Sustainable Production of 5-Hydroxymethylfurfural from Pectin-Free Sugar Beet Pulp in a Simple Aqueous Phase System-Optimization with Doehlert Design
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Reactor and Experimental Procedure
2.3. Separation of Products of Pectin-Free Sugar Beet Pulp Hydrothermal Treatment
2.4. Isolation of 5-HMF
2.5. Analyses and Analytical Methods
2.6. Modeling and Optimization Method
3. Results and Discussion
3.1. Composition of Pectin-Free Sugar Beet Pulp
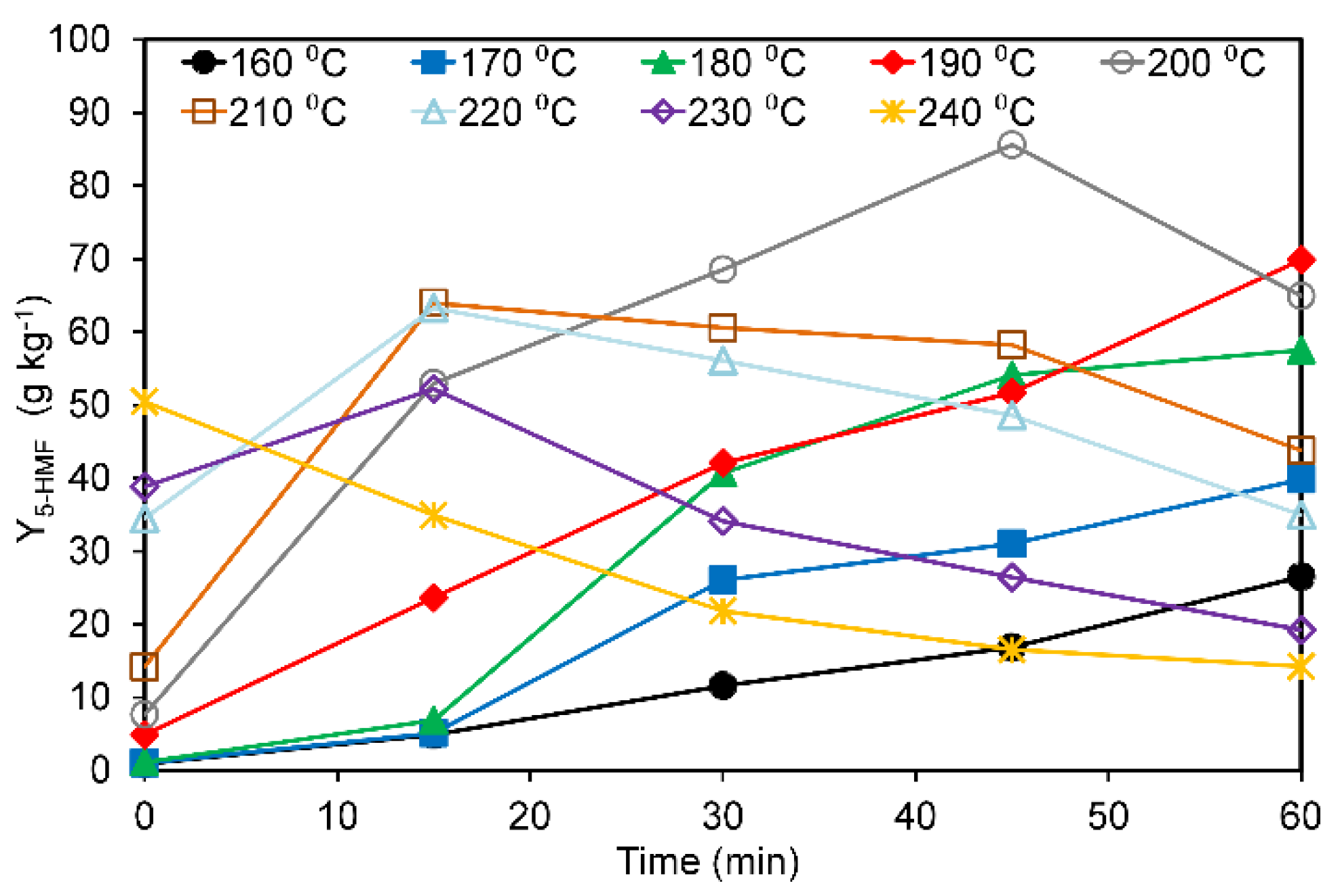
3.2. Effects of Hydrothermal Treatment on Pectin-Free Sugar Beet Pulp
3.3. Modeling of the Hydrothermal Treatment on Pectin-Free Sugar Beet Pulp, Using Optimal Experimental Design
3.4. Isolation of 5-HMF from the Hydrozthermal Conversion of Aqueous Product Fractions
3.5. Composition of Product Fractions and Material Balance
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Menegazzo, F.; Ghedini, E.; Signoretto, M. 5-hydroxymethylfurfural (HMF) production from real biomass. Molecules 2018, 23, 2201. [Google Scholar] [CrossRef] [Green Version]
- Nishimura, S.; Shibata, A.; Ebitan, K. Direct hydroxymethylation of furaldehydes with aqueous formaldehyde over a reusable sulfuric functionalized resin catalyst. ACS Omega 2018, 3, 5988–5993. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, S.; Shibata, A. Hydroxymethylation of furfural to HMF with aqueous formaldehyde over zeolite beta catalyst. Catalysts 2019, 9, 314. [Google Scholar] [CrossRef] [Green Version]
- Teong, S.P.; Yi, G.; Zhang, Y. Hydroxymethylfurfural production from bioresources: Past, present and future. Green Chem. 2014, 16, 2015–2026. [Google Scholar] [CrossRef]
- Yu, I.K.M.; Tsang, D.C.W. Conversion of biomass to hydroxymethylfurfural: A review of catalytic systems and undergoing mechanisms. Bioresour. Technol. 2017, 238, 716–732. [Google Scholar] [CrossRef] [PubMed]
- Van Putten, R.J.; Van der Waal, J.C.; De Jong, E.; Rasrenda, C.B.; Heeres, H.J.; De Vries, J.G. Hydroxymethylfurfural, a versatile platform chemical made from renewable resources. Chem. Rev. 2013, 113, 1499–1597. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, J.; Su, D. 5-Hydroxymethylfurfural: A key intermediate for efficient biomass conversion. J. Energy Chem. 2015, 24, 548–551. [Google Scholar] [CrossRef]
- Rout, P.K.; Nannaware, A.D.; Prakash, O.; Kalra, A.; Rajasekhran, R. Synthesis of hydroxymethylfurfural from cellulose using green processes: A promising biochemical and biofuel feedstock. Chem. Eng. Sci. 2016, 142, 318–346. [Google Scholar] [CrossRef]
- Perez, G.P.; Mukherjee, A.; Dumont, M.-J. Insights into HMF catalysis. J. Ind. Eng. Chem. 2019, 70, 1–34. [Google Scholar] [CrossRef]
- Dutta, S.; De, S.; Saha, B. Advances in biomass transformation to 5-hydroxymethylfurfural and mechanistic aspects. Biomass Bioenergy 2013, 55, 355–369. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, C.; Li, D.; Liu, Q.; Tan, J.; Wang, C.; Cai, C.; Ma, L. Recent advances in catalytic conversion of biomass to 5-hydroxymethylfurfural and 2,5-dimethylfuran. Renew. Sustain. Energy Rev. 2019, 103, 227–247. [Google Scholar] [CrossRef]
- Rosatella, A.A.; Simeonov, S.P.; Frade, R.F.M.; Afonso, C.A.M. 5-Hydroxymethylfurfural (HMF) as a building block platform: Biological properties, synthesis and synthetic applications. Green Chem. 2011, 13, 754–793. [Google Scholar] [CrossRef]
- Addepally, U.; Thulluri, C. Recent progress in production of fuel liquid hydrocarbons from biomass-derived furanics via strategic catalytic routes. Fuel 2015, 159, 935–942. [Google Scholar] [CrossRef]
- Kruse, A.; Dahmen, N. Hydrothermal biomass conversion: Quo vadis? J. Supercrit. Fluids 2018, 134, 114–123. [Google Scholar] [CrossRef]
- Attard, T.M.; Clark, J.H.; McElroy, C.R. Recent developments in key biorefinery areas. Curr. Opin. Green Sustain. Chem. 2020, 21, 64–74. [Google Scholar] [CrossRef]
- González-García, S.; Gullón, B.; Moreira, M. Environmental assessment of Biorefinery processes for the valorization of lignocellulosic wastes into oligosaccharides. J. Clean. Prod. 2018, 172, 4066–4073. [Google Scholar] [CrossRef] [Green Version]
- Da Silva, T.L.; Moniz, P.; Silva, C.; Reis, A. The dark side of microalgae biotechnology: A heterotrophic biorefinery platform directed to ω-3 rich lipid production. Microorganisms 2019, 7, 670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ubando, A.T.; Felix, C.B.; Chen, W.H. Biorefineries in circular bioeconomy: A comprehensive review. Bioresour. Technol. 2020, 299, 122585. [Google Scholar] [CrossRef]
- Ruiz, H.A.; Conrad, M.; Sun, S.N.; Sanchez, A.; Rocha, G.J.M.; Romaní, A.; Castro, E.; Torres, A.; Rodríguez-Jasso, R.M.; Andrade, L.P.; et al. Engineering aspects of hydrothermal pretreatment: From batch to continuous operation, scale-up and pilot reactor under biorefinery concept. Bioresour. Technol. 2020, 299, 122685. [Google Scholar] [CrossRef] [Green Version]
- Lachos-Perez, D.; Brown, A.B.; Mudhoo, A.; Martinez, J.; Timko, M.T.; Rostagno, M.A.; Forester-Carneiro, T. Applications of subcritical and supercritical water conditions for extraction, hydrolysis, gasification, and carbonization of biomass: A critical review. Biofuel Res. J. 2017, 14, 611–626. [Google Scholar] [CrossRef]
- Knez, Ž.; Knez Hrnčič, M.; Čolnik, M.; Škerget, M. Chemicals and value added compounds from biomass using sub- and supercritical water. J. Supercrit. Fluids 2018, 133, 591–602. [Google Scholar] [CrossRef]
- Pińkowska, H.; Krzywonos, M.; Wolak, P.; Złocińska, A. Pectin and neutral monosaccharides production from the simultaneous hydrothermal extraction of waste biomass from refining of sugar—Optimizatuion with the use of Doehlert design. Molecules 2019, 24, 472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pińkowska, H.; Wolak, P.; Oliveros, E. Hydrothermolysis of rapeseed cake in subcritical water. Effect on reaction temperature and holding time on product composition. Biomass Bioenergy 2014, 64, 50–61. [Google Scholar] [CrossRef]
- Overend, R.P.; Chornet, E.; Gascoigne, J.A. Fractionation of lignocellulosics by steam/aqueous pretreatments. Philos. T. R. Soc. Lond. 1987, A321, 523–536. [Google Scholar]
- De Farias Silva, C.E.; Bertucco, A. Severity factor as an efficient control parameter to predict biomass solubilization and saccharification during acidic hydrolysis of microalgal biomass. Bioenergy Res. 2018, 11, 491–504. [Google Scholar] [CrossRef]
- Liu, F.; Sivoththaman, S.; Tan, Z. Solvent extraction of 5-HMF from simulated hydrothermal conversion product. Sustain. Environ. Res. 2014, 24, 149–157. [Google Scholar]
- Carter, B.; Gilcrease, P.C.; Menkhaus, T.J. Removal and recovery of furfural, 5-hydroxymethylfurfural, and acetic acid from aqueous solutions using a soluble cationic polyelectrolyte. Biotechnol. Bioeng. 2011, 108, 2046–2052. [Google Scholar] [CrossRef]
- Undersander, D.; Mertens, D.R.; Thiex, N. Forage Analyses Procedures; The National Forage Testing Association: Omaha, NE, USA, 1993; p. 147. Available online: https://www.foragetesting.org/lab-procedures (accessed on 26 August 2020).
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Report No. TP-510-42622; National Renewable Energy Laboratory: Golden, CO, USA, 2005; p. 8. Available online: https://www.nrel.gov/docs/gen/fy08/42622.pdf (accessed on 26 August 2020).
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Carrier, M.; Loppinet-Serani, A.; Denux, D.; Lasnier, J.M.; Ham-Pichavant, F.; Cansell, F.; Aymonier, C. Thermogravimetric analysis as a new method to determine the lignocellulosic composition of biomass. Biomass Bioenergy 2011, 35, 298–307. [Google Scholar] [CrossRef]
- Alcazar, A.; Jurado, J.M.; Pablos, F.; Gonzalez, A.G.; Martin, M.J. HPLC determination of 2-furaldehyde and 5-hydroxymethyl-2-furaldehyde in alcoholic beverages. Microchem. J. 2006, 82, 22–28. [Google Scholar] [CrossRef]
- Pińkowska, H.; Wolak, P.; Oliveros, E. Production of xylose and glucose from rapeseed straw in subcritical water—Use of Doehlert design for optimizing the reaction conditions. Biomass Bioenergy 2013, 58, 188–197. [Google Scholar] [CrossRef]
- Ferreira, S.L.C.; Dos Santos, W.N.L.; Quitella, C.M.; Neto, B.B.; Bosque-Sendra, J.M. Doehlert matrix: A chemometric tool for analytical chemistry—Review. Talanta 2004, 63, 1061–1067. [Google Scholar] [CrossRef]
- Wang, Z.W.; Zhu, M.Q.; Li, M.F.; Wang, J.Q.; Sun, R.C. Comprehensive evaluation of the liquid fraction during the hydrothermal treatment of rapeseed straw. Biotechnol. Biofuels 2016, 9, 142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.; He, Q.S.; Yang, L. A review on hydrothermal co-liquefaction of biomass. Appl. Energy 2019, 250, 926–945. [Google Scholar] [CrossRef]
- Yu, Y.; Lou, X.; Wu, H. Some recent advances in hydrolysis of biomass in hot-compressed water and its comparisons with other hydrolysis methods. Energy Fuels 2008, 22, 46–60. [Google Scholar] [CrossRef]
- Möller, M.; Harnisch, F.; Schröder, U. Microwave-assisted hydrothermal degradation of fructose and glucose in subcritical water. Biomass Bioenergy 2012, 39, 389–398. [Google Scholar] [CrossRef]
- Promdej, C.; Matsumura, Y. Temperature effect on hydrothermal decomposition of glucose in sub- and supercritical Water. Ind. Eng. Chem. Res. 2011, 50, 8492–8497. [Google Scholar] [CrossRef]
- Blumenthal, L.C.; Jens, C.M.; Ulbrich, J.; Schwering, F.; Langrehr, V.; Turek, T.; Kunz, U.; Leonhard, K.; Palkovits, R. Systematic identification of solvents optimal for the extraction of 5-hydroxymethylfurfural from aqueous reactive solutions. ACS Sustain. Chem. Eng. 2016, 4, 228–235. [Google Scholar] [CrossRef]
- Ma, H.; Wang, F.; Yu, Y.; Wang, L.; Li, X. Autocatalytic production of 5-hydroxymethylfurfural from fructose-based carbohydrates in a biphasic system and its purification. Ind. Eng. Chem. Res. 2015, 54, 2657–2666. [Google Scholar] [CrossRef]
- Sindermann, E.C.; Holbach, A.; Hahn, A.; Kockmann, N. Single phase coutercurrent extraction of 5-hydroxymethylfurfural from aqueous phase system. Chem. Eng. J. 2016, 283, 251–259. [Google Scholar] [CrossRef]
- Rihko-Struckmann, L.K.; Molnar, M.; Pirwitz, K.; Fachet, M.; McBride, K.; Zinser, A.; Sundmacher, K. Recovery and separation of carbohydrate derivatives from the lipid extracted alga Dunaliella by mild liquefaction. ACS Sustain. Chem. Eng. 2017, 5, 588–595. [Google Scholar] [CrossRef]
- Deng, F.; Aita, G.M. Detoxification of dilute ammonia pretreated energy cane bagasse enzymatic hydrolysate by soluble polyelectrolyte flocculants. Ind. Crops Prod. 2018, 112, 681–690. [Google Scholar] [CrossRef]
- Carter, B.; Squillace, P.; Gilcrease, P.C.; Menkhaus, T.J. Detoxification of a lignocellulosic biomass slurry by soluble polyelectrolyte adsorption for improved fermentation efficiency. Biotechnol. Bioeng. 2011, 108, 2053–2060. [Google Scholar] [CrossRef]
- Sharma, H.B.; Sarmah, A.K.; Dubey, B. Hydrothermal carbonization of renewable waste biomass for solid biofuel production: A discussion on process mechanism, the influence of process parameters, environmental performance and fuel properties of hydrochar. Renew. Sustain. Energy Rev. 2020, 123, 109761. [Google Scholar] [CrossRef]
- Petersen, M.Ø.; Larson, J.; Thomsen, M.H. Optimization of hydrothermal pretreatment of wheat straw for production of bioethanol at low water consumption without addition of chemicals. Biomass Bioenergy 2009, 33, 834–840. [Google Scholar] [CrossRef]
- Barbier, J.; Charon, N.; Dupassieux, N.; Loppinet-Serani, A.; Mahé, L.; Ponthus, J.; Courtiade, M.; Ducrozet, A.; Quoineaud, A.A.; Cansell, F. Hydrothermal conversion of lignin compounds. A detailed study of fragmentation and condensation reaction pathways. Biomass Bioenergy 2012, 46, 479–491. [Google Scholar] [CrossRef]
- Ruiz, H.A.; Rodríguez-Jasso, R.M.; Fernandes, B.D.; Vicente, A.A.; Teixeira, J.A. Hydrothermal processing, as an alternative for upgrading agriculture residues and marine biomass according to the biorefinery concept: A review. Renew. Sustain. Energy Rev. 2013, 21, 35–51. [Google Scholar] [CrossRef] [Green Version]
- Wahyudiono; Sasaki, M.; Goto, M. Recovery of phenolic compounds through the decomposition of lignin in near and supercritical water. Chem. Eng. Process. 2008, 47, 1609–1619. [Google Scholar] [CrossRef]
- Ponnusamy, V.K.; Nagappan, S.; Bhosale, R.R.; Lay, C.H.; Nguyen, D.D.; Pugazhendhi, A.; Chang, S.W.; Kumar, G. Review on sustainable production of biochar through hydrothermal liquefaction: Physico-chemical properties and applications. Bioresour. Technol. 2020, 310, 123414. [Google Scholar] [CrossRef]
- Fang, Z.; Minowa, T.; Smith, R.L.; Ogi, T.; Koziński, J.A. Liquefaction and gasification of cellulose with Na2CO3 and Ni in subcritical water at 350 °C. Ind. Eng. Chem. Res. 2004, 43, 2454–2463. [Google Scholar] [CrossRef]
- Rogalinski, T.; Herrmann, S.; Brunner, G. Production of amino acids from bovine serum albumin by continuous sub-critical water hydrolysis. J. Supercrit. Fluids 2005, 36, 49–58. [Google Scholar] [CrossRef]
- Sato, N.; Quitain, A.T.; Kang, K.; Daimon, H.; Fujie, K. Reaction kinetics of amino acid decomposition in high-temperature and high-pressure water. Ind. Eng. Chem. Res. 2004, 43, 3217–3222. [Google Scholar] [CrossRef]
- Sinağ, A.; Gülbay, S.; Uskan, B.; Canel, M. Biomass decomposition in near critical water. Energy Convers. Manag. 2010, 51, 612–620. [Google Scholar] [CrossRef]
- Peterson, A.; Lachance, R.P.; Tester, J.W. Kinetic evidence of the Maillard reaction in hydrothermal biomass processing: Glucose-glycine interactions in high-temperature, high-pressure water. Ind. Eng. Chem. Res. 2010, 49, 2107–2117. [Google Scholar] [CrossRef]
- Yang, D.-P.; Li, Z.; Liu, M.; Zhang, X.; Chen, Y.; Xue, H.; Ye, E.; Luque, R. Biomass-derived carbonaceous materials: Recent progress in synthetic approaches, advantages, and applications. ACS Sustain. Chem. Eng. 2019, 7, 4564–4585. [Google Scholar] [CrossRef]
- Peterson, A.A.; Vogel, F.; Lachance, R.P.; Fröling, M.; Antal, M.J.; Tester, J.W. Thermochemical biofuel production in hydrothermal media: A review of sub- and supercritical water technologies. Energy Environ. Sci. 2008, 1, 32–65. [Google Scholar] [CrossRef]
- Lü, X.; Saka, S. New insights on monosaccharides’ isomerization, dehydration and fragmentation in hot-compressed water. J. Supercrit. Fluids 2012, 61, 146–156. [Google Scholar] [CrossRef]
- Kruse, A.; Funke, A.; Titiric, M.M. Hydrothermal conversion of biomass to fuels and energetic materials. Curr. Opin. Chem. Biol. 2013, 17, 515–521. [Google Scholar] [CrossRef]
- Bayu, A.; Abudula, A.; Guan, G. Reaction pathways and selectivity in chemo-catalytic conversion of biomass-derived carbohydrates to high-value chemicals: A review. Fuel Process. Technol. 2019, 196, 106162. [Google Scholar] [CrossRef]
- Wang, T.; Zhaia, Y.; Zhu, Y.; Li, C.; Zeng, G. A review of the hydrothermal carbonization of biomass waste for hydrochar formation: Process conditions, fundamentals, and physicochemical properties. Renew. Sustain. Energy Rev. 2018, 90, 223–247. [Google Scholar] [CrossRef]
- Woiciechowski, A.L.; Neto, C.J.; de Souza Vandenberghe, L.P.; de Carvalho Neto, D.P.; Sydney, A.C.; Letti, L.A.; Karp, S.G.; Torres, L.A.; Soccol, C.R. Lignocellulosic biomass: Acid and alkaline pretreatments and their effects on biomass recalcitrance—Conventional processing and recent advances. Bioresour. Technol. 2020, 304, 122848. [Google Scholar] [CrossRef] [PubMed]
- Cocero, M.J.; Cabeza, Á.; Abad, N.; Adamovic, T.; Vaquerizo, L.; Martínez, C.M.; Pazo-Cepeda, M.V. Understanding biomass fractionation in subcritical & supercritical water. J. Supercrit. Fluids 2018, 133, 550–565. [Google Scholar]
- Kruse, A.; Dinjus, E. Hot compressed water as reaction medium and reactant. Properties and synthesis reactions. J. Supercrit. Fluids 2007, 39, 362–380. [Google Scholar] [CrossRef]
- Okolie, J.A.; Nanda, S.; Dalaia, A.K.; Berruti, F.; Kozinski, J.A. A review on subcritical and supercritical water gasification of biogenic, polymeric and petroleum wastes to hydrogen-rich synthesis gas. Renew. Sustain. Energy Rev. 2020, 119, 109546. [Google Scholar] [CrossRef]
- Kang, S.; Fu, J.; Zhang, G. From lignocellulosic biomass to levulinic acid: A review on acid-catalyzed hydrolysis. Renew. Sustain. Energy Rev. 2018, 94, 340–362. [Google Scholar] [CrossRef]
- Mika, L.T.; Cséfalvay, E.; Németh, A. Catalytic conversion of carbohydrates to initial platform chemicals: Chemistry and sustainability. Chem. Rev. 2018, 118, 505–613. [Google Scholar] [CrossRef]
- Cheng, L.; Ye, X.P.; He, R.; Liu, S. Investigation of rapid conversion of switchgrass in subcritical water. Fuel Process. Technol. 2009, 90, 301–311. [Google Scholar] [CrossRef]
- Aida, T.M.; Sato, Y.; Watanabe, M.; Tajima, K.; Nonaka, T.; Hattori, H.; Arai, K. Dehydration of D-glucose in high temperature water at pressures up to 80 MPa. J. Supercrit. Fluids 2007, 40, 381–388. [Google Scholar] [CrossRef]
- Aida, T.M.; Tajima, K.; Watanabe, M.; Saito, Y.; Kuroda, K.; Nonaka, T.; Hattori, H.; Smith, R.L.; Arai, K. Reactions of D-fructose in water at temperature up to 400 °C and pressures up to 100 MPa. J. Supercrit. Fluids 2007, 42, 110–119. [Google Scholar] [CrossRef]
- Möller, M.; Nilges, P.; Harnisch, F.; Schröder, U. Subcritical water as reaction environment: Fundamentals of hydrothermal biomass transformation. ChemSusChem. 2011, 4, 566–579. [Google Scholar] [CrossRef]
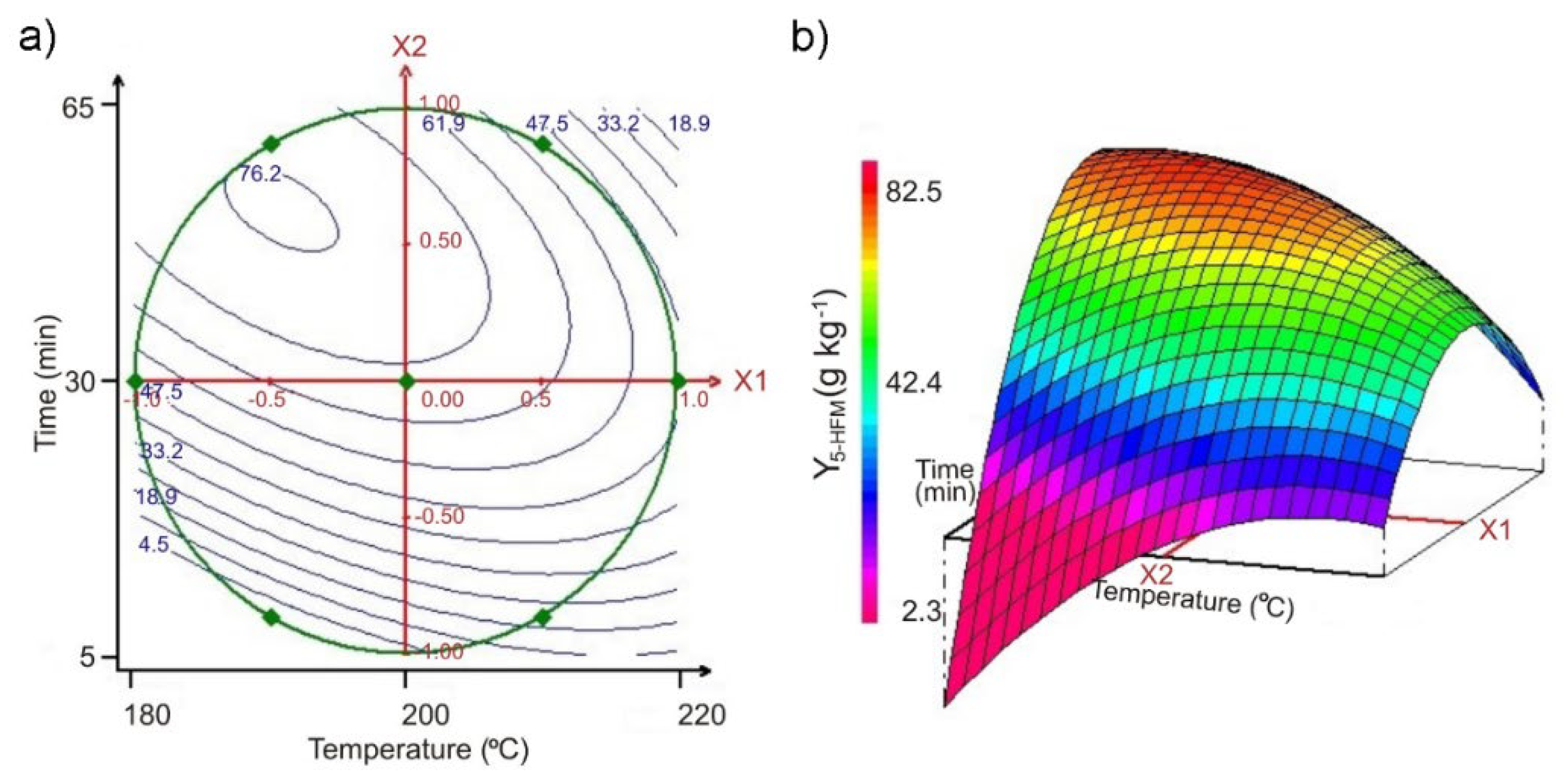
| Experiment | x1 | x2 | u1 T (°C) | u2 | Y5-HMF-exp. (2) (g·kg−1) | Y5-HMF-cal. (3) (g·kg−1) | |
|---|---|---|---|---|---|---|---|
| th (min) | t (min) | ||||||
| 1 | +1 | 0 | 220 | 30 | 34.8 | 56.1 | 48.6 |
| 2 | −1 | 0 | 180 | 30 | 31.8 | 40.5 | 48.0 |
| 3 | +0.5 | +0.866 | 210 | 60 | 63.5 | 43.5 | 51.0 |
| 4 | −0.5 | −0.866 | 190 | 0 | 3.1 | 4.5 | 1.4 |
| 5 | +0.5 | −0.866 | 210 | 0 | 2.8 | 14.1 | 21.6 |
| 6 | −0.5 | +0.866 | 190 | 60 | 62.1 | 82.5 | 75.0 |
| 7 | 0 | 0 | 200 | 30 | 31.5 | 66.4 | 69.9 |
| 7′ (1) | 0 | 0 | 200 | 30 | 32.4 | 74.9 | 69.9 |
| 7′′ (1) | 0 | 0 | 200 | 30 | 31.8 | 68.5 | 69.9 |
| Experiment | Log Ro | YWI a (g·kg−1) | Content in the WI Fractions (g·kg−1) | YWS b | YG c (g·kg−1) | |||
|---|---|---|---|---|---|---|---|---|
| H d | C e | L f | P g | |||||
| 1 | 5.08 | 410.9 | 0.0 | 185.3 | 545.2 | 78.1 | 213.0 | 376.1 |
| 2 | 3.86 | 453.1 | 18.2 | 229.2 | 429.2 | 87.5 | 330.9 | 216.0 |
| 3 | 5.04 | 408.1 | 0.0 | 173.4 | 551.8 | 90.5 | 197.6 | 394.3 |
| 4 | 3.14 | 518.9 | 89.1 | 241.6 | 361.3 | 115.8 | 358.0 | 123.1 |
| 5 | 3.67 | 457.4 | 39.5 | 217.1 | 370.6 | 106.2 | 370.7 | 171.9 |
| 6 | 4.44 | 456.3 | 3.8 | 209.7 | 529.6 | 92.6 | 243.8 | 299.0 |
| 7 h | 4.45 | 443.1 | 21.1 | 211.3 | 500.1 | 78.8 | 232.4 | 324.5 |
| Component | Experiment | ||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 a | |
| Pentoses | 0.5 | 2.9 | 0.1 | 3.1 | 2.6 | 1.1 | 1.2 |
| Hexoses | 6.7 | 2.6 | 5.6 | 1.9 | 3.2 | 2.5 | 3.5 |
| 2-FA | 96.1 | 51.7 | 76.5 | 5.2 | 22.2 | 63.9 | 65.0 |
| 5-HMF | 56.1 | 40.5 | 43.5 | 4.5 | 14.1 | 82.5 | 69.9 |
| Unidentified b | 53.6 | 233.2 | 71.9 | 343.3 | 328.6 | 93.8 | 92.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pińkowska, H.; Krzywonos, M.; Wolak, P.; Seruga, P.; Górniak, A.; Złocińska, A.; Ptak, M. Sustainable Production of 5-Hydroxymethylfurfural from Pectin-Free Sugar Beet Pulp in a Simple Aqueous Phase System-Optimization with Doehlert Design. Energies 2020, 13, 5649. https://doi.org/10.3390/en13215649
Pińkowska H, Krzywonos M, Wolak P, Seruga P, Górniak A, Złocińska A, Ptak M. Sustainable Production of 5-Hydroxymethylfurfural from Pectin-Free Sugar Beet Pulp in a Simple Aqueous Phase System-Optimization with Doehlert Design. Energies. 2020; 13(21):5649. https://doi.org/10.3390/en13215649
Chicago/Turabian StylePińkowska, Hanna, Małgorzata Krzywonos, Paweł Wolak, Przemysław Seruga, Agata Górniak, Adrianna Złocińska, and Michał Ptak. 2020. "Sustainable Production of 5-Hydroxymethylfurfural from Pectin-Free Sugar Beet Pulp in a Simple Aqueous Phase System-Optimization with Doehlert Design" Energies 13, no. 21: 5649. https://doi.org/10.3390/en13215649







