Highly Ordered TiO2 Nanotube Electrodes for Efficient Quasi-Solid-State Dye-Sensitized Solar Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Anodic TiO2 Nanotube (NT) Electrodes
2.2. Preparation of Polymer Gel Electrolyte
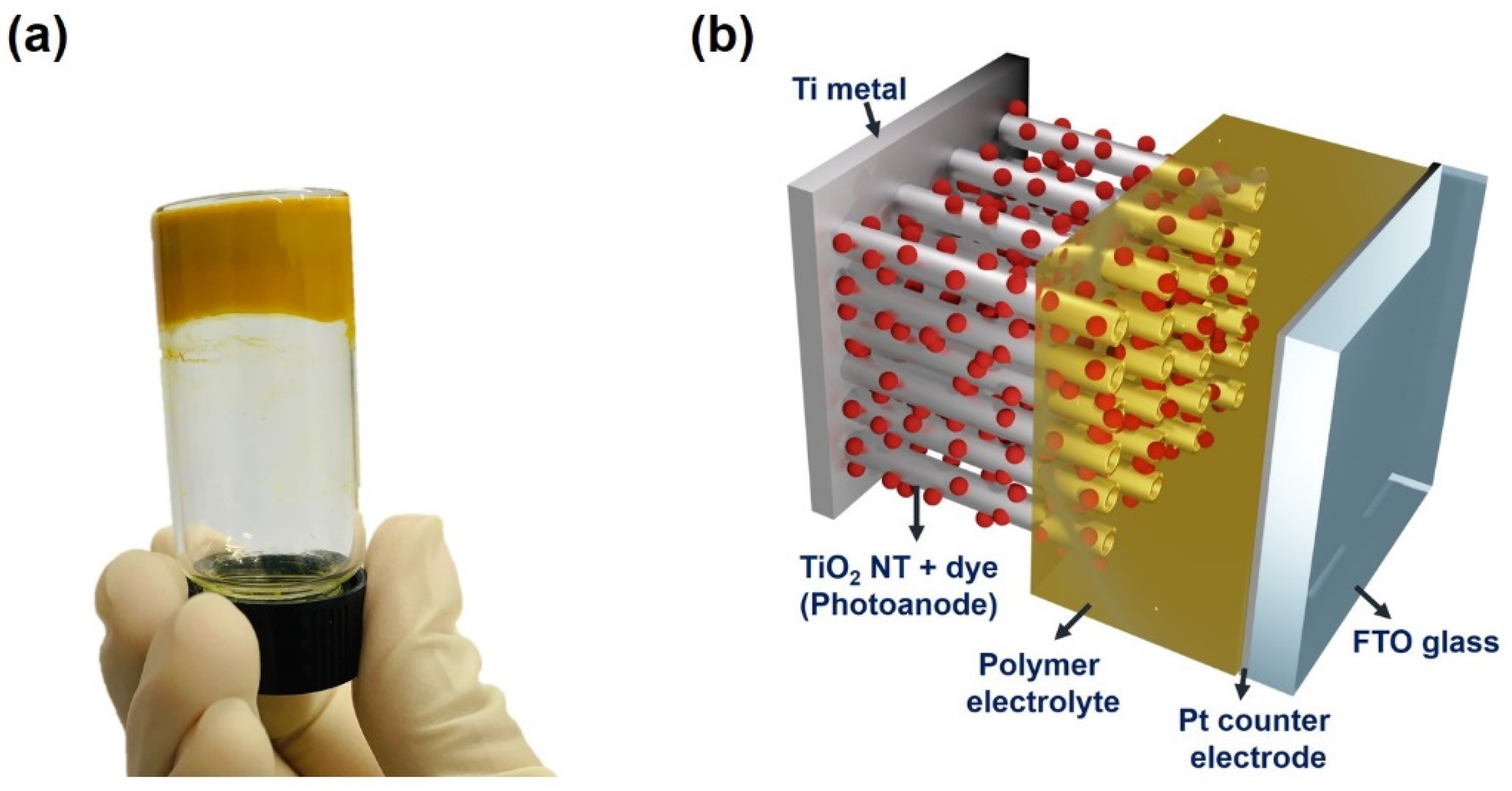
2.3. Fabrication of Dye-Sensitized Solar Cell
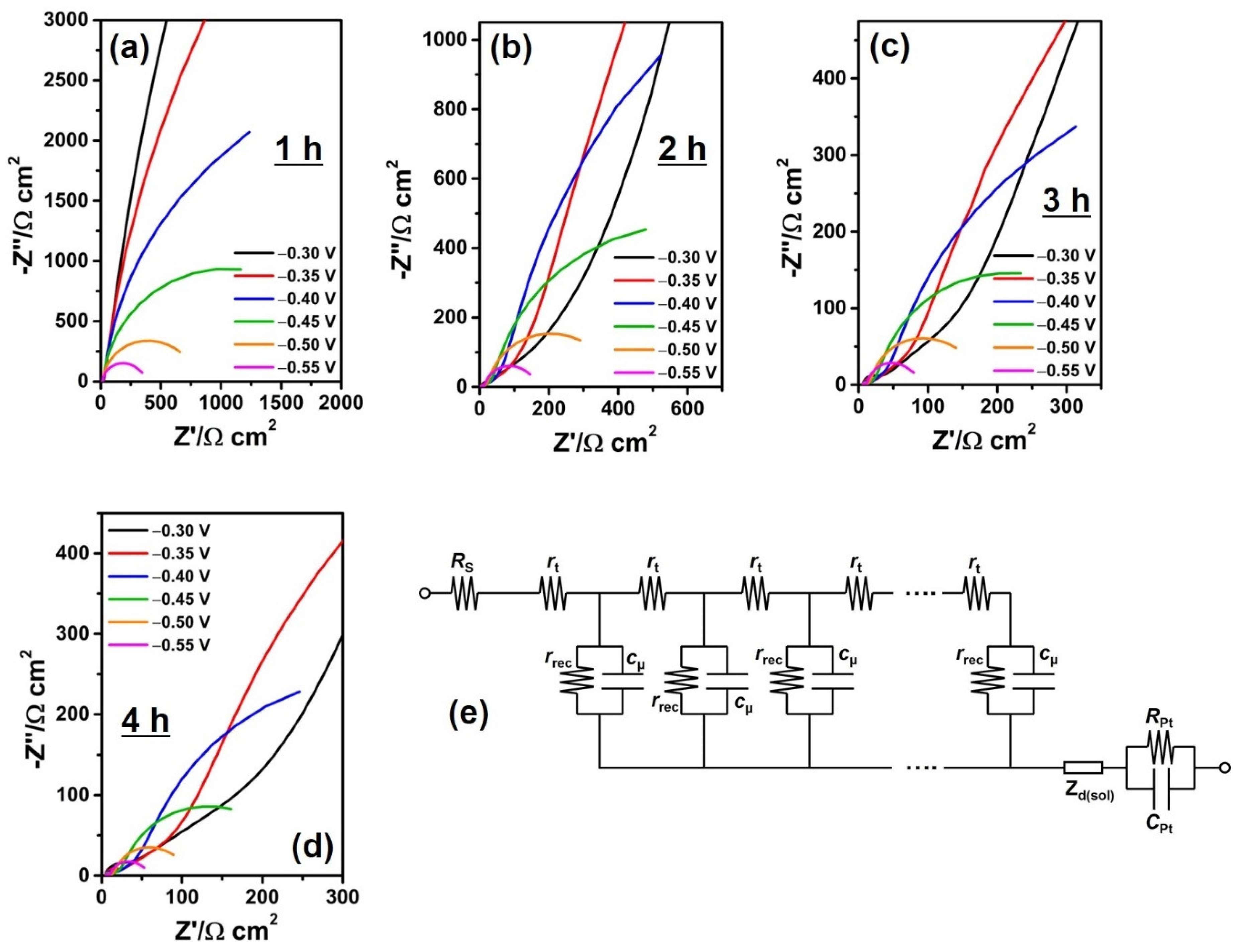
2.4. Characterization
3. Results and Discussion
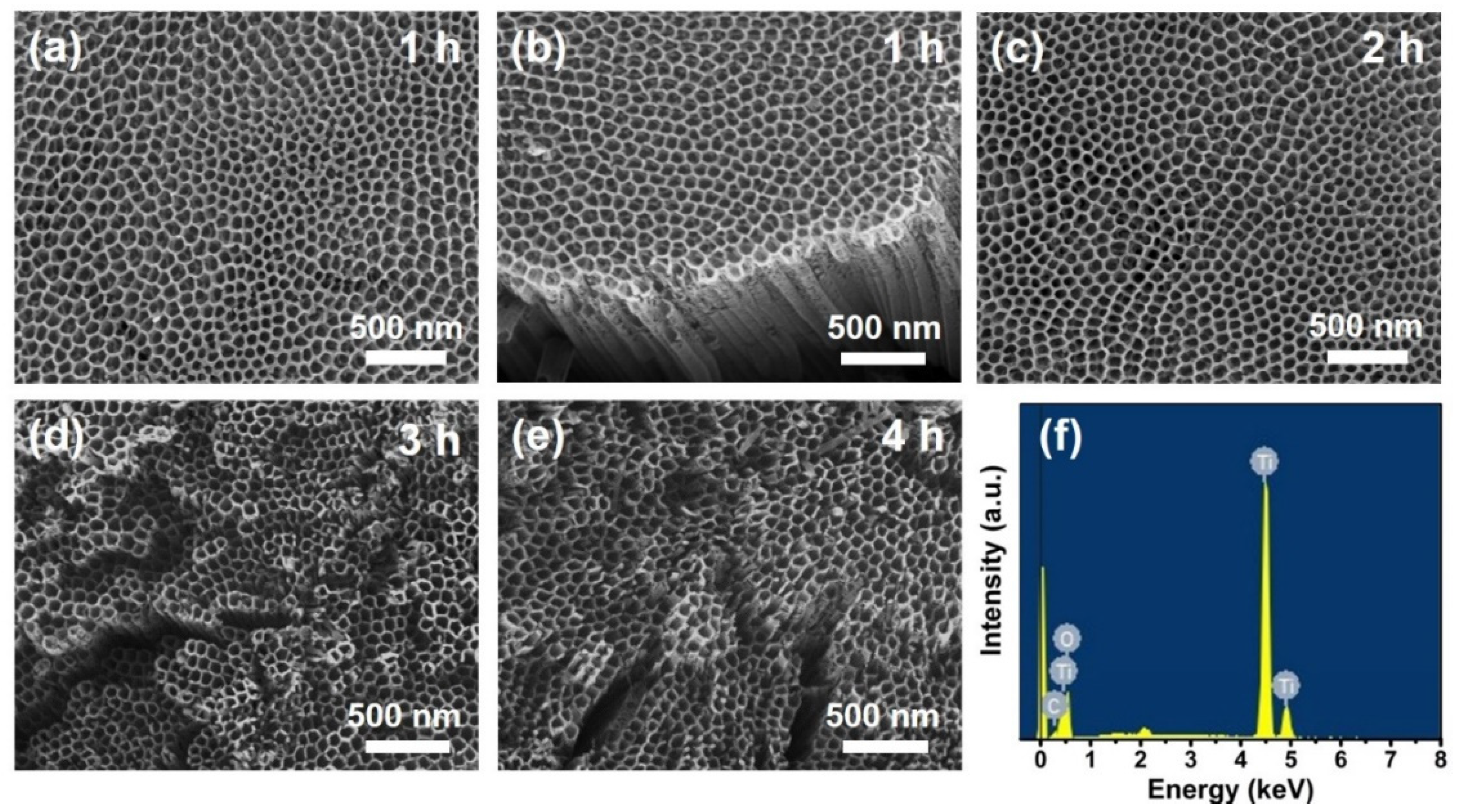
Morphology and Structure of the Anodic TiO2 NT Electrodes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- O’Regan, B.; Grätzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Lee, M.M.; Joël, T.; Tsutomu, M.; Murakani, T.N.; Snaith, H.J. Efficient hybrid solar cells based on meso-superstructured organometal halide perovskites. Science 2012, 338, 643–647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, B.-M.; Lee, M.-H.; Dilimon, V.S.; Kim, J.S.; Nam, J.S.; Cho, Y.-G.; Noh, H.K.; Roh, D.-H.; Kwon, T.-H.; Song, H.-K. Indoor-light-energy-harvesting dye-sensitized photo-rechargeable battery. Energy Environ. Sci. 2020, 13, 1473–1480. [Google Scholar] [CrossRef]
- Jiang, H.; Ren, Y.; Zhang, W.; Wu, Y.; Socie, E.C.; Carlsen, B.I.; Moser, J.-E.; Tian, H.; Zakeeuddin, S.M.; Zhu, W.-H.; et al. Phenanthrene-fused-quinoxaline as a key building block for highly efficient and stable sensitizers in copper-electrolyte-based dye-sensitized solar cells. Angew. Chem. Int. Ed. 2020, 59, 9324–9329. [Google Scholar] [CrossRef] [PubMed]
- Rajaramanan, T.; Natarajan, M.; Ravirajan, P.; Senthilnanthanan, M.; Velauthapillai, D. Ruthenium (Ru) doped titanium dioxide (P25) electrode for dye sensitized solar cells. Energies 2020, 13, 1532. [Google Scholar] [CrossRef] [Green Version]
- Chou, J.-C.; Ko, C.-C.; Chang, J.-X.; Lai, C.-H.; Nien, Y.-H.; Kuo, P.-Y.; Chen, H.-H.; Hsu, H.-H.; Hu, G.-M. Dye-sensitized solar cells using aluminum-doped zinc oxide/titanium dioxide photoanodes in parallel. Energies 2019, 12, 3469. [Google Scholar] [CrossRef] [Green Version]
- Cooper, C.B.; Beard, E.J.; Vázquez-Mayagoitia, Á.; Stan, L.; Stenning, G.B.G.; Nye, D.W.; Vigil, J.A.; Tomar, T.; Jia, J.; Bodedla, G.B.; et al. Design-to-device approach affords panchromatic co-sensitized solar cells. Adv. Energy Mater. 2018, 9. [Google Scholar] [CrossRef] [Green Version]
- Hagfeldt, A.; Boschloo, G.; Sun, L.; Kloo, L.; Pettersson, H. Dye-sensitized solar cells. Chem. Rev. 2010, 11, 6595–6663. [Google Scholar] [CrossRef]
- Yoo, K.; Kim, J.-Y.; Lee, J.A.; Kim, J.S.; Lee, D.-K.; Kim, K.; Kim, J.Y.; Kim, B.S.; Kim, H.; Kim, W.M.; et al. Completely transparent conducting oxide-Free and flexible dye-sensitized solar cells fabricated on plastic substrates. ACS Nano 2015, 9, 3760–3771. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Kang, S.H.; Kim, H.S.; Sung, Y.-E. Preparation of highly ordered mesoporous Al2O3/TiO2 and its application in dye-sensitized solar cells. Langmuir 2010, 26, 2864–2870. [Google Scholar] [CrossRef]
- Lee, M.-W.; Kim, J.-Y.; Lee, H.-G.; Cha, H.G.; Lee, D.-H.; Ko, M.J. Effects of structure and electronic properties of D-π-A organic dyes on photovoltaic performance of dye-sensitized solar cells. J. Energy Chem. 2021, 54, 208–216. [Google Scholar] [CrossRef]
- Kang, J.S.; Choi, H.C.; Kim, J.; Park, H.; Kim, J.-Y.; Choi, J.-W.; Yu, S.-H.; Lee, K.J.; Kang, Y.S.; Park, S.H.; et al. Multidimensional anodized yitanium foam photoelectrode for efficient utilization of photons in mesoscopic solar cells. Small 2017. [Google Scholar] [CrossRef]
- Mor, G.K.; Shankar, K.; Paulose, M.; Varghese, O.K.; Grimes, C.A. Use of highly-ordered TiO2 nanotube arrays in dye-sensitized solar cells. Nano Lett. 2006, 6, 215–218. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Neale, N.R.; Miedaner, A.; Frank, A.J. Enhanced charge-collection efficiencies and light scattering in dye-sensitized solar cells using oriented TiO2 nanotubes arrays. Nano Lett. 2007, 7, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Vinzant, T.B.; Neale, N.R.; Frank, A.J. Removing structural disorder from TiO2 oriented nanotube arrays: reducing the dimensionality of transport and recombination in dye-sensitized solar cells. Nano Lett. 2007, 7, 3739–3746. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-Y.; Shin, J.Y.; Kim, D.H.; Sung, Y.-E.; Ko, M.J. Long vertically aligned TiO2 nanotube electrodes prepared via two-step anodization for highly efficient photovoltaics. Isr. J. Chem. 2015, 55, 1034–1040. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Shin, K.-Y.; Raza, M.H.; Pinna, N.; Sung, Y.-E. Vertically aligned TiO2ZnO nanotube arrays prepared by atomic layer deposition for photovoltaic applications. Korean J. Chem. Eng. 2019, 36, 1157–1163. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Lee, K.J.; Kang, S.H.; Shin, J.; Sung, Y.-E. Enhanced photovoltaic properties of a cobalt bipyridyl redox electrolyte in dye-sensitized solar cells employing vertically aligned TiO2 nanotube electrodes. J. Phys. Chem. C 2011, 115, 19979–19985. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Lee, K.-H.; Shin, J.; Park, S.H.; Kang, J.S.; Han, K.S.; Sung, M.M.; Pinna, N.; Sung, Y.-E. Highly ordered and vertically oriented TiO2/Al2O3 nanotube electrodes for application in dye-sensitized solar cells. Nanotechnology 2014, 25. [Google Scholar] [CrossRef]
- Kuang, D.; Brillet, J.; Chen, P.; Takata, M.; Uchida, S.; Miura, H.; Sumioka, K.; Zakeeruddin, S.M.; Grätzel, M. Application of highly ordered TiO2 nanotube arrays in flexible dye-sensitized solar cells. ACS Nano 2008, 2, 1113–1116. [Google Scholar] [CrossRef]
- Jin, L.N.; Qian, X.Y.; Wang, J.G.; Aslan, H.; Dong, M. MIL-68 (In) nano-rods for the removal of Congo red dye from aqueous solution. J. Colloid Interface Sci. 2015, 453, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.H.; Kim, J.-Y.; Kim, Y.; Kim, H.S.; Sung, Y.-E. Surface modification of stretched TiO2 nanotubes for solid-state dye-sensitized solar cells. J. Phys. Chem. C 2007, 111, 9614–9623. [Google Scholar] [CrossRef]
- Stergiopoulos, T.; Ghicov, A.; Likodimos, V.; Tsoukleris, D.S.; Kunze, J.; Schmuki, P.; Falaras, P. Dye-sensitized solar cells based on thick highly ordered TiO2 nanotubes produced by controlled anodic oxidation in non-aqueous electrolytic media. Nanotechnology 2008, 19. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Brillet, J.; Bala, H.; Wang, P.; Zakeeruddin, S.M.; Grätzel, M. Solid-state dye-sensitized solar cells using TiO2 nanotube arrays on FTO glass. J. Mater. Chem. 2009, 19, 5325–5328. [Google Scholar] [CrossRef]
- Pugliese, D.; Lamberti, A.; Bella, F.; Sacco, A.; Bianco, S.; Tresso, E. TiO2 nanotubes as flexible photoanode for back-illuminated dye-sensitized solar cells with hemi-squaraine organic dye and iodine-free transparent electrolyte. Org. Electron. 2014, 15, 3715–3722. [Google Scholar] [CrossRef]
- Seidalilir, Z.; Malekfar, R.; Wu, H.-P.; Shiu, J.-W.; Diau, W.-G. High-performance and stable gel-state dye-sensitized solar cells using anodic TiO2 nanotube arrays and polymer-based gel electrolytes. ACS Appl. Mater. Interfaces 2015, 7, 12731–12739. [Google Scholar] [CrossRef]
- Krumpmann, A.; Dervaux, J.; Derue, L.; Douhéret, O.; Lazzaroni, R.; Snyders, R.; Decroly, A. Influence of a sputtered compact TiO2 layer on the properties of TiO2 nanotube photoanodes for solid-state DSSCs. Mater. Des. 2017, 120, 298–306. [Google Scholar] [CrossRef]
- Xiao, B.-C.; Lin, L.-Y. Tuning electrolyte configuration and composition for fiber-shaped dye-sensitized solar cell with poly (vinylidene fluoride-co-hexafluoropropylene) gel electrolyte. J. Colloid Interface Sci. 2020, 571, 126–133. [Google Scholar] [CrossRef]
- Praveen, E.; Peter, I.J.; Kumar, A.M.; Ramachandran, K.; Jayakumar, K. Boosting of power conversion efficiency of 2D ZnO nanostructures-based DSSC by the Lorentz force with chitosan polymer electrolyte. J. Inorg. Organomet. Polym. Mater. 2020, 30. [Google Scholar] [CrossRef]
- Selvanathan, V.; Yahya, R.; Alharbi, H.F.; Alharthi, N.H.; Alharthi, Y.S.; Ruslan, M.H.; Amin, N.; Akhtaruzzaman, M. Organosoluble starch derivative as quasi-solid electrolytes in DSSC: Unravelling the synergy between electrolyte rheology and photovoltaic properties. Sol. Energy 2020, 197, 144–153. [Google Scholar] [CrossRef]
- Galliano, S.; Bella, F.; Bonomo, M.; Viscardi, G.; Gerbaldi, C.; Boschloo, G.; Barolo, C. Hydrogel electrolytes based on xanthan gum: Green route towards stable dye-sensitized solar cells. Nanomaterials 2020, 10, 1585. [Google Scholar] [CrossRef] [PubMed]
- Stergiopoulos, T.; Arabatzis, I.M.; Katsaros, G.; Falaras, P. Binary Polyethylene oxide/titania solid-state redox electrolyte for highly efficient nanocrystalline TiO2 photoelectrochemical cells. Nano Lett. 2002, 2, 1259–1261. [Google Scholar] [CrossRef]
- Ghicov, A.; Albu, S.P.; Hahn, R.; Kim, D.; Stergiopoulos, T.; Kunze, J.; Schiller, C.-A.; Falaras, P.; Schmuki, P. TiO2 nanotubes in dye-sensitized solar cells: Critical factors for the conversion efficiency. Chem. Asian J. 2009, 4, 520–525. [Google Scholar] [CrossRef] [PubMed]
- Nazeeruddin, K.M.; Zakeeruddin, S.M.; Humphry-Baker, R.; Jirousek, M.; Liska, P.; Vlachopoulos, N.; Shklover, V.; Fischer, C.-H.; Grätzel, M. Acid−base equilibria of (2,2‘-Bipyridyl-4,4‘-dicarboxylic acid)ruthenium(II) complexes and the effect of protonation on charge-transfer sensitization of nanocrystalline titania. Inorg. Chim. 1999, 38, 6298–6305. [Google Scholar] [CrossRef]
- Lenzmann, F.; Krueger, J.; Burnside, S.; Brooks, K.; Grätzel, M.; Gal, D.; Rühle, S.; Cahen, D. Surface photovoltage spectroscopy of dye-sensitized solar cells with TiO2, Nb2O5, and SrTiO3 nanocrystalline photoanodes: Indication for electron injection from higher excited dye states. J. Phys. Chem. B 2001, 105, 6347–6352. [Google Scholar] [CrossRef]
- Ziółek, M.; Cohen, B.; Yang, X.; Sun, L.; Paulose, M.; Varghese, O.K.; Grimes, C.A.; Douhal, A. Femtosecond to millisecond studies of electron transfer processes in a donor- (π-spacer)–acceptor series of organic dyes for solar cells interacting with titania nanoparticles and ordered nanotube array films. Phys. Chem. Chem. Phys. 2012, 14, 2816–2831. [Google Scholar] [CrossRef]
- Zukalová, M.; Procházka, J.; Zukal, A.; Yum, J.H.; Kavan, L. Structural parameters controlling the performance of organized mesoporous TiO2 films in dye sensitized solar cells. Inorg. Chim. Acta 2008, 361, 656–662. [Google Scholar] [CrossRef]
- Fang, K.; Yang, Y.; Fu, L.; Zheng, H.; Yuan, J.; Niu, L. Highly selective H2O2 sensor based on 1-D nanoporous Pt@C hybrids with core–shell structure. Sen. Actuators B 2014, 191, 401–407. [Google Scholar] [CrossRef]
- Wang, Z.; Li, P.; Chen, Y.; He, J.; Zhang, W.; Schmidt, O.G.; Li, Y. Pure thiophene–sulfur doped reduced graphene oxide: Synthesis, structure, and electrical properties. Nanoscale 2014, 6, 7281–7287. [Google Scholar] [CrossRef] [Green Version]
- Fabregat-Santiago, F.; Bisquert, J.; Garcia-Belmonte, G.; Boschloo, G.; Hagfeldt, A. Influence of electrolyte in transport and recombination in dye-sensitized solar cells studied by impedance spectroscopy. Sol. Energy Mater. Sol. Cells 2005, 87, 117–131. [Google Scholar] [CrossRef]
- Jennings, J.R.; Liu, Y.; Safari-Alamuti, F.; Wang, Q. Dependence of dye-sensitized solar cell impedance on photoelectrode thickness. J. Phys. Chem. C 2012, 116, 1556–1562. [Google Scholar] [CrossRef]
- Qiu, J.; Zhuge, F.; Lou, K.; Li, X.; Gao, Z.; Gan, X.; Yu, W.; Kim, H.-K.; Hwang, Y.-H. A facile route to aligned TiO2 nanotube arrays on transparent conducting oxide substrates for dye-sensitized solar cells. J. Mater. Chem. 2011, 21, 5062–5068. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, J.; Hao, X.; Wang, Y.; Chen, Y.; Li, P. Enhanced power density of a supercapacitor by introducing 3D-interfacial graphene. New J. Chem. 2020, 44, 13377–13381. [Google Scholar] [CrossRef]
- Hsiao, P.T.; Liou, Y.J.; Teng, H. Electron transport patterns in TiO2 nanotube arrays based dye-sensitized solar cells under frontside and backside illuminations. J. Phys. Chem. C 2011, 115, 15018–15024. [Google Scholar] [CrossRef]



| Anodization Time | JSC (mA/cm2) | VOC (mV) | FF | η (%) | Adsorbed Amount of Dye (×10−8 mol cm−2) |
|---|---|---|---|---|---|
| 1 h | 5.91 ± 0.21 | 539 ± 4 | 55.0 ± 2.1 | 1.75 ± 0.09 | 6.6 |
| 2 h | 8.05 ± 0.24 | 523 ± 6 | 52.1 ± 1.2 | 2.19 ± 0.08 | 12.3 |
| 3 h | 7.61 ± 0.30 | 503 ± 3 | 51.7 ± 0.6 | 1.98 ± 0.05 | 16.7 |
| 4 h | 7.48 ± 0.57 | 479 ± 6 | 53.7 ± 3.7 | 1.92 ± 0.01 | 20.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, A.R.; Kim, J.-Y. Highly Ordered TiO2 Nanotube Electrodes for Efficient Quasi-Solid-State Dye-Sensitized Solar Cells. Energies 2020, 13, 6100. https://doi.org/10.3390/en13226100
Lee AR, Kim J-Y. Highly Ordered TiO2 Nanotube Electrodes for Efficient Quasi-Solid-State Dye-Sensitized Solar Cells. Energies. 2020; 13(22):6100. https://doi.org/10.3390/en13226100
Chicago/Turabian StyleLee, A Reum, and Jae-Yup Kim. 2020. "Highly Ordered TiO2 Nanotube Electrodes for Efficient Quasi-Solid-State Dye-Sensitized Solar Cells" Energies 13, no. 22: 6100. https://doi.org/10.3390/en13226100
APA StyleLee, A. R., & Kim, J.-Y. (2020). Highly Ordered TiO2 Nanotube Electrodes for Efficient Quasi-Solid-State Dye-Sensitized Solar Cells. Energies, 13(22), 6100. https://doi.org/10.3390/en13226100





