1.20 | 1.40 | 0.57 | 0.58 | 0.58 |
| Fsteam (g.min−1) | 0.20 | 0.20 | 0.25 | 0.20 | 0.20 |
| Foxygen (NL.min−1) | 0 | 0.25 | 0 | 0 | 0.25 |
| (S/B)/(S/B)st | 1.24 | 1.13 | 1.06 | 0.87 | 0.87 |
| Run | Gas Production (mmol/gbiomass,dry) | Mass Balance (g) | Closure | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| H2 | CO | CO2 | CH4 | CnHm | Reactants | Products | |||||
| Biomass | H2O | O2 | Gas | Residues | % | ||||||
| #1 | 33.88 | 27.49 | 2.26 | 1.67 | 0.86 | 30.00 | 6.70 | 0.00 | 26.95 | 7.66 | 94.8 |
| #2 | 19.26 | 20.46 | 8.73 | 2.47 | 1.04 | 27.86 | 5.19 | 7.00 | 26.97 | 12.05 | 96.0 |
| Run | Energy breakdown (kJ) | CCE | CGE | SFE | ||
|---|---|---|---|---|---|---|
| Biomass | Syngas | Solar | ||||
| #1 | 460 | 520 | 2498 | 83.3% | 112.9% | 17.6% |
| #2 | 427 | 362 | 1457 | 84.6% | 84.2% | 17.6% |
| Run | Gas Production (mmol/gSRF) | Mass Balance (g) | Closure | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| H2 | CO | CO2 | CH4 | CnHm | Reactants | Products | |||||
| SRF | H2O | O2 | Gas | Residues | % | ||||||
| #3 | 44.73 | 25.25 | 5.76 | 1.79 | 1.19 | 20.00 | 8.85 | 0.00 | 20.23 | 4.95 | 87.7 |
| #4 | 30.98 | 20.22 | 7.87 | 1.70 | 0.80 | 20.00 | 6.54 | 8.51 | 18.63 | 11.87 | 87.3 |
| Run | Energy Breakdown (kJ) | CCE | CGE | SFE | ||
|---|---|---|---|---|---|---|
| SRF | Syngas | Solar | ||||
| #3 | 375 | 391 | 2102 | 88.1% | 104.5% | 15.8% |
| #4 | 375 | 292 | 2086 | 78.5% | 78.0% | 11.9% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boujjat, H.; Rodat, S.; Abanades, S. Solar-hybrid Thermochemical Gasification of Wood Particles and Solid Recovered Fuel in a Continuously-Fed Prototype Reactor. Energies 2020, 13, 5217. https://doi.org/10.3390/en13195217
Boujjat H, Rodat S, Abanades S. Solar-hybrid Thermochemical Gasification of Wood Particles and Solid Recovered Fuel in a Continuously-Fed Prototype Reactor. Energies. 2020; 13(19):5217. https://doi.org/10.3390/en13195217
Chicago/Turabian StyleBoujjat, Houssame, Sylvain Rodat, and Stéphane Abanades. 2020. "Solar-hybrid Thermochemical Gasification of Wood Particles and Solid Recovered Fuel in a Continuously-Fed Prototype Reactor" Energies 13, no. 19: 5217. https://doi.org/10.3390/en13195217
APA StyleBoujjat, H., Rodat, S., & Abanades, S. (2020). Solar-hybrid Thermochemical Gasification of Wood Particles and Solid Recovered Fuel in a Continuously-Fed Prototype Reactor. Energies, 13(19), 5217. https://doi.org/10.3390/en13195217





