The Effect of Light Wavelength on CO2 Capture, Biomass Production and Nutrient Uptake by Green Microalgae: A Step Forward on Process Integration and Optimisation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microorganisms and Culture Medium
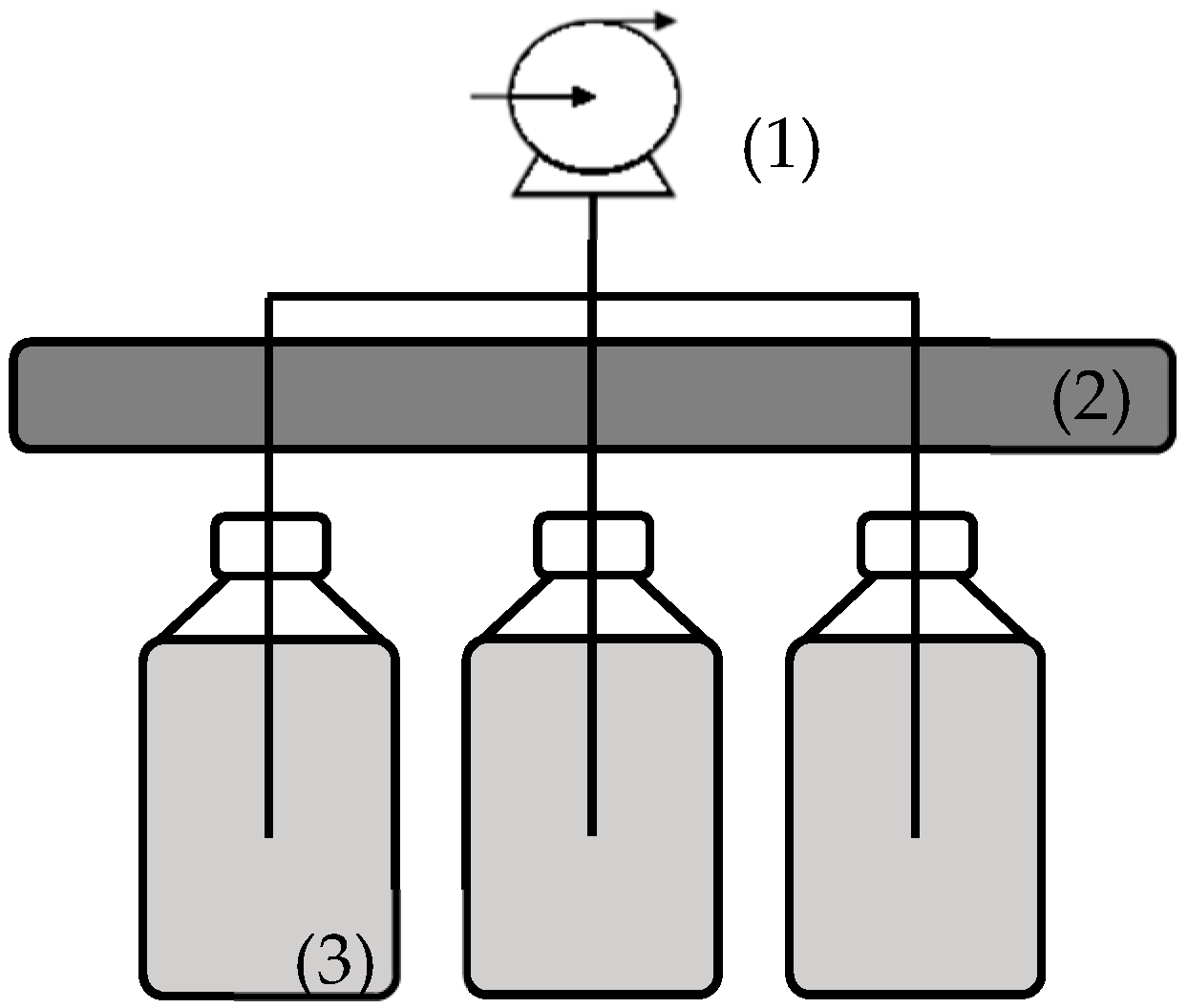
2.2. Experimental Setup and Cultivation Conditions
2.3. Growth Monitoring and Kinetic Growth Parameters
2.4. Carbon Dioxide Fixation Rate
2.5. Nutrients Removal
2.6. Statistical Analysis
3. Results and Discussion
3.1. Microalgal Growth
3.2. Carbon Dioxide Fixation Rates
3.3. Nutrient Removal
3.4. Integrated Evaluation of the Effect of Light Wavelength on Microalgal Growth and Carbon and Nutrient Uptake
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- De Morais, M.G.; de Morais, E.G.; Duarte, J.H.; Deamici, K.M.; Mitchell, B.G.; Costa, J.A.V. Biological CO2 mitigation by microalgae: Technological trends, future prospects and challenges. World J. Microbiol. Biotechnol. 2019, 35, 78. [Google Scholar] [CrossRef] [PubMed]
- Tavoni, M.; Socolow, R. Modeling meets science and technology: An introduction to a special issue on negative emissions. Clim. Chang. 2013, 118, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Solomon, S.; Plattner, G.-K.; Knutti, R.; Friedlingstein, P. Irreversible climate change due to carbon dioxide emissions. Proc. Natl. Acad. Sci. USA 2009, 106, 1704–1709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esteves, A.F.; Santos, F.M.; Pires, J.C.M. Carbon dioxide as geothermal working fluid: An overview. Renew. Sustain. Energy Rev. 2019, 114, 109331. [Google Scholar] [CrossRef]
- Lam, M.K.; Lee, K.T.; Mohamed, A.R. Current status and challenges on microalgae-based carbon capture. Int. J. Greenh. Gas Control 2012, 10, 456–469. [Google Scholar] [CrossRef]
- Ahmed, R.; Liu, G.; Yousaf, B.; Abbas, Q.; Ullah, H.; Ali, M.U. Recent advances in carbon-based renewable adsorbent for selective carbon dioxide capture and separation—A review. J. Clean. Prod. 2019, 242, 118409. [Google Scholar] [CrossRef]
- Pires, J.; Martins, F.; Alvim-Ferraz, M.; Simões, M. Recent developments on carbon capture and storage: An overview. Chem. Eng. Res. Des. 2011, 89, 1446–1460. [Google Scholar] [CrossRef]
- Klinthong, W.; Yang, Y.-H.; Huang, C.-H.; Tan, C.-S. A review: Microalgae and their applications in CO2 capture and renewable energy. Aerosol Air Qual. Res. 2015, 15, 712–742. [Google Scholar] [CrossRef]
- Cheah, W.Y.; Show, P.L.; Chang, J.-S.; Ling, T.C.; Juan, J.C. Biosequestration of atmospheric CO2 and flue gas-containing CO2 by microalgae. Bioresour. Technol. 2015, 184, 190–201. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, W.; Wang, J.; Chen, Y.; Shen, S.; Liu, T. Utilization of simulated flue gas for cultivation of Scenedesmus dimorphus. Bioresour. Technol. 2013, 128, 359–364. [Google Scholar] [CrossRef]
- Wijffels, R.H.; Barbosa, M.J. An outlook on microalgal biofuels. Science 2010, 329, 796–799. [Google Scholar] [CrossRef] [Green Version]
- Milano, J.; Ong, H.C.; Masjuki, H.; Chong, W.; Lam, M.K.; Loh, P.K.; Vellayan, V. Microalgae biofuels as an alternative to fossil fuel for power generation. Renew. Sustain. Energy Rev. 2016, 58, 180–197. [Google Scholar] [CrossRef]
- Parmar, A.; Singh, N.K.; Pandey, A.; Gnansounou, E.; Madamwar, D. Cyanobacteria and microalgae: A positive prospect for biofuels. Bioresour. Technol. 2011, 102, 10163–10172. [Google Scholar] [CrossRef]
- Saratale, R.G.; Kumar, G.; Banu, R.; Xia, A.; Periyasamy, S.; Saratale, G.D. A critical review on anaerobic digestion of microalgae and macroalgae and co-digestion of biomass for enhanced methane generation. Bioresour. Technol. 2018, 262, 319–332. [Google Scholar] [CrossRef]
- Lucas, B.F.; de Morais, M.G.; Santos, T.D.; Costa, J.A.V. Spirulina for snack enrichment: Nutritional, physical and sensory evaluations. LWT 2018, 90, 270–276. [Google Scholar] [CrossRef]
- Schulze, P.S.; Pereira, H.G.; Santos, T.F.; Schueler, L.; Guerra, R.; Barreira, L.A.; Perales, J.A.; Varela, J.C. Effect of light quality supplied by light emitting diodes (LEDs) on growth and biochemical profiles of Nannochloropsis oculata and Tetraselmis chuii. Algal Res. 2016, 16, 387–398. [Google Scholar] [CrossRef]
- Schulze, P.S.C. Effects of Light Quality Supplied by Light Emitting Diodes (LEDs) on Microalgal Production. Master’s Thesis, Universidade do Algarve Faculdade de Ciências e Tecnologia, Algarve, Portugal, 2014. [Google Scholar]
- Gonçalves, A.L.; Rodrigues, C.M.; Pires, J.C.; Simões, M. The effect of increasing CO2 concentrations on its capture, biomass production and wastewater bioremediation by microalgae and cyanobacteria. Algal Res. 2016, 14, 127–136. [Google Scholar] [CrossRef]
- Rocha, R.P.; Soares, O.S.G.; Gonçalves, A.G.; Órfão, J.J.; Pereira, M.F.R.; Figueiredo, J.L. Different methodologies for synthesis of nitrogen doped carbon nanotubes and their use in catalytic wet air oxidation. Appl. Catal. A Gen. 2017, 548, 62–70. [Google Scholar] [CrossRef]
- Zwietering, M.H.; Jongenburger, I.; Rombouts, F.M.; Van’t Riet, K. Modeling of the bacterial growth curve. Appl. Environ. Microbiol. 1990, 56, 1875–1881. [Google Scholar] [CrossRef] [Green Version]
- Mohsenpour, S.F.; Richards, B.; Willoughby, N. Spectral conversion of light for enhanced microalgae growth rates and photosynthetic pigment production. Bioresour. Technol. 2012, 125, 75–81. [Google Scholar] [CrossRef]
- Li, D.; Yuan, Y.; Cheng, D.; Zhao, Q. Effect of light quality on growth rate, carbohydrate accumulation, fatty acid profile and lutein biosynthesis of Chlorella sp. AE10. Bioresour. Technol. 2019, 291, 121783. [Google Scholar] [CrossRef] [PubMed]
- Schulze, P.S.C.; Barreira, L.A.; Pereira, H.G.C.; Perales, J.A.; Varela, J.C.S. Light emitting diodes (LEDs) applied to microalgal production. Trends Biotechnol. 2014, 32, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, V.D.; Fagundes-Klen, M.R.; Trigueros, D.E.G.; Schuelter, A.R.; Kroumov, A.D.; Módenes, A.N. Combination of Light Emitting Diodes (LEDs) for photostimulation of carotenoids and chlorophylls synthesis in Tetradesmus sp. Algal Res. 2019, 43, 101649. [Google Scholar] [CrossRef]
- Zhao, Y.; Sun, S.; Hu, C.; Zhang, H.; Xu, J.; Ping, L. Performance of three microalgal strains in biogas slurry purification and biogas upgrade in response to various mixed light-emitting diode light wavelengths. Bioresour. Technol. 2015, 187, 338–345. [Google Scholar] [CrossRef]
- Assunção, J.; Batista, A.P.; Manoel, J.; da Silva, T.L.; Marques, P.; Reis, A.; Gouveia, L. CO2 utilization in the production of biomass and biocompounds by three different microalgae. Eng. Life Sci. 2017, 17, 1126–1135. [Google Scholar] [CrossRef] [Green Version]
- Masojídek, J.; Torzillo, G.; Koblížek, M. Photosynthesis in Microalgae. In Handbook of Microalgal Culture: Applied Phycology and Biotechnology; Blackwell Science Ltd.: Oxford, UK, 2013; pp. 21–36. [Google Scholar]
- Richmond, A.; Hu, Q. Biological principles of mass cultivation of photoautotrophic microalgae. In Handbook of Microalgal Culture: Applied Phycology and Biotechnology; Blackwell Science Ltd.: Oxford, UK, 2013; pp. 171–204. [Google Scholar]
- Ho, S.-H.; Chen, C.-Y.; Chang, J.-S. Effect of light intensity and nitrogen starvation on CO2 fixation and lipid/carbohydrate production of an indigenous microalga Scenedesmus obliquus CNW-N. Bioresour. Technol. 2012, 113, 244–252. [Google Scholar] [CrossRef]
- Chaudhary, R.; Dikshit, A.K.; Tong, Y.W. Carbon-dioxide biofixation and phycoremediation of municipal wastewater using Chlorella vulgaris and Scenedesmus obliquus. Environ. Sci. Pollut. Res. 2018, 25, 20399–20406. [Google Scholar] [CrossRef]
- Razzak, S. In situ biological CO2 fixation and wastewater nutrient removal with Neochloris oleoabundans in batch photobioreactor. Bioprocess Biosyst. Eng. 2019, 42, 93–105. [Google Scholar] [CrossRef]
- Kowallik, W. Blue light effects on respiration. Annu. Rev. Plant Physiol. 1982, 33, 51–72. [Google Scholar] [CrossRef]
- Kamiya, A.; Saitoh, T. Blue-light-control of the uptake of amino acids and of ammonia in Chlorella mutants. Physiol. Plant. 2002, 116, 248–254. [Google Scholar] [CrossRef]
- Phukan, M.M.; Chutia, R.S.; Konwar, B.; Kataki, R. Microalgae Chlorella as a potential bio-energy feedstock. Appl. Energy 2011, 88, 3307–3312. [Google Scholar] [CrossRef]
- Tibbetts, S.M.; Milley, J.E.; Lall, S.P. Chemical composition and nutritional properties of freshwater and marine microalgal biomass cultured in photobioreactors. J. Appl. Phycol. 2015, 27, 1109–1119. [Google Scholar] [CrossRef] [Green Version]
- Directive 1991/271/EEC. Directive 1991/271/EEC. Directive of the European Council oh 21 May 1991 concerning urban wastewater treatment. In 1991/271/EEC, Official Journal of the European Union L 0271; European Comission: Brussels, Belgium, 1991. [Google Scholar]
- Directive 1998/15/EC. Directive 1998/15/EC. Directive of the European Commissin of 27 February 1998 amending Council Directive 91/271/EEC with respect to certain requirements established in Annex I thereof. In 1998/15/CE, Official Journal of the European Union L67/29; European Comission: Brussels, Belgium, 1998. [Google Scholar]
- Kim, T.-H.; Lee, Y.; Han, S.-H.; Hwang, S.-J. The effects of wavelength and wavelength mixing ratios on microalgae growth and nitrogen, phosphorus removal using Scenedesmus sp. for wastewater treatment. Bioresour. Technol. 2013, 130, 75–80. [Google Scholar] [CrossRef] [PubMed]


| Microalgae | Calibration Curves | R2 | LOD (mgdw L−1) | LOQ (mgdw L−1) |
|---|---|---|---|---|
| C. vulgaris | 0.999 | 5.46 | 18.8 | |
| T. obliquus | 0.999 | 1.26 | 4.21 | |
| N. oleoabundans | 0.997 | 7.03 | 23.4 |
| Parameters | Light | C. vulgaris | T. obliquus | N. oleoabundans |
|---|---|---|---|---|
| µ (d−1) | White | 0.084 ± 0.007 | 0.12 ± 0.02 | 0.264 ± 0.005 |
| Red | 0.090 ± 0.007 | 0.08 ± 0.01 | 0.17 ± 0.03 | |
| Blue | 0.0657 ± 0.0008 | 0.106 ± 0.006 | ND | |
| Xmax (mgdw L−1) | White | 128 ± 5 | 79 ± 9 | 80 ± 4 |
| Red | 116 ± 2 | 66 ± 2 | 73 ± 8 | |
| Blue | 111 ± 2 | 75 ± 1 | 34 ± 4 | |
| Pmax (mgdw L−1 d−1) | White | 14 ± 4 | 8.8 ± 0.9 | 10.6 ± 0.3 |
| Red | 10.2 ± 0.4 | 8 ± 1 | 10 ± 1 | |
| Blue | 11 ± 3 | 5.9 ± 0.2 | ND | |
| P (mgdw L−1 d−1) | White | 6.8 ± 0.5 | 4 ± 1 | 5.5 ± 0.5 |
| Red | 4.6 ± 0.5 | 3.0 ± 0.2 | 4.2 ± 0.3 | |
| Blue | 4.53 ± 0.07 | 3.70 ± 0.08 | ND |
| Parameters | Light | C. vulgaris | T. obliquus | N. oleoabundans |
|---|---|---|---|---|
| C (wt.%) | White | 46 ± 2 | 48 ± 2 | 46.0 ± 0.6 |
| Red | 45.6 ± 0.9 | 45.8 ± 0.9 | 40 ± 1 | |
| Blue | 43 ± 1 | 43.6 ± 0.7 | ND | |
| H (wt.%) | White | 6.8 ± 0.3 | 7.0 ± 0.2 | 6.7 ± 0.1 |
| Red | 6.7 ± 0.1 | 6.9 ± 0.2 | 6.0 ± 0.1 | |
| Blue | 6.5 ± 0.2 | 6.6 ± 0.2 | ND | |
| N (wt.%) | White | 7.9 ± 0.3 | 8.7 ± 0.1 | 7.9 ± 0.1 |
| Red | 7.5 ± 0.1 | 8.6 ± 0.2 | 7.4 ± 0.2 | |
| Blue | 8.0 ± 0.3 | 8.4 ± 0.3 | ND | |
| S (wt.%) | White | 0.5 ± 0.1 | 0.6 ± 0.1 | 0.8 ± 0.1 |
| Red | 0.4 ± 0.1 | 0.6 ± 0.1 | 0.8 ± 0.1 | |
| Blue | 0.6 ± 0.1 | 0.5 ± 0.1 | ND |
| Microalgae | Light | RE (%) | λ (d) | k (d−1) | R2 | RMSE (mg L−1) 2 | |
|---|---|---|---|---|---|---|---|
| N | C. vulgaris | White | 36 ± 1 | 0.804 | 0.316 | 0.993 | 0.786 |
| Red | 23 ± 1 | 0 | 0.328 | 0.992 | 0.611 | ||
| Blue | 20 ± 5 | 0.917 | 0.319 | 0.994 | 0.485 | ||
| T. obliquus | White | 23 ± 3 | 0.164 | 0.355 | 0.974 | 0.846 | |
| Red | 20 ± 5 | 0.157 | 0.322 | 0.993 | 0.467 | ||
| Blue | 20 ± 2 | 2.06 | 0.397 | 0.995 | 0.522 | ||
| N. oleoabundans | White | 21 ± 2 | 0.368 | 0.318 | 0.992 | 0.450 | |
| Red | 17 ± 1 | 0.333 | 0.339 | 0.995 | 0.361 | ||
| Blue | ND | ND | ND | ND | ND | ||
| P | C. vulgaris | White | 20 ± 9 | 1.79 | 0.394 | 0.990 | 0.168 |
| Red | 9 ± 1 | 0 | 0.213 | 0.934 | 0.222 | ||
| Blue | 7 ± 5 | 0 | 0.250 | 0.965 | 0.129 | ||
| T. obliquus | White | 17 ± 4 | 0 | 0.517 | 0.968 | 0.188 | |
| Red | 18 ± 4 | 0 | 0.276 | 0.980 | 0.213 | ||
| Blue | 13 ± 1 | 2.41 | 0.270 | 0.976 | 0.230 | ||
| N. oleoabundans | White | 16 ± 2 | 0 | 0.269 | 0.970 | 0.209 | |
| Red | 14 ± 2 | 0 | 0.310 | 0.976 | 0.176 | ||
| Blue | ND | ND | ND | ND | ND |
| Microalgae | Light | Effects |
|---|---|---|
| C. vulgaris | White | High biomass productivities; High C, H and N contents; High CO2 uptake rates; High N and P uptake. |
| Red | High specific growth rates; High C and H contents. | |
| Blue | High N and S contents. | |
| T. obliquus | White | High specific growth rates and biomass productivities; High C, H, N and S contents; High CO2 uptake rates; High N uptake. |
| Red | High P uptake. | |
| N. oleoabundans | White | High specific growth rates and biomass productivities; High C, H, N and S contents; High CO2 uptake rates; High N and P uptake. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esteves, A.F.; Soares, O.S.G.P.; Vilar, V.J.P.; Pires, J.C.M.; Gonçalves, A.L. The Effect of Light Wavelength on CO2 Capture, Biomass Production and Nutrient Uptake by Green Microalgae: A Step Forward on Process Integration and Optimisation. Energies 2020, 13, 333. https://doi.org/10.3390/en13020333
Esteves AF, Soares OSGP, Vilar VJP, Pires JCM, Gonçalves AL. The Effect of Light Wavelength on CO2 Capture, Biomass Production and Nutrient Uptake by Green Microalgae: A Step Forward on Process Integration and Optimisation. Energies. 2020; 13(2):333. https://doi.org/10.3390/en13020333
Chicago/Turabian StyleEsteves, Ana F., Olívia S. G. P. Soares, Vítor J. P. Vilar, José C. M. Pires, and Ana L. Gonçalves. 2020. "The Effect of Light Wavelength on CO2 Capture, Biomass Production and Nutrient Uptake by Green Microalgae: A Step Forward on Process Integration and Optimisation" Energies 13, no. 2: 333. https://doi.org/10.3390/en13020333







