Characteristics of Aerosol Formation and Emissions During Corn Stalk Pyrolysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
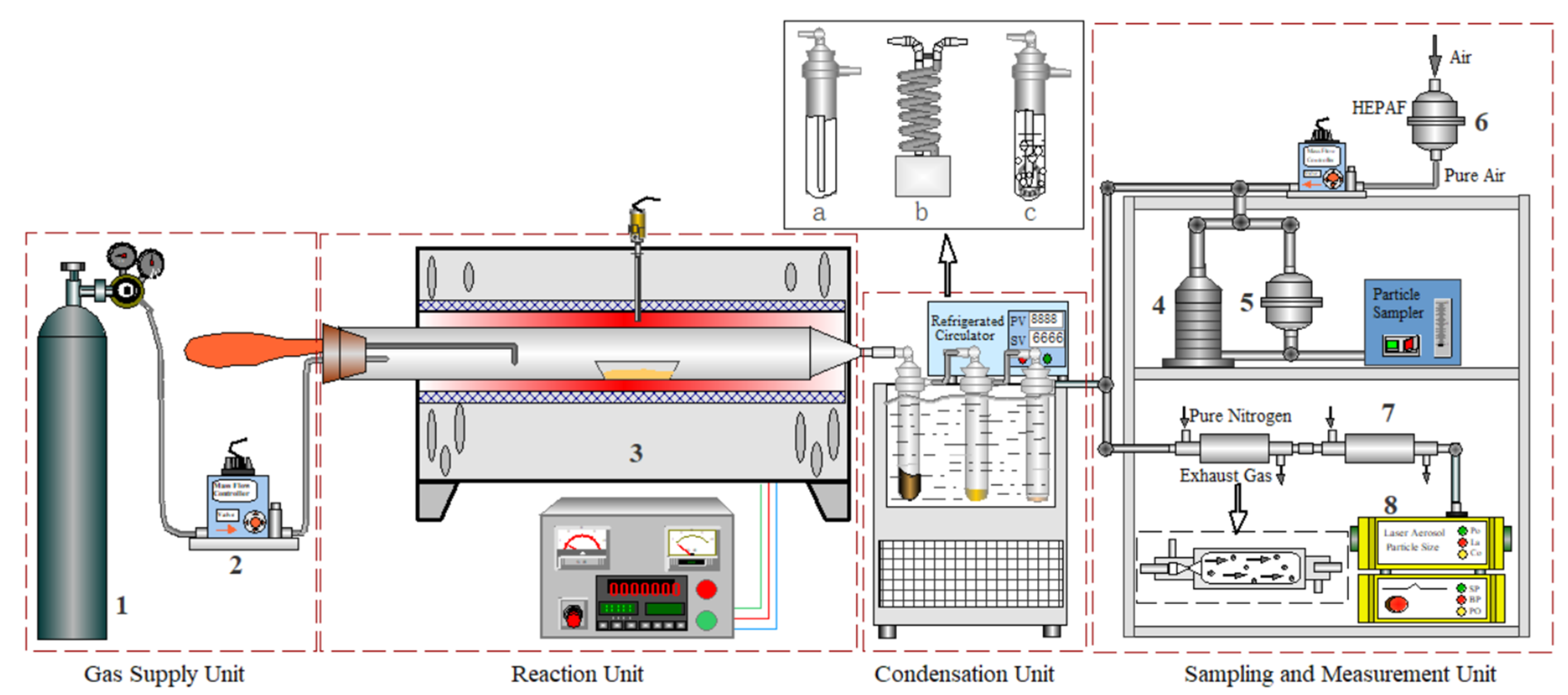
2.2. Experimental Process
2.3. Sampling Characterization
3. Results
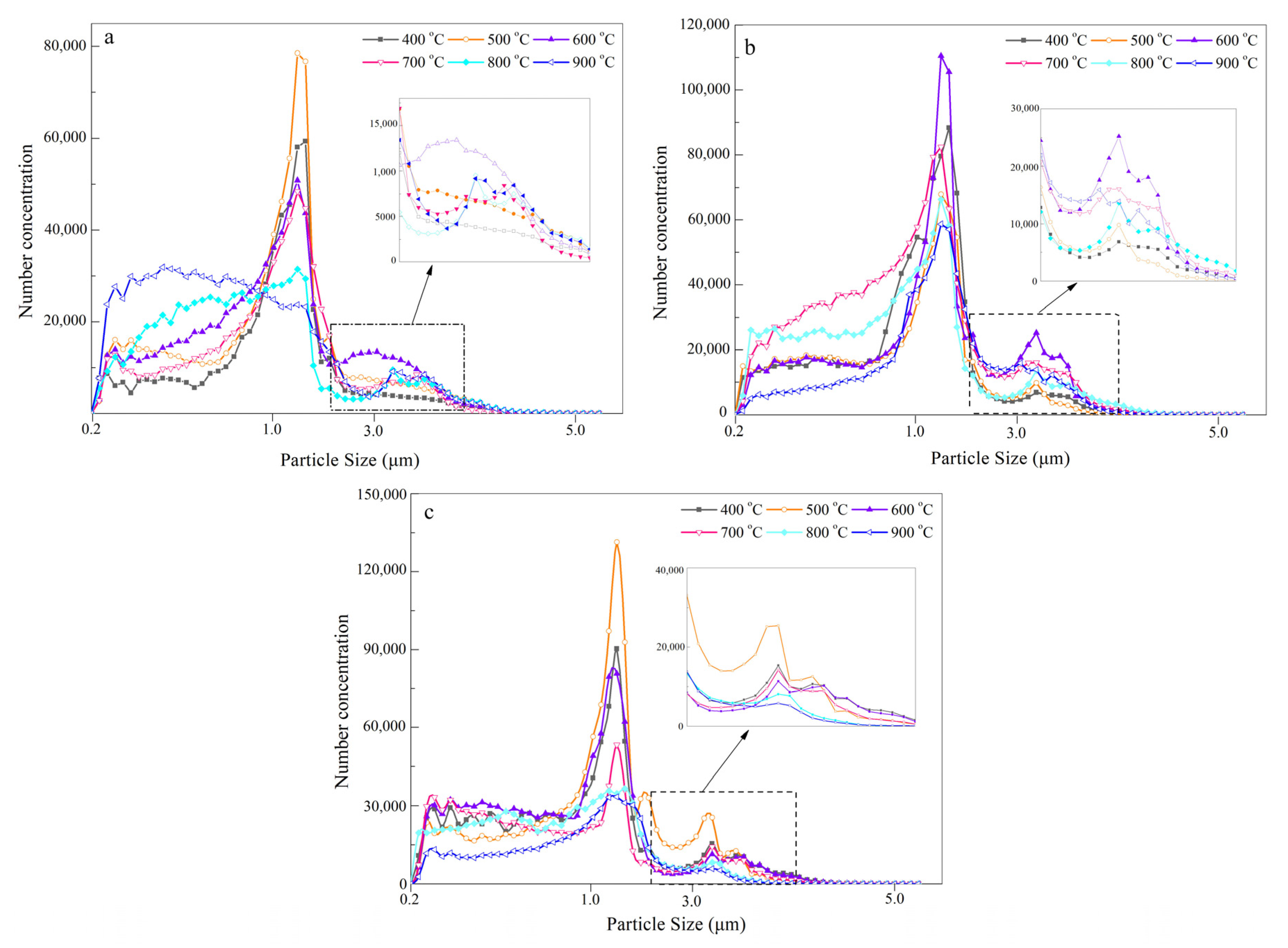
3.1. The Thermal Conversion and Particle Size Distribution at Different Temperature
3.2. The Morphologies and Compositions of Aerosols Produced during Biomass Pyrolysis
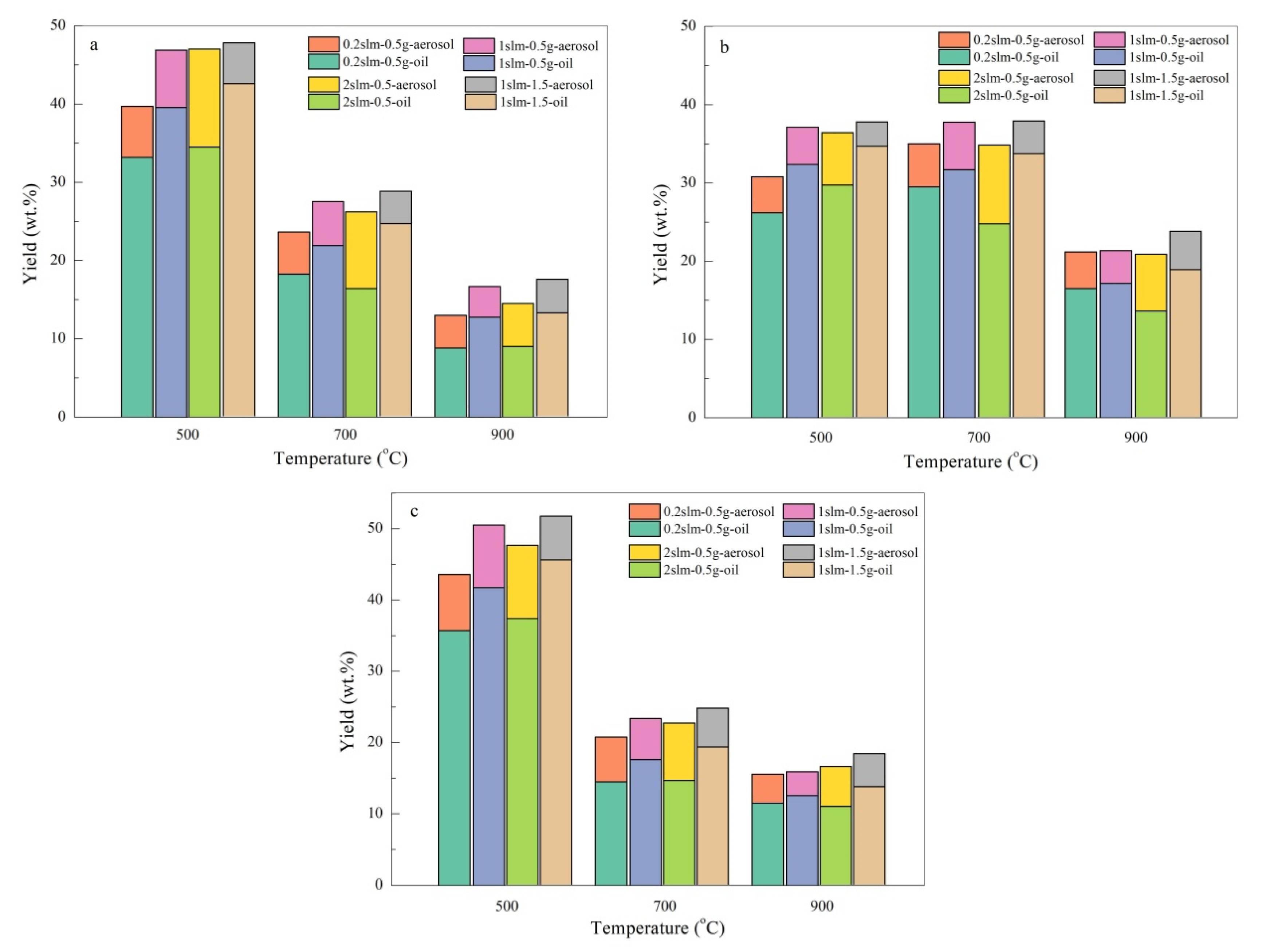
3.3. The Effects of Gas Flow, Mass of Feedstock and Condensation Method on the Balance of Bio-Oil and Aerosols
4. Conclusions
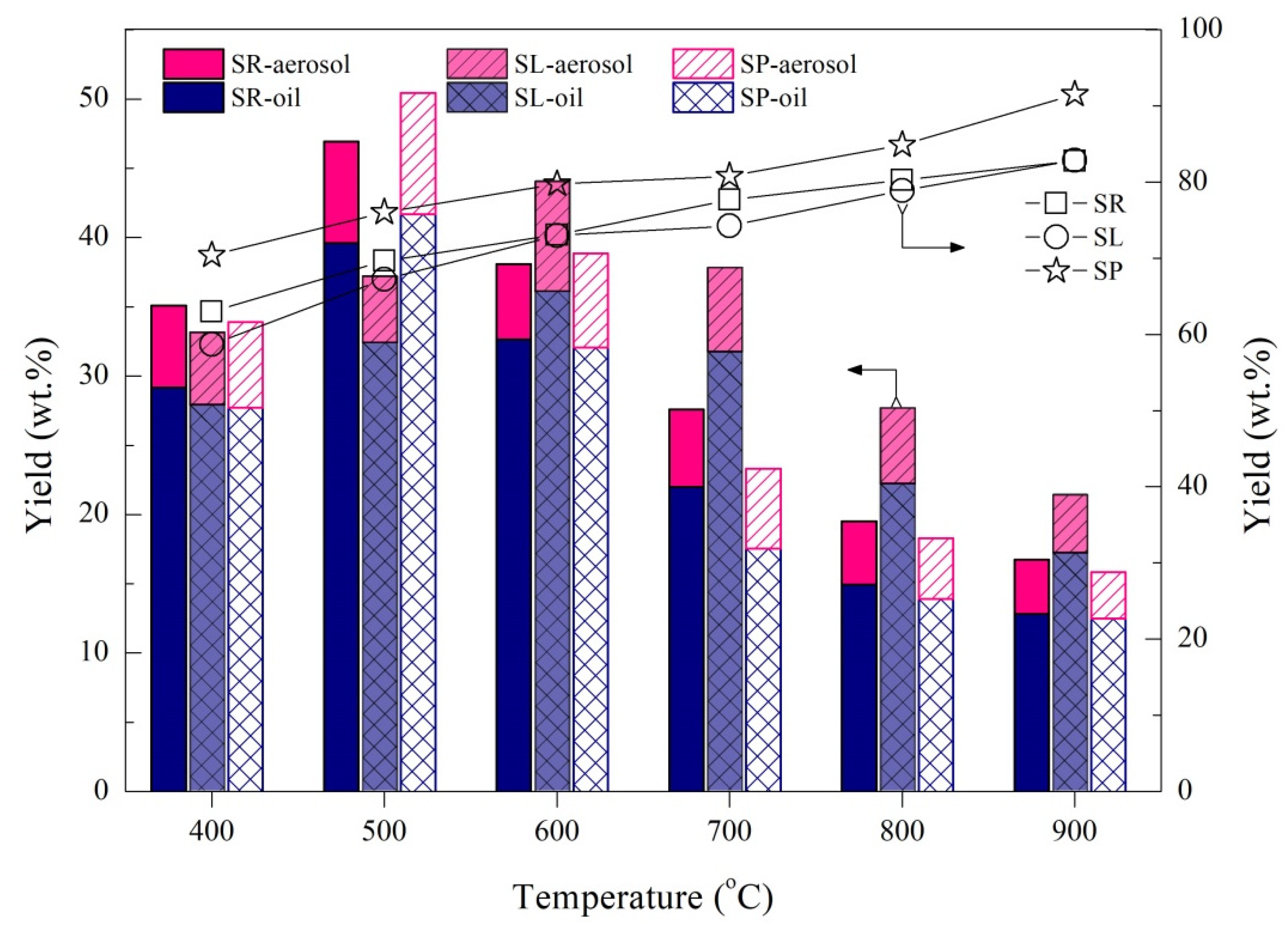
- As the temperature increases, the aerosol yield presents a regular change pattern that increases initially and decreases afterwards in the range of 3.4–8.7 wt.% that corresponds with bio-oil, but the maximum peak varies for SR and SP as 500 °C and for SL as 600 °C owing to the heterogeneous structure of the tissues.
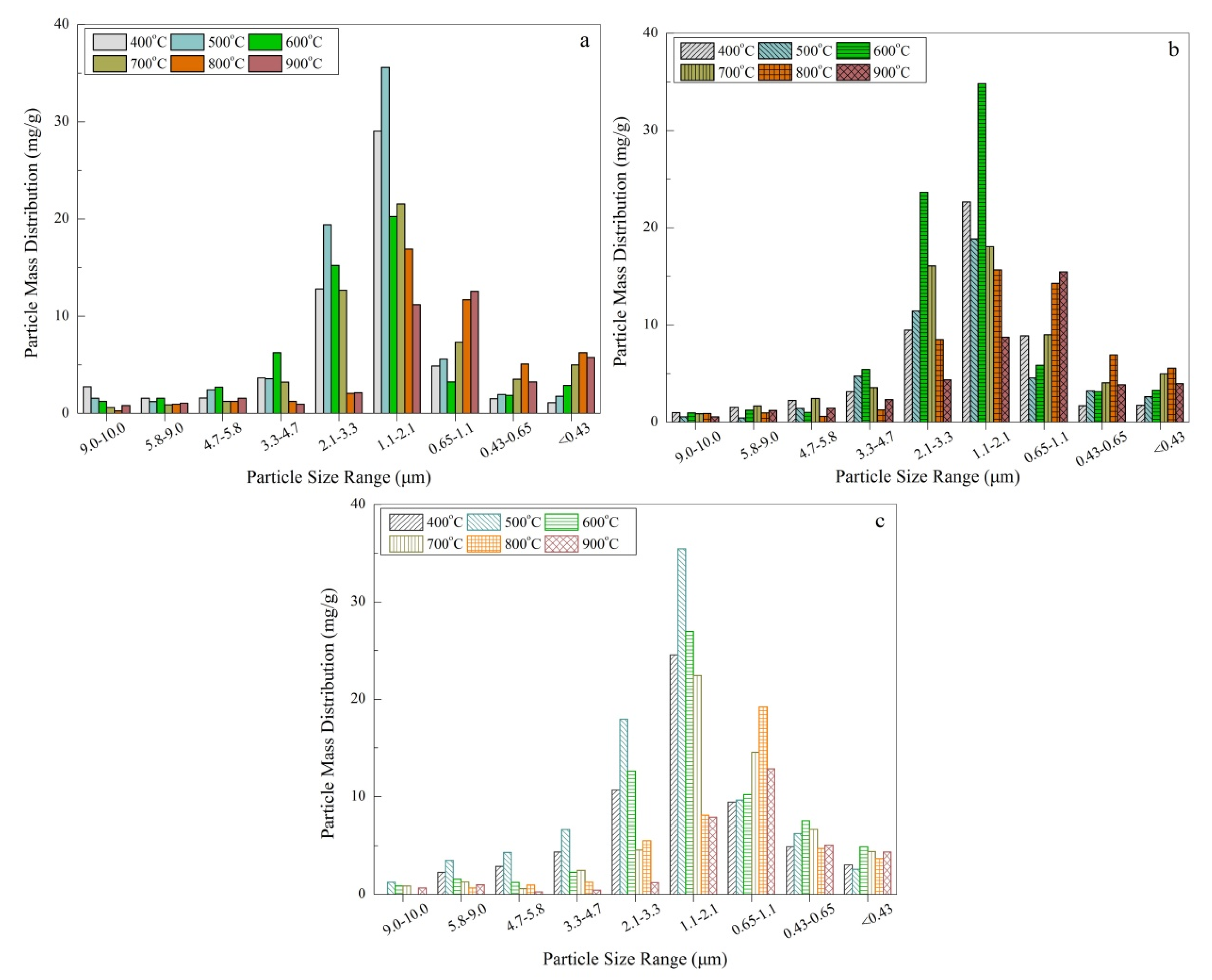
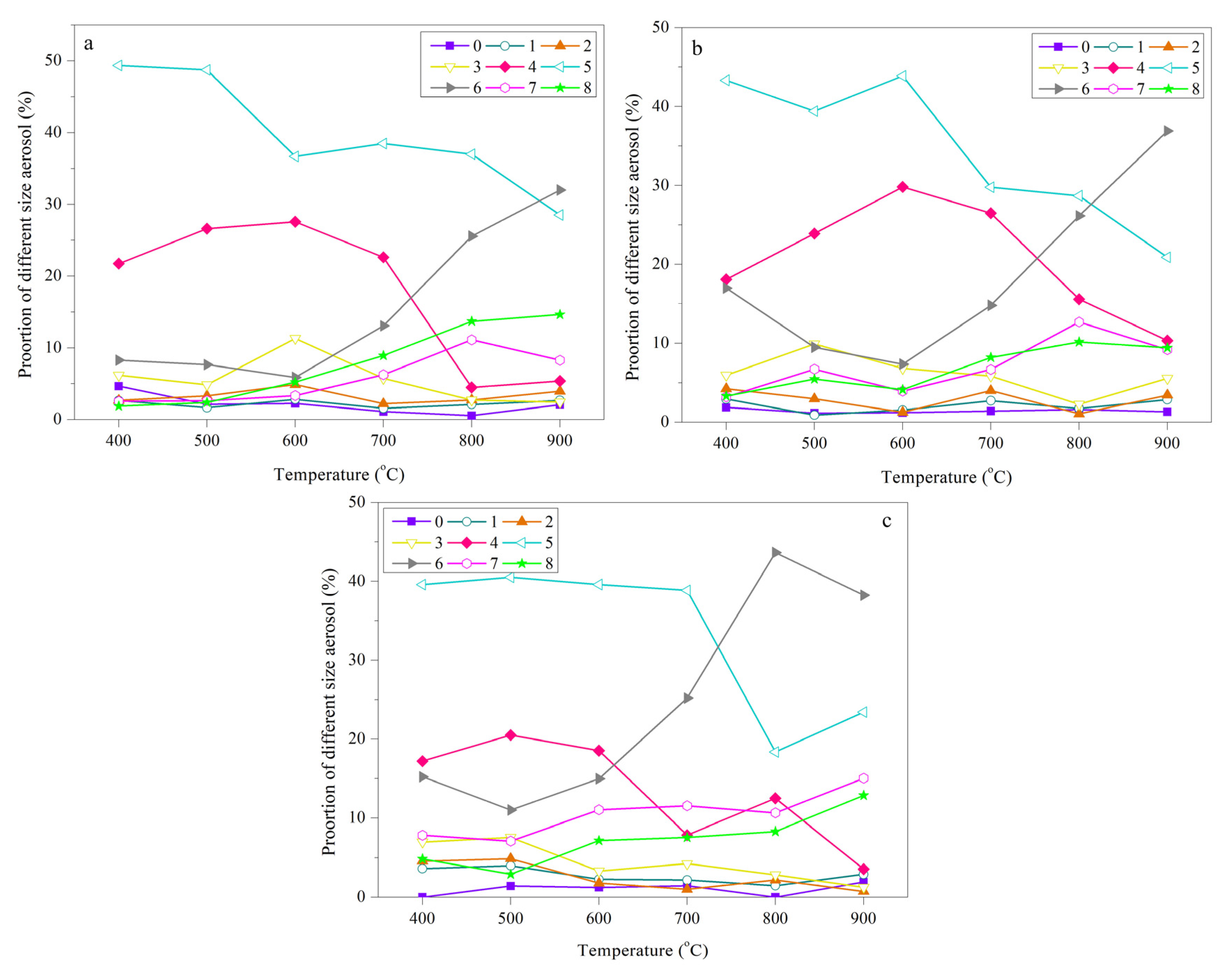
- The mass-based size distribution reveals a unimodal mode and the peak is located in the range of 1.1–2.1 μm. The maximum of a five stage classification is 35.5 mg/g and 35.1 mg/g, respectively for SR and SP. However, for SL, a yield of 34.0 mg/g was obtained. With increasing temperature the main distribution of aerosols presents a trend from coarse mode to accumulation mode. The number-based distribution confirmed the previous inference. It also further reveals that the maximum aerosol aerodynamic diameter range is mainly concentrated at about 1.0 μm.
- The emitted aerosols show three different appearances: bead-like droplets strung on the fibres; an elongated-like liquid laid on the intersection of fibres and solid crystals. During migration, homogenous or heterogeneous aggregation can occur. Additionally, aerosols mainly consist of a large proportion of organic matter and a little amount of inorganic matter. The former has a large amount of OC and VOCs exceed 50% compared with negligible EC. The ejection of AAEMs mainly happens at high temperatures in excess 700 °C.
- Approximate gas flow and high vapor concentration can promote the yield of bio-oil, while the amount of aerosol collected is reduced. At 500 °C for SR pyrolysis, the increased in bio-oil yield is about 3 wt.% and the aerosol reduction is approximately 1.3 wt.%. The longer and larger surface efficiently boosts the capture of aerosols. The direct condensation with organic solvent achieves an analogous effect, but it also brings up a new aerosol problem that can perhaps cause more complex composition issues.
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- McKay, H. Environmental, economic, social and political drivers for increasing use of woodfuel as a renewable resource in Britain. Biomass Bioenergy 2006, 30, 308–315. [Google Scholar] [CrossRef]
- Kan, T.; Strezov, V.; Evans, T.J. Lignocellulosic biomass pyrolysis: A review of product properties and effects of pyrolysis parameters. Renew. Sustain. Energy Rev. 2016, 57, 1126–1140. [Google Scholar] [CrossRef]
- Fahmy, T.Y.; Fahmy, Y.; Mobarak, F.; El-Sakhawy, M. Biomass pyrolysis past present and future. Environ. Dev. Sustain. 2018, 22, 17–32. [Google Scholar] [CrossRef]
- Williams, A.; Jones, J.M.; Ma, L.; Pourkashanian, M. Pollutants from the combustion of solid biomass fuels. Prog. Energy Combust. Sci. 2012, 38, 113–137. [Google Scholar] [CrossRef]
- García, R.; Gil, M.V.; Rubiera, F.; Pevida, C. Pelletization of wood and alternative residual biomass blends for producingindustrial quality pellets. Fuel 2019, 251, 739–753. [Google Scholar] [CrossRef]
- Qureshi, K.M.; Kay Lup, A.N.; Khan, S.; Abnisa, F.; Daud, W.M.A.W. A technical review on semi-continuous and continuous pyrolysis process of biomass to bio-oil. J. Anal. Appl. Pyrolysis 2018, 131, 52–75. [Google Scholar] [CrossRef]
- Poshina, D.N.; Raik, S.V.; Poshin, A.N.; Skorik, Y.A. Accessibility of chitin and chitosan in enzymatic hydrolysis: A review. Polym. Degrad. Stab. 2018, 156, 269–278. [Google Scholar] [CrossRef]
- Bridgwater, A.V. Review of fast pyrolysis of biomass and product upgrading. Biomass Bioenergy 2012, 38, 68–94. [Google Scholar] [CrossRef]
- Tobias-Pröll, J. karl Steam gasification of biomass in dual fluidized bed gasifiers: A review. Renew. Sustain. Energy Rev. 2018, 98, 64–78. [Google Scholar]
- Iisa, K.; Johansson, A.-C.; Pettersson, E.; French, R.J.; Orton, K.A.; Wiinikka, H. Chemical and physical characterization of aerosols from fast pyrolysis of biomass. J. Anal. Appl. Pyrolysis 2019, 142, 104606. [Google Scholar] [CrossRef]
- Vamvuka, D.; Sfakiotakis, S.; Pantelaki, O. Evaluation of gaseous and solid products from the pyrolysis of waste biomass blends for energetic and environmental applications. Fuel 2019, 236, 574–582. [Google Scholar] [CrossRef]
- Ordou, N.; Agranovski, I.E. Mass distribution and elemental analysis of the resultant atmospheric aerosol particles generated in controlled biomass burning processes. Atmos. Res. 2017, 198, 108–112. [Google Scholar] [CrossRef]
- Zhang, G.; Ma, Z.; Shen, J.; Zhang, K.; Wang, J.; Chi, Z. Experimental study on eliminating fire smokes using acoustic agglomeration technology. J. Hazard. Mater. 2020, 382, 121089. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.-Y.; Hsiao, T.-C.; Hong, B.-L. Improving the removal efficiency of fine particulate matters in air pollution control devices: Design and performance of an electrostatic aerosol particle agglomerator. J. Taiwan Inst. Chem. Eng. 2020, 107, 110–118. [Google Scholar] [CrossRef]
- Nussbaumer, T. Overview on Technologies for Biomass Combustion and Emission Levels of Particulate Matter; Swiss Federal Office for the Environment (FOEN): Zürich, Switzerland, 2010. [Google Scholar]
- Patiño, D.; Pérez-Orozco, R.; Porteiro, J.; Lapuerta, M. Characterization of biomass PM emissions using thermophoretic sampling: Composition and morphological description of the carbonaceous residues. J. Aerosol Sci. 2019, 127, 49–62. [Google Scholar] [CrossRef]
- Nussbaumer, T. Aerosols from Biomass Combustion. Technical Report on Behalf of the IEA Bioenergy Task 32; Verenum Research: Zurich, Switzerland; Lucerne University of Applied Sciences and Arts: Horw, Switzerland, 2017; p. 32. [Google Scholar]
- Bond, T.C.; Bergstrom, R.W. Light absorption by carbonaceous particles: An investigative review. Aerosol Sci. Technol. 2006, 40, 27–67. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Tan, H.; Bai, S.; Mikuli, H.; Yang, F. Evolution of PM2.5 from biomass high-temperature pyrolysis in an entrained flow reactor. J. Anal. Appl. Pyrolysis 2019, 92, 1548–1556. [Google Scholar] [CrossRef]
- Wang, X.; Bai, S.; Jin, Q.; Li, S.; Li, Y.; Li, Y.; Tan, H. Soot formation during biomass pyrolysis: Effects of temperature, waterleaching, and gas-phase residence time. J. Anal. Appl. Pyrolysis 2018, 134, 484–494. [Google Scholar] [CrossRef]
- Samburova, V.; Connolly, J.; Gyawali, M.; Yatavelli, R.L.N.; Watts, A.C.; Chakrabarty, R.K.; Zielinska, B.; Moosmüller, H.; Khlystov, A. Polycyclic aromatic hydrocarbons in biomass-burning emissions and their contribution to light absorption and aerosol toxicity. Sci. Total Environ. 2016, 568, 391–401. [Google Scholar] [CrossRef] [Green Version]
- Guillaume Schmidt, G.T.; Gontrand Leyssens, C.S.; Genevray, P.; Cazier, F.; Dewaele, D.; Coralie Vandenbilcke, E.F.; Denance, Y.; Dreff-Lorimier, C. Le Wood washing: Influence on gaseous and particulate emissions during wood combustion in a domestic pellet stove. Fuel Process. Technol. 2018, 174, 104–117. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Shen, Z.; Zhang, L.; Zhang, Q.; Lei, Y.; Cao, J.; Huang, Y.; Liu, S.; Zheng, C.; Xu, H.; et al. Impact of primary and secondary air supply intensity in stove on emissions of size-segregated particulate matter and carbonaceous aerosols from apple tree wood burning. Atmos. Res. 2018, 202, 33–39. [Google Scholar] [CrossRef]
- Hu, Z.; Wang, X.; Adewale, A.; Ruan, R.; Tan, H. Aggravated fine particulate matter emissions from heating-upgraded biomass and biochar combustion: The effect of pretreatment temperature. Fuel Process. Technol. 2018, 171, 1–9. [Google Scholar] [CrossRef]
- Morgalla, M.; Lin, L.; Seemann, M.; Strand, M. Characterization of particulate matter formed during wood pellet gasification in an indirect bubbling fluidized bed gasifier using aerosol measurement techniques. Fuel Process. Technol. 2015, 138, 578–587. [Google Scholar] [CrossRef]
- Zhao, H.; Song, Q.; Wu, X.; Yao, Q. Study on the Transformation of Inherent Potassium during the FastPyrolysis Process of Rice Straw. Energy Fuels 2015, 29, 6404–6411. [Google Scholar] [CrossRef]
- Zhao, H.; Song, Q.; Wu, X.; Yao, Q. Transformation of alkali and alkaline earth metallic species during pyrolysis and CO2 gasification of rice straw char. J. Fuel Chem. Technol. 2018, 46, 27–33. [Google Scholar] [CrossRef]
- Yang, Y.; Lin, X.; Li, S.; Luo, M.; Yin, J.; Wang, Y. Formation factors and emission characteristics of ultrafine particulate matters during Na-rich char gasification. Fuel 2019, 253, 781–791. [Google Scholar] [CrossRef]
- Mugica-Álvarez, V.; Hernández-Rosas, F.; Magaña-Reyes, M.; Herrera-Murillo, J.; Rosa, N.; Gutiérrez-Arzaluz, M.; Figueroa-Lara, J.; González-Cardoso, G. Sugarcane burning emissions: Characterization and emission factors. Atmos. Environ. 2018, 193, 262–272. [Google Scholar] [CrossRef]
- Teixeira, A.R.; Gantt, R.; Joseph, K.E.; Maduskar, S.; Paulsen, A.D.; Krumm, C.; Zhu, C.; Dauenhauer, P.J. Spontaneous Aerosol Ejection: Origin of Inorganic Particles in Biomass Pyrolysis. ChemSusChem 2016, 9, 1322–1328. [Google Scholar] [CrossRef]
- Dauenhauer, P.J.; Colby, J.L.; Balonek, C.M.; Suszynskib, W.J.; Schmidt, L.D. Reactive boiling of cellulose for integrated catalysis through an intermediate liquid. Green Chem. 2009, 11, 1555–1561. [Google Scholar] [CrossRef]
- Jendoubi, N.; Broust, F.; Commandre, J.M.; Mauviel, G.; Lédé, J. Inorganics distribution in bio oils and char produced by biomass fast pyrolysis: The key role of aerosols. J. Anal. Appl. Pyrolysis 2011, 92, 59–67. [Google Scholar] [CrossRef]
- Ko, J.H.; Wang, J.; Xu, Q. Characterization of particulate matter formed during sewage sludge pyrolysis. Fuel 2018, 224, 210–218. [Google Scholar]
- Chen, Y.; Bond, T.C. Light absorption by organic carbon from wood combustion. Atmos. Chem. Phys. 2010, 10, 1773–1778. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Chen, Y.; Bond, T.C. Light absorption of organic aerosol from pyrolysis of corn stalk. Atmos. Environ. 2016, 144, 249–256. [Google Scholar] [CrossRef] [Green Version]
- Yao, Z.; You, S.; Dai, Y.; Wang, C.-H. Particulate emission from the gasification and pyrolysis of biomass: Concentration, size distributions, respiratory deposition-based control measure evaluation. Environ. Pollut. 2018, 242, 1108–1118. [Google Scholar] [CrossRef] [Green Version]
- Subramanian, R.; Roden, C.A.; Boparai, P.; Bond, T.C. Yellow Beads and Missing Particles: Trouble Ahead for Filter-Based Absorption Measurements. Aerosol Sci. Technol. 2007, 41, 630–637. [Google Scholar] [CrossRef] [Green Version]
- Xie, M.; Shen, G.; Holder, A.L.; Hays, M.D.; Jetter, J.J. Light absorption of organic carbon emitted from burning wood, charcoal, and kerosene in household cookstoves. Environ. Pollut. 2018, 240, 60–67. [Google Scholar] [CrossRef]
- Chen, H.; Chen, X.; Qiao, Z.; Liu, H. Release and transformation characteristics of K and Cl during straw torrefaction and mild pyrolysis. Fuel 2016, 167, 31–39. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, H.; Sárossy, Z.; Dong, C.; Glarborg, P. Release and transformation of chlorine and potassium during pyrolysis of KCl doped biomass. Fuel 2017, 197, 422–432. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Xie, X.; Zhao, J.; Wei, X. The alkali metal occurrence characteristics and its release and conversion during wheat straw pyrolysis. Renew. Energy 2020, 151, 255–262. [Google Scholar] [CrossRef]
- Deng, L.; Ye, J.; Jin, X.; Che, D. Transformation and release of potassium during fixed-bed pyrolysis of biomass. J. Energy Inst. 2018, 91, 630–637. [Google Scholar] [CrossRef]
- Papari, S.; Hawboldt, K. A review on condensing system for biomass pyrolysis process. Fuel Process. Technol. 2018, 180, 1–13. [Google Scholar] [CrossRef]
- Papari, S.; Hawboldt, K.; Helleur, R.J. Pyrolysis: A theoretical and experimental study on the conversion of softwood sawmill residues to biooil. Ind. Eng. Chem. Res. 2015, 54, 605–611. [Google Scholar] [CrossRef]
- SeokChoi, H.; Seok Choi, Y.; Chae Park, H. Fast pyrolysis characteristics of lignocellulosic biomass with varying reaction conditions. Renew. Energy 2012, 42, 131–135. [Google Scholar]


| Species | Unit | SR | SL | SP | Species | Unit | SR | SL | SP |
|---|---|---|---|---|---|---|---|---|---|
| Moisture | wt.% | 6.17 | 7.92 | 8.71 | Cellulose | wt.% | 45.06 | 33.90 | 39.26 |
| Ash | wt.% | 6.29 | 10.05 | 4.23 | Hemicellulose | wt.% | 23.96 | 31.57 | 28.87 |
| Volatile matter | wt.% | 77.06 | 73.57 | 82.34 | Lignin | wt.% | 7.87 | 2.85 | 1.20 |
| Fixed carbon a | wt.% | 10.48 | 8.46 | 4.72 | Extractives | wt.% | 16.82 | 21.63 | 26.44 |
| C | wt.% | 43.82 | 47.26 | 42.53 | K | mg/kg | 26,590.0 | 13,543.7 | 31,953.3 |
| H | wt.% | 5.6 | 5.93 | 5.76 | Na | mg/kg | 82.2 | 153.5 | 208.0 |
| N | wt.% | 50.04 | 46.61 | 50.98 | Ca | mg/kg | 2100.8 | 6339.5 | 2974.6 |
| O a | wt.% | 0.54 | 0.2 | 0.73 | Mg | mg/kg | 1696.9 | 4434.5 | 2154.4 |
| Temperature (°C) | OC (μg·cm−1) | EC (μg·cm−1) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| VOCs | NVOCs | ||||||||
| SR | SL | SP | SR | SL | SP | SR | SL | SP | |
| 500 | 102.17 | 162.34 | 134.11 | 51.08 | 94.97 | 103.02 | 1.47 | 0.55 | 1.02 |
| 700 | 146.47 | 192.75 | 82.37 | 76.68 | 167.98 | 68.29 | 7.71 | 1.27 | 1.53 |
| 900 | 46.52 | 67.83 | 35.85 | 50.64 | 78.19 | 57.08 | 8.44 | 4.78 | 5.11 |
| Temperature (°C) | Product | Unit | K | Na | Ca | Mg | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SR | SL | SP | SR | SL | SP | SR | SL | SP | SR | SL | SP | |||
| 500 | Bio-oil | μg·g−1 | 212.7 | 67.7 | 479.3 | 10.4 | 27.8 | 50.5 | -- | -- | 4.3 | -- | 35.5 | 10.8 |
| Aerosol | μg·g−1 | 425.4 | 162.5 | 287.6 | 15.0 | 24.7 | 31.8 | -- | -- | 17.8 | -- | 62.1 | 23.7 | |
| 700 | Bio-oil | μg·g−1 | 930.7 | 433.4 | 1437.8 | 18.4 | 25.9 | 42.6 | 37.8 | 76.0 | 25.9 | 56.0 | 110.8 | 86.2 |
| Aerosol | μg·g−1 | 1808.1 | 623.0 | 3035.6 | 20.3 | 44.8 | 69.3 | 67.2 | 221.8 | 154.7 | 110.3 | 239.5 | 213.3 | |
| 900 | Bio-oil | μg·g−1 | 1196.6 | 623.0 | 2428.5 | 13.3 | 39.9 | 28.1 | 73.5 | 304.3 | 103.4 | 76.4 | 243.8 | 140.0 |
| Aerosol | μg·g−1 | 6700.6 | 2424.3 | 10,928.0 | 23.1 | 35.6 | 77.6 | 273.1 | 640.3 | 550.3 | 307.1 | 585.4 | 415.8 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, N.; Zhang, J.; Li, Z.; Li, Y. Characteristics of Aerosol Formation and Emissions During Corn Stalk Pyrolysis. Energies 2020, 13, 5924. https://doi.org/10.3390/en13225924
Li N, Zhang J, Li Z, Li Y. Characteristics of Aerosol Formation and Emissions During Corn Stalk Pyrolysis. Energies. 2020; 13(22):5924. https://doi.org/10.3390/en13225924
Chicago/Turabian StyleLi, Ning, Jiale Zhang, Zhihe Li, and Yongjun Li. 2020. "Characteristics of Aerosol Formation and Emissions During Corn Stalk Pyrolysis" Energies 13, no. 22: 5924. https://doi.org/10.3390/en13225924
APA StyleLi, N., Zhang, J., Li, Z., & Li, Y. (2020). Characteristics of Aerosol Formation and Emissions During Corn Stalk Pyrolysis. Energies, 13(22), 5924. https://doi.org/10.3390/en13225924





