Abstract
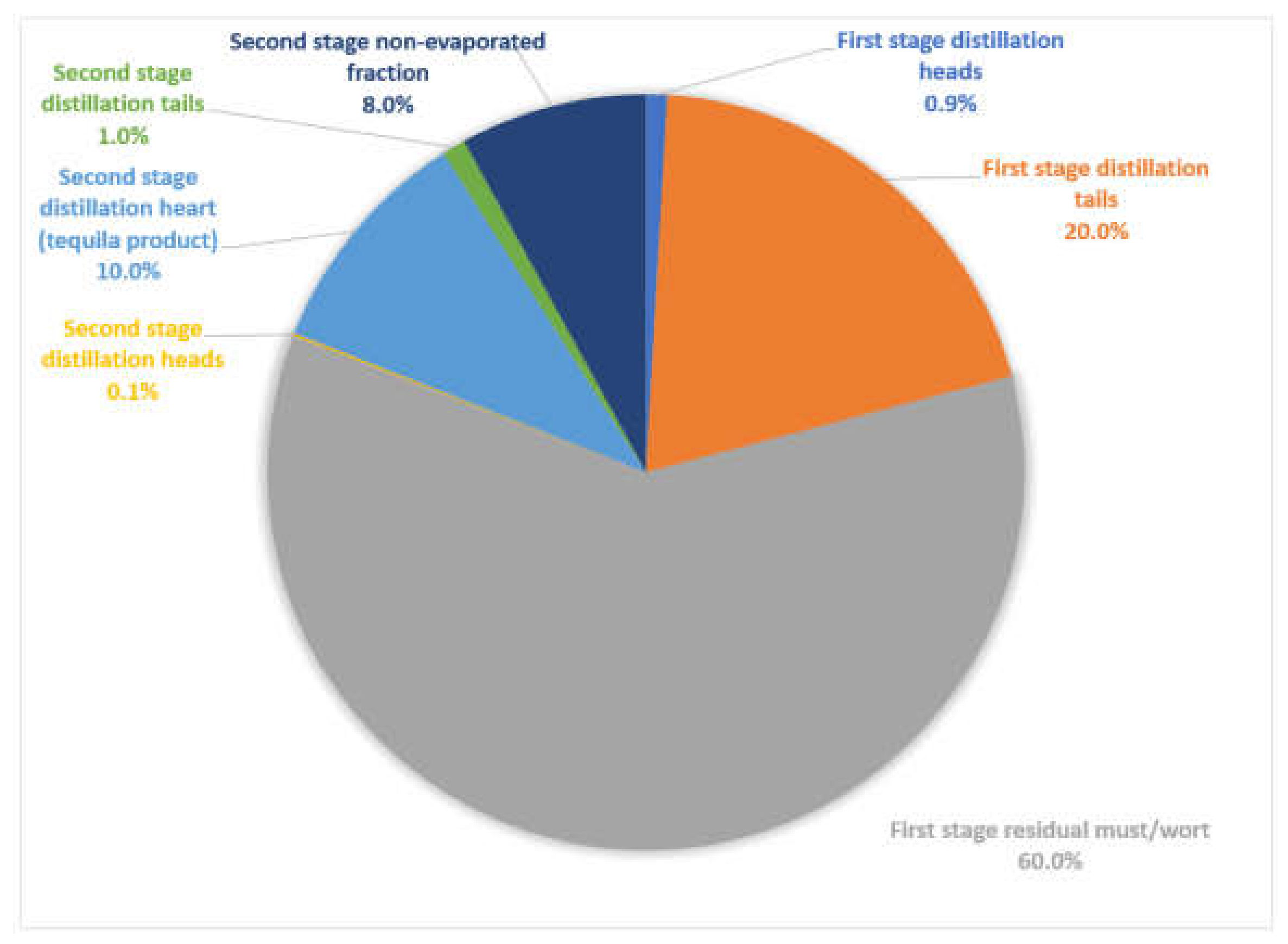
Tequila vinasses is a mixture made from up to six still distillation two-stage process residual effluents. First stage fractions: residual must (60%), heads (0.9%) and tails (20.0%); second stage fractions: non-evaporated (8.0%), heads (0.1%) and tails (1.0%); the result is a more complex effluent for its treatment or biorefining. The objectives of this study were to: (a) characterize the five still distillation volatile streams in the Tequila 100% Agave processing; compounds: methanol, ethanol, acetaldehyde, ethyl acetate, sec-butanol, n-propanol, iso-butanol, n-butanol, iso-amyl, n-amyl, and ethyl lactate were detected by gas chromatography; calculated chemical oxygen demand from chemical composition had very high values (53,760–1,239,220 mg/L); measurement of pH (3.24–4.80), color (38.6 UC Pt-Co max), turbidity (46.1 max), electrical conductivity (3.30–172.20 μS/cm), and solid content (0 mg/L) was also made; (b) report an energy analysis (2.02 × 109 KWh) and CO2 production (429 × 106 kg) in the Tequila industry during 2019; (c) up to date residues (365.2 × 106 kg agave bagasse, 1146.1 × 106 kg agave leaves and 3300.0 × 106 L agave vinasse) in 2019; (d) economic analysis, current tequila vinasses treatment price is 16.00 USD/m3 but could reach a considerable fraction value if is bio-refined, a break down component analysis reach for five volatile streams $51.23–$140.00 USD/m3.
1. Introduction
1.1. Tequila Process
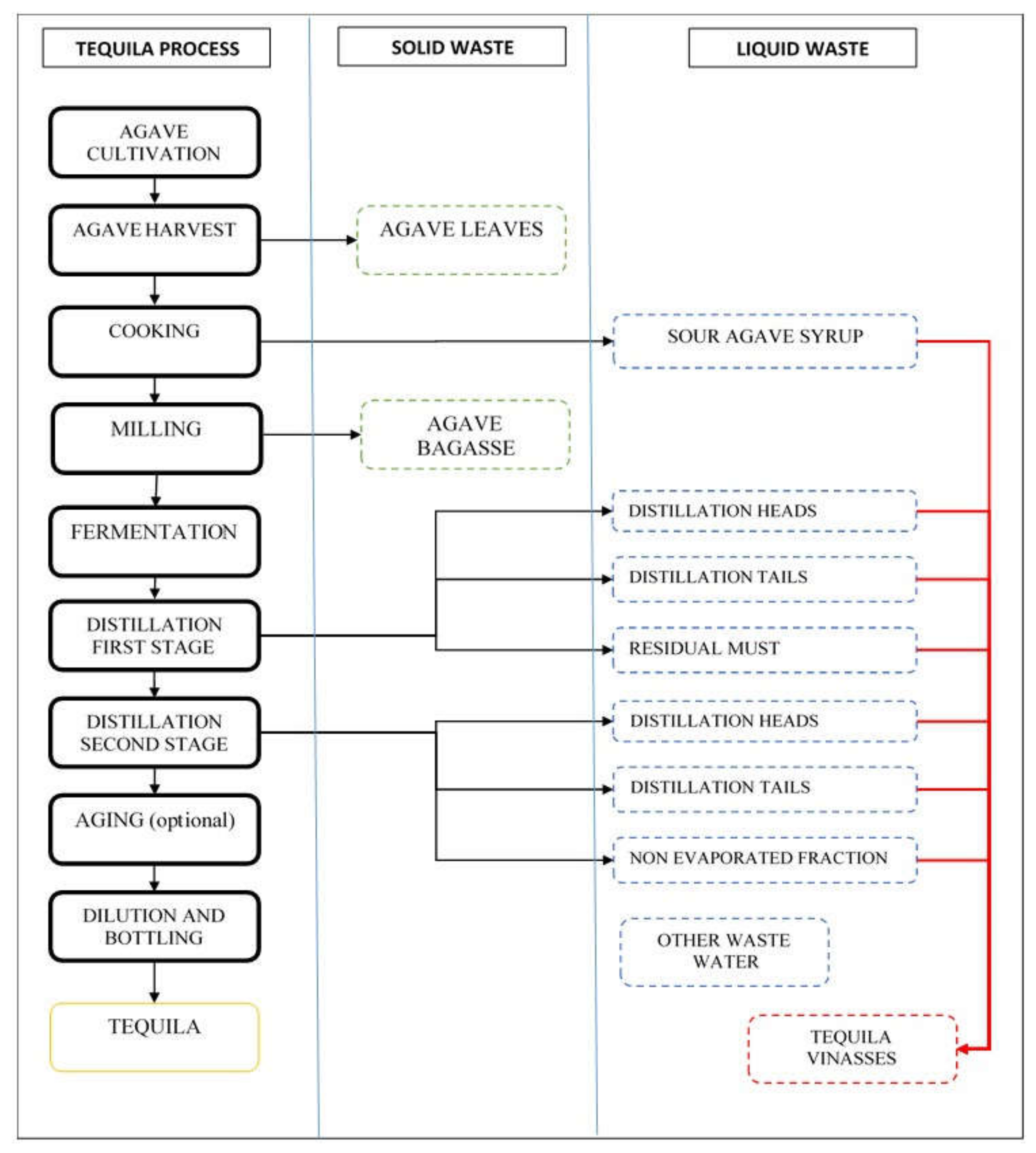
Tequila is a Mexican beverage protected by Origin Denomination, its production and commercialization are verified and certified by Tequila Regulating Council (CRT) [1,2]. The tequila production process consists of Agave tequilana Weber sugars extraction, by hydrolysis or cooking followed by milling to obtain agave juice for further fermentation, distillation, mixing, aging, and bottling. Tequila has two categories: “Tequila 100% agave” and “Tequila” (which is made with a minimum 51% agave reducing sugars and generally completed with cane sugar). It also produces the following residues: agave leaves, agave bagasse, and tequila vinasses [1,2,3,4].
1.2. Tequila Residues
Agave leaves are generally left in fields after agave harvest, to reincorporate nutrients by natural decomposition or stirring them with soil. In tequila factories, agave bagasse or wet agave bagasse are agave fibers after juice extraction with no further use in the tequila processing. Tequila vinasses are produced in the tequila distillation process, which can be done in a distillation tower or distillation still. Tequila vinasses are reported as residual must or wort, considering them one residual stream. Distillation has two purposes: separate and increase the ethanol and light flavor compounds concentration, and remove toxic substances such as methanol, superior alcohols, residual organic matter, and minerals [3,4].
1.3. Tequila Distillation
Tequila distillation by distillation still covers a two-stage process. The first stage starts by boiling the agave must. The first distilled fraction is the first stage heads, this is a residual stream. The next distillation fraction is the first stage heart which will be directed to distillation second stage. The last first stage distillation fraction is the first stage tails; this is also a residual stream. In the still will remain the residual must, also called tequila vinasses, or raw tequila vinasses. Distillation second stage uses the first stage distillation heart in the still. Second stage heads is the first fraction obtained, also a residual stream. The next distillation fraction is second stage heart which is the Tequila, this fraction will be leaded to aging and/or diluting. The last distillation fraction is the second stage distillation tails, the last residual distilled stream. In the still will be left the second stage non-evaporated fraction [3,4,5].
1.4. Tequila Industry Products and Residues
Tequila production by category in 2019 was 138.3 × 106 “Tequila” liters and 191.7 × 106 “Tequila 100% agave” liters according to CRT data [6]. It is important to point out that “Tequila” (51% agave) is 41.91% of total tequila production meanwhile “Tequila 100% agave” is 58.09%. Most publications in this field refer to tequila as only to tequila 100% agave, making no distinction per tequila category.
For each “Tequila 100% agave” liter, 4.37 kg agave leaves [7] and 1.4 kg agave bagasse [8] are produced; also a production of 2.23 kg agave leaves and 0.7 kg agave bagasse per “Tequila” liter since the agave amount required is reduced to 51%. Based on CRT data [6], estimated agave leaves amount in 2019 are 837.7 × 106 kg for “Tequila 100% agave” and 308.4 × 106 kg for “Tequila”, a total amount of 1146.1 × 106 kg agave leaves; an estimation in agave bagasse yielding in 2019 are 268.4 × 106 kg for “Tequila 100% agave” and 96.8 × 106 kg for “Tequila”, a total amount of 365.2 × 106 kg agave bagasse. Tequila vinasses yield rate is considered to be 10–12 L per tequila liter produced [8] regardless of the tequila category. According to CRT data [6], 330.0 × 106 tequila liters were produced in 2019, leading to a minimum production of 3300.0 × 106 L tequila vinasses. See Table 1 for further reference.

Table 1.
Tequila, agave, and residues estimate data in 2019. Adapted from CRT [6].
1.5. Objectives
The objectives of the present work read as follows:
- (a)
- Characterize five still distillation residual effluents to consider these effluents for further biorefinery processes. Residual must is out of the scope for this investigation.
- (b)
- Make a tequila industry level energy analysis and CO2 production.
- (c)
- Point out tequila categories and their respective residues.
- (d)
- Propose an economic analysis to encourage biorefining of analyzed streams.
1.6. Scientific Contribution
It is important to point out that the tequila industry residual liquid streams is not only one (tequila vinasses); during two-stage still distillation process there are six residual streams (heads, tails and stillage in first and second stage) that have not been characterized separately; these streams could lead to separate processes making an alternative for biorefining and water recover; also a CO2 production and economic analysis for treatment vs. refined price is included.
In Section 2 will be described the materials and methods for analysis used, chemical oxygen demand calculation and document research for energy and economic analysis.
Section 3 shows the results for chemical composition reported in tables and respective figures for a clearer understanding, same applies to physic-chemical properties.
2. Materials and Methods
2.1. Materials
The most common tequila production in medium size factories uses cooking in an autoclave, for thermal efficiency improvement, mills, and distillation stills constructed in stainless steel to avoid corrosion and increase lifetime.
Revising tequila processing it was noted that “tequila vinasses” are not yielded from a single stream. Practically three or more effluents are combined to form the final residue by mixing. The three participant factories used a dump duct where all residual streams were directed and then discharged in pools outside the factory for cooling. See Figure 1. All effluents are not produced simultaneously thus it could be possible to lead each stream to a different vessel. When agave prices are high, some producers use distillation heads and tails for mixing in cheap or low-quality tequila, complying with tequila Mexican Official Norm NOM-142-SSA1/SCFI-2014 “Alcoholic beverages-Sanitary specifications-Sanitary and commercial labeling” [5].
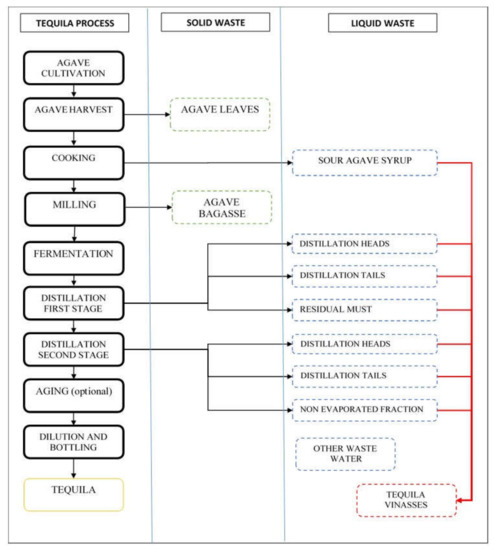
Figure 1.
Tequila traditional production process (still distillation) residual streams.
Samples of all distilled (evaporated-condensed) streams were taken directly from the still distillation process of tequila 100% agave from three different tequila factories. From first stage distillation heads (3 samples) and tails (3 samples) were collected from the spillage pipe located at the end of the still condenser and acts as a manually operated separation valve; from second stage heads (3 samples) and tails (3 samples) also collected from the spillage pipe and a non-evaporated fraction (3 samples) collected from still output pipe. Samples were collected in sterilized glass bottles during distillation and taken to refrigeration in the laboratory at 4 °C. Making a total of five samples per tequila factory, 15 samples in total.
It was conducted an analysis regarding volume balance in the three factories for a two-stage still distillation process to know the distillation streams proportions and an economic analysis.
2.2. Methods
Samples were analyzed by gas chromatography according to Mexican Official Norm NOM-142-SSA1/SCFI-2014 “Alcoholic beverages. Sanitary specifications. Sanitary and commercial labeling.” to report ethanol, methanol, acetaldehyde, ethyl acetate, sec-butanol, n-propanol, iso-butanol, n-butanol, iso-amyl, n-amyl and ethyl lactate.
Measurement of pH was made according to the Standard Method for the Examination of Water and Wastewater 4500-H+B: pH in Water by Potentiometry. Electrical conductivity was obtained according to Standard Method for the Examination of Water and Wastewater 2510 Conductivity (2017). Color Pt-Co according to Standard Method for the Examination of Water and Wastewater 2120 Color (2017). Turbidity was taken according to Standard Method for the Examination of Water and Wastewater 2130 Turbidity (2017). Solids content measurements were also taken for total solids (TS), total suspended solids (TSS), total dissolved solids (TDS), and total volatile solids (TVS) according to Standard Method for the Examination of Water and Wastewater 2540 Solids (2017).
Chemical oxygen demand is calculated based on chemical composition, it is the oxygen required to oxidize organic matter. The following chemical reactions and equations apply:
Methanol:
Ethanol:
Acetaldehyde:
Ethyl acetate:
n-propanol:
Sec-butanol/iso-butanol/n-butanol:
Iso-amyl/n-amyl:
Ethyl lactate:
Regarding Energy a survey of fuel oil consumption was made. The participant tequila factories use fuel oil steam boilers. The amount of fuel used per tequila liter produced is reported. CO2 production is calculated based on fuel oil chemical composition.
With respect to economic analysis, a survey for vinasses treatment cost is reported. Two online reference websites for chemical prices were consulted to obtain analyzed compounds pricing and a mixture price for each stream was calculated.
3. Results
3.1. Tequila Distillation by Two-Stage Distillation Still Process Volume Balance
Several tequila factories use stainless steel distillation stills of 5000 L capacity. As stated before, two stages are required for this distillation method. In Table 2 is reported the volume balance for each stage, considering as basis a batch of 5000 L agave must, which will feed the distillation first stage still. The second stage still is also performed with 5000 L and the process will begin once several batches of first stage distillation heart currents are gathered; to be concise, in Table 2 is reported the resulting streams proportionally. From a 5000 L agave must basis, first stage heads represents 0.8–1.0% total volume, first stage heart 16–19%, first stage tails 19–21%, residual must 59–60%, second stage heads 0.08–0.10%, second stage heart 8.0–9.5%, second stage tails 0.8–1.0% and second stage non-evaporated fraction 5.5–8.4%. In addition, see Figure 2 for streams proportion.

Table 2.
Volume balance for still distillation process for 1 batch of 5000 L fermented agave must.
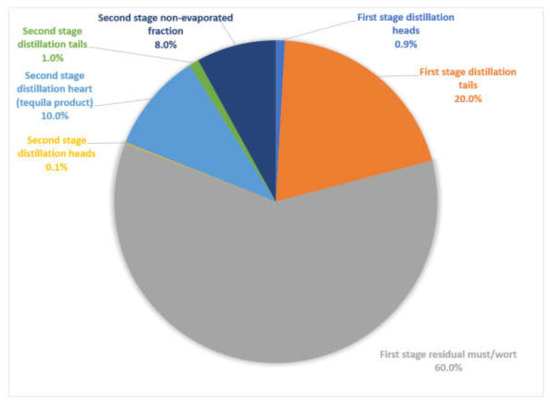
Figure 2.
Tequila traditional production process (still distillation) streams produced.
3.2. Chemical Composition and Calculated Chemical Oxigen Demand (COD)
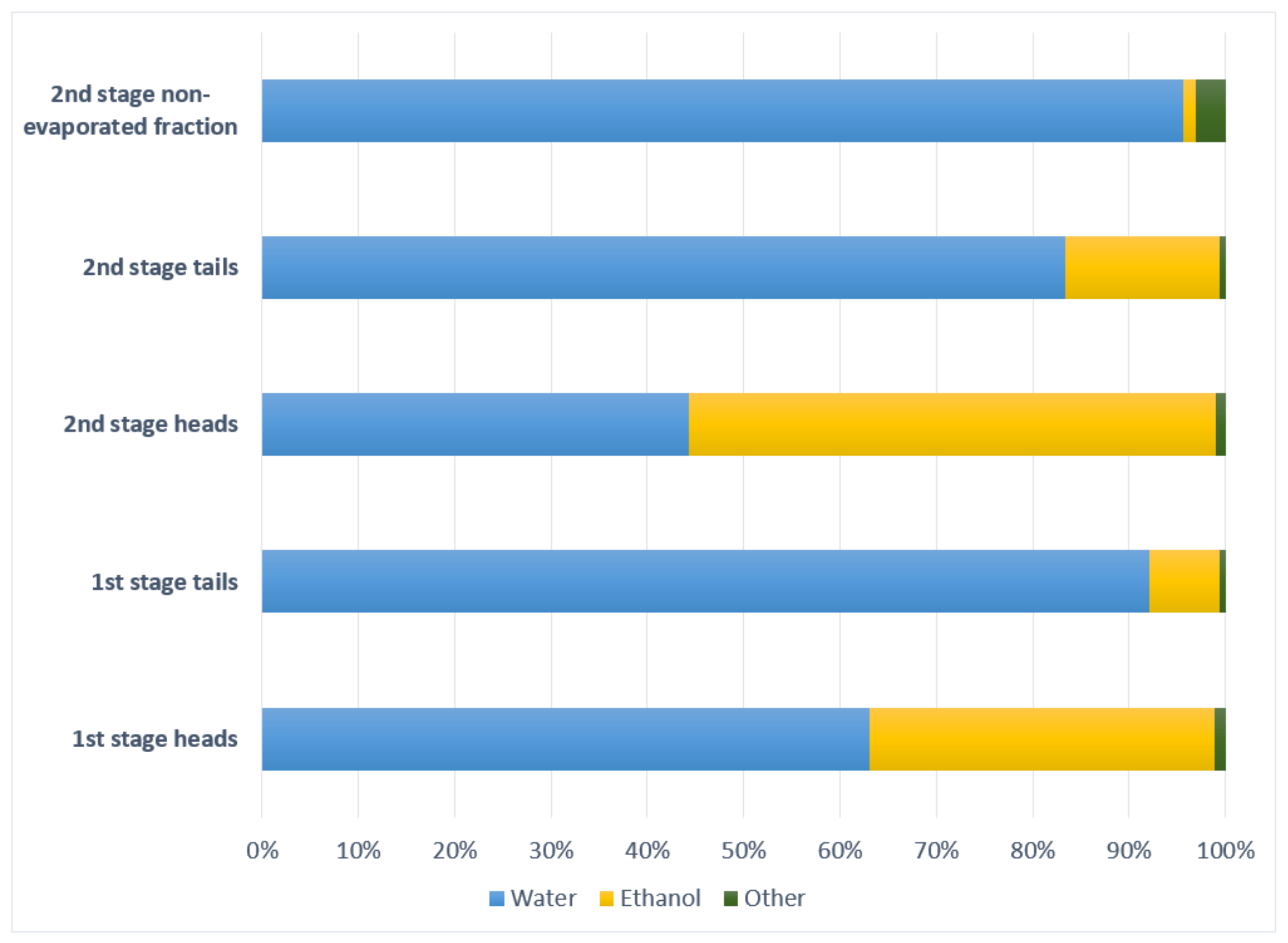
Results for the five streams are noted below. First and second stage heads were high in ethanol, 330,860–560,070 mg/L, while first and second stage distillation tails had between 60,410–203,210 mg/L. The second stage non-evaporated fraction had a small alcoholic content, below 23,460 mg/L. Methanol had high values in all streams, representing toxicity risk of biotreatment. The highest value obtained is 13,296.07 mg/L in the second stage non-evaporated fraction and the lower value in the first stage heads with 1258.81 mg/L.
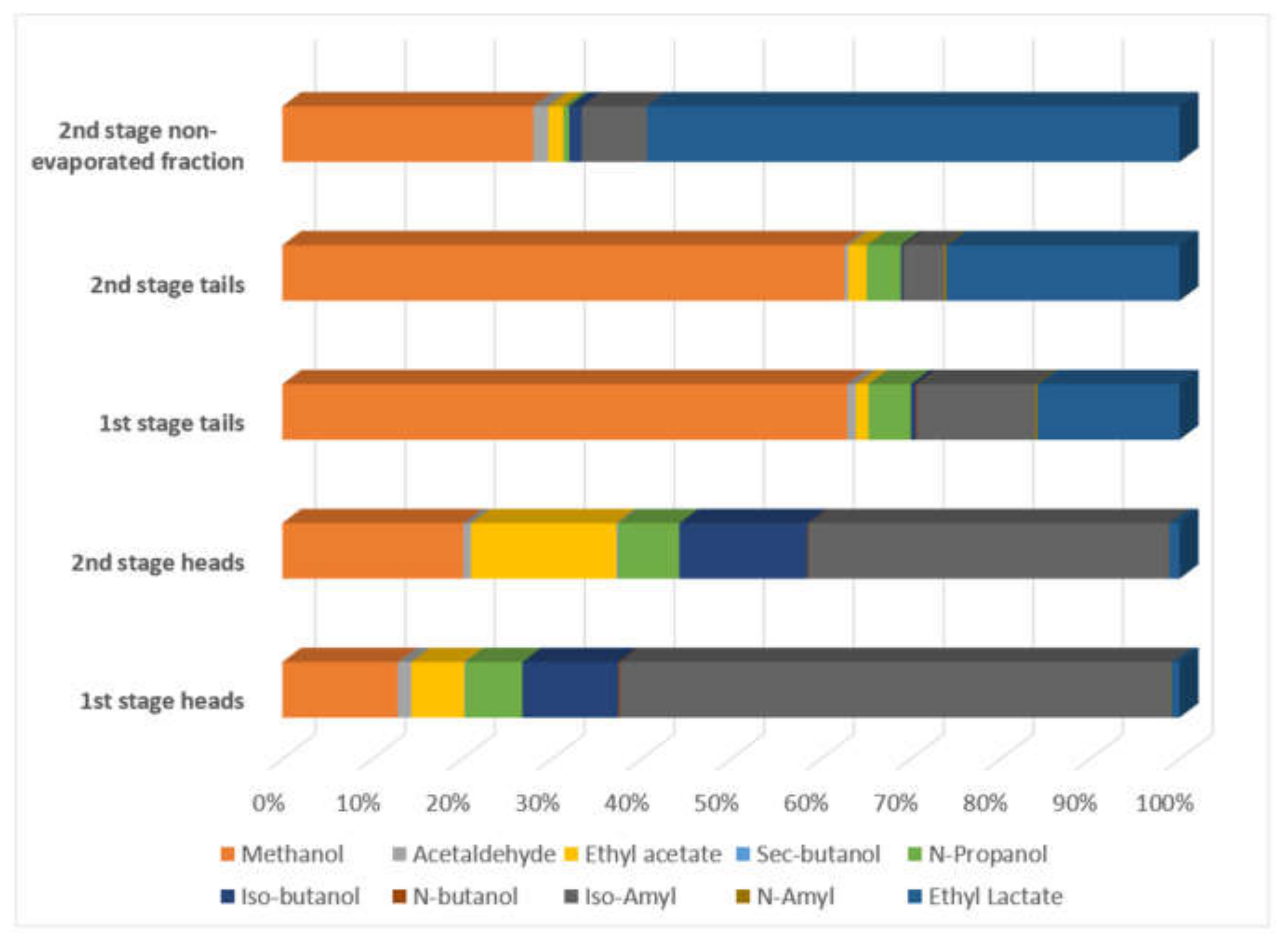
Acetaldehyde had a relatively low concentration in first and second stage heads and tails with values below 314.75 mg/L, major values in the second stage non-evaporated fraction up to 1046.04 mg/L. Ethyl acetate had low values in distillation first and second stage tails with values between 77.23–151.55 mg/L, medium values in distillation first stage heads and second stage non-evaporated fraction with values 274.21–766.40 mg/L. The second stage distillation heads had the highest value with 1890.76 mg/L.
Sec-butanol had low values, with a higher value of 21.5 mg/L and non-detected in three streams. N-Propanol, Iso-butanol, N-butanol, and Iso-Amyl is reported with a higher concentration in first and second stage distillation heads in comparison with first and second stage distillation tails and second stage non-evaporated fraction. N-Amyl has low concentration values with a maximum of 28.04 mg/L. Ethyl lactate has high values in second stage non evaporated fraction with values up to 35,050.58 mg/L and more related to water content.
COD calculated from chemical composition had very high values; lower values were for second stage non-evaporated fraction (53,800–91,600 mg/L), following first stage tails (137,200–184,800 mg/L), second stage tails (257,300–429,800 mg/L), first stage heads (707,900–831,400 mg/L) and finally second stage heads (1,196,600–1,239,200 mg/L). For further details please refer to Table 3 and Figure 3 and Figure 4.

Table 3.
Chemical composition and calculated COD.
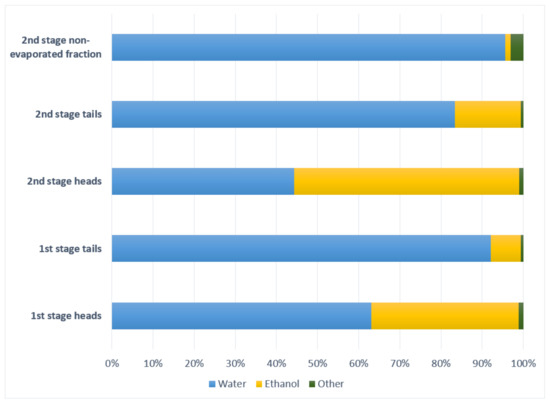
Figure 3.
Chemical composition: mean mass proportion of major components.
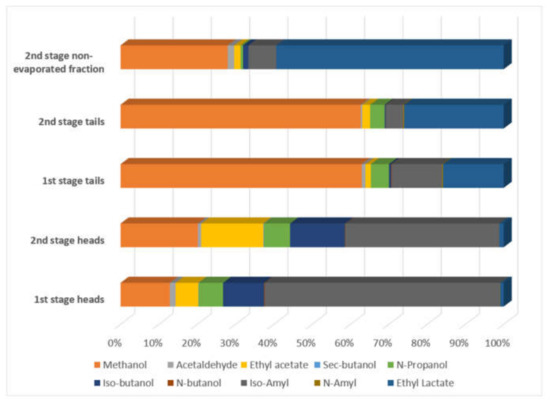
Figure 4.
Chemical composition: mean mass proportion of minor components.
3.3. Physic-Chemical Properties
All streams were acidic with pH values between 3.28 and 4.80. Electrical conductivity is proportional to water content, with values from 3.30 and 172.20 μS/cm. Regarding color, it was noted that all tequila factories had different ranges; Tequila factory 1 streams had color values 1–14 UC Pt-Co, Tequila factory 2 between 1–385 UC Pt-Co, and Tequila factory 3 between 6–24 UC Pt-Co. Turbidity values were between 0.31–15.38 NTU. None of them suitable for direct disposal according to Mexican norms, but with potential for further use. For results further details please refer to Table 4.

Table 4.
pH, conductivity, color, and turbidity determination.
Regarding solids content, all considered effluents were evaporated either distillation first or the second stage, therefore they have no solids. All effluents were tested to prove this, and the results were zero solids in all samples.
3.4. Energy Consumption and CO2 Production
The three tequila factories reported energy consumption of 0.5 L fuel oil per tequila liter produced (regardless of tequila category), which is an approximate of 22 × 106 J per tequila liter; extrapolating this data to 2019 tequila production (see Table 1), results in 165 × 106 L fuel oil and 7.26 × 1015 J (2.02 × 109 KWh) energy consumption. Regarding CO2 production an approximate of 2.6 kg CO2 per each fuel oil liter, leading to a yield of 1.3 Kg CO2 per tequila liter produced. A total of 429 × 106 kg CO2 generated in 2019.
3.5. Economic Analysis
Considering indicative prices reported by Chembid [9] and Alibaba [10], see Table 5, it is possible to make an approximate mixture price, stating base for its refining or biotransformation into a more valuable substance. Presently, the cost for tequila vinasses treatment (evaporation/oxidation in large pools) in the Jalisco Highlands region in Mexico is 16.00 USD/m3, with no further benefit.

Table 5.
Commercial reference lowest prices for chemical compounds reported in USD/kg from Chembid [9] and Alibaba [10].
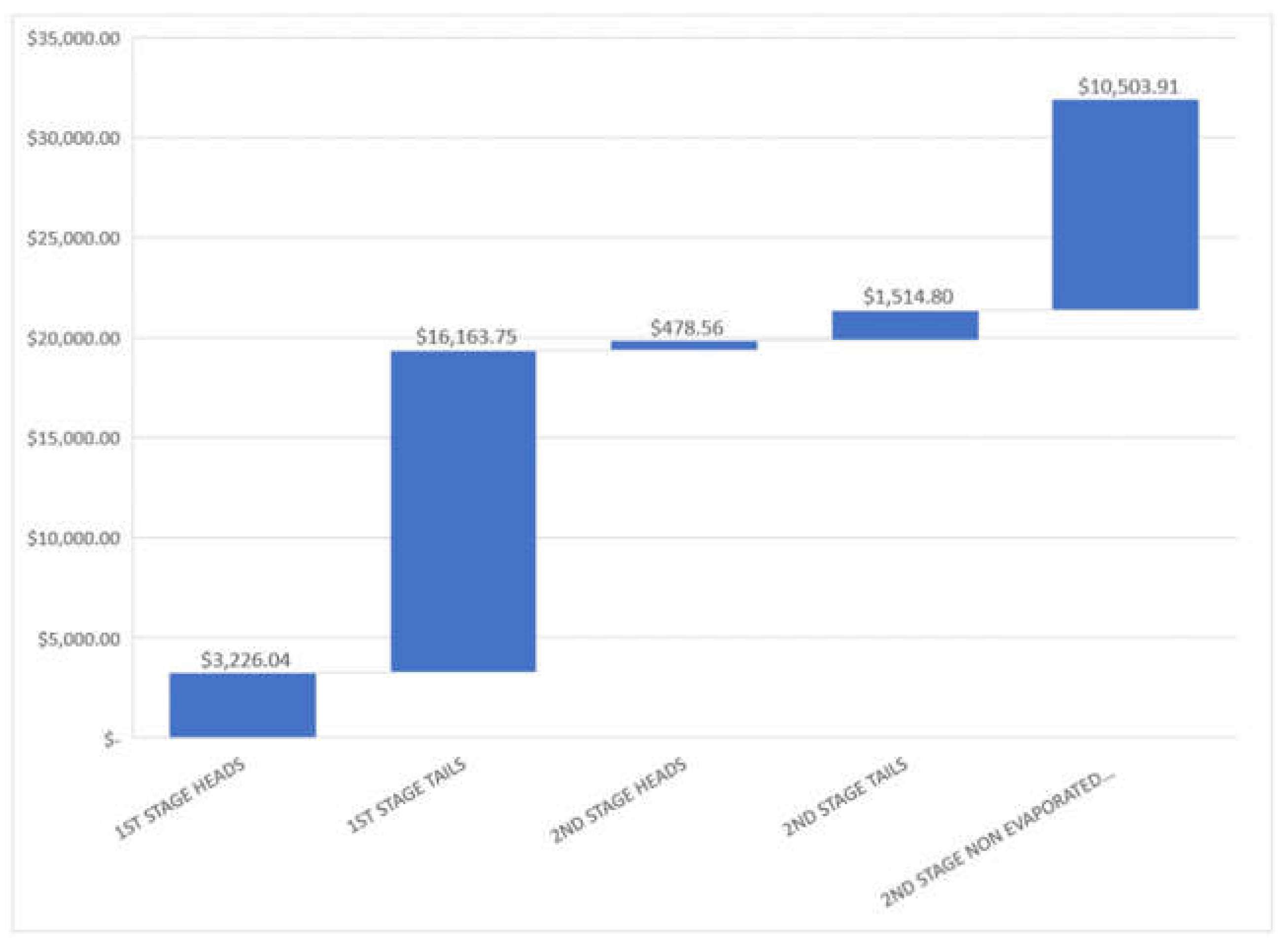
For a medium size tequila factory, it is common an approximate production of 1 × 105 L tequila (40%A.V.) per month, this quantity will be used for analysis. In Table 6 is indicated the approximate volume for each stream and the range of price calculated, Figure 5 shows a mean pricing for each stream.

Table 6.
Approximate volume proportion of residual streams studied and mixture price for a 100,000 tequila 40% A.V. liter production.
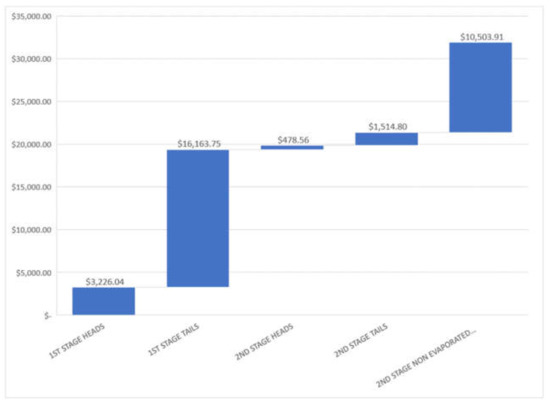
Figure 5.
Mean mixture pricing in USD for residual streams studied for a batch of for a 100,000 tequila 40%A.V. liter production in a month.
4. Discussion
4.1. Biogas Production
Several investigations were published to summarize the sustainable process for biorefining tequila wastes; López-López et al., Alemán-Nava et al., Nava-Cruz et al., García-Depraect et al., and Sanchez et al. [11,12,13,14,15,16] made the analysis of several treatments but no biorefinery proposal in concrete is reported yet. Tequila vinasses are considered to be a single waste stream produced in the tequila distillation process; however, the six still distillation waste effluents have not been taken in consideration separately.
Tequila vinasses, the only liquid residual stream reported have been bioprocessed in several reactors. Mendez-Acosta, et al. [17] reported a 70% COD removal and >60% CH4 production in a CSTR-type digester using a mixture 70% water–30% tequila vinasses with 10,000 mg/L COD, 7.4 pH and alkalinity factor α close to 0.3. Lizarraga-Palauzelos, et al. [18], reported an 80% COD removal and >70% CH4 production in an up-flow fixed-bed bioreactor using a mixture 70% water–30% tequila vinasses with 25,000 mg/L COD and 6.5 pH. Arreola-Vargas, et al. [19], reported up to 85% COD removal and 77–95% CH4 production in an Anaerobic Sequencing Batch Reactor using a mixtures water–tequila vinasses with 8000 mg/L COD, with pH-temperature combinations: 7.0–32 °C, 7.0–38 °C, 8.0–32 °C, 8.0–38 °C.
Buitrón and Carvajal [20], obtained biogas with hydrogen content 20.4–38.0% using a sequencing batch reactor fed with tequila vinasses diluted to from 500–3000 mg/L COD at two temperatures (25 °C and 35 °C) with two HRT (12 h and 24 h). COD removal reached around 1000 mg/L. Buitrón, et al. [21], reported hydrogen and methane production using a two-stage processes (H2-SBR and CH4-UASB) with COD feed 2300 mg/L and COD removals in the 73–75% range. Buitrón, et al. [22], reported biogas production using 4.7 pH with 35–36 °C in a fixed-bed reactor with 8500 mg/L COD with glucose-tequila vinasses feed.
Considering these concentrations it can be possible to perform batch or sequential reactors to produce biogas, using as basis distillation first stage tails and second stage non-evaporated fraction with a diluted reflow to maintain COD in the 25,000 mg/L feed range.
4.2. Chemical Composition
Regarding chemical composition, Rodríguez-Félix, et al. [23] report n-butanol, n-propanol, ethyl lactate, and ethyl acetate content in tequila vinasses as a whole, these molecules are coincident in this investigation. Darici, et al. [24] reported ethanol, methanol, acetaldehyde, ethyl acetate, n-propanol, sec-butanol, iso-butanol, n-butanol, and iso-amyl.
Darici, et al. [24] reported triple distillation in pot (still) for Raki manufacturing compared with double distillation for Tequila; they deducted that esters and aldehydes are reduced while odor active compounds are powered. However they do not report non-evaporated fractions or stillage.
Ethanol (boiling point 78.37 °C) is present in major proportion in second stage heads, followed by first stage heads, second stage tails, first stage tails and second stage non-evaporated fraction, is proportional with temperature and coincident with Darici, et al. [24].
Methanol, although has a lower boiling point (64.7 °C) than ethanol, is present in mayor proportion in first and second stage tails and second stage non-evaporated fraction, being the minor proportion reported in first and second stage heads, contrary to Darici, et al. [24] reported data.
Acetaldehyde is a volatile compound with a boiling point of 20.2 °C, is present in more volatile fractions as first and second stage heads with major proportion in comparison with first and second stage tails, being consistent with Darici, et al. [24]; however the second stage non evaporated fraction had a major proportion due a peak in one of the gas chromatography analysis reported.
Ethyl acetate (boiling point 77.1 °C) is more concentrated in second stage heads, followed by first stage heads, second stage non-evaporated fraction, second stage tails, and first stage tails, contrary to Darici, et al. [24] reported.
n-propanol (boiling point 97 °C), coincident with Darici, et al. [24], was more concentrated in first stage heads, followed by second stage heads, first and second stage tails and second stage non-evaporated fraction.
Iso-amyl (boiling point 132 °C), n-butanol (boiling point 117.7 °C), Iso-butanol (boiling point 108 °C), sec-butanol (boiling point 100 °C) had more concentration in first and second stage heads, followed by second stage non-evaporated fraction, first and second stage tails, coincident with Darici, et al. [24]. n-butanol was not detected in second stage non-evaporated fraction. Sec-butanol was only detected in first and second stage heads.
n-amyl (boiling point 138 °C) has major concentration in first stage tails, followed by second stage tails, first and second stage heads. Not detected in second stage non evaporated fraction.
Ethyl lactate (boiling point 154 °C) was more concentrated in second stage non-evaporated fraction, followed by second stage tails, first stage tails, first stage heads and second stage tails.
4.3. Physic-Chemical Properties
All streams were acidic with pH, levels are consistent with reported data for tequila vinasses with range 3.24–4.80 [11,12,13,14,15,16,17,18,19], this levels are not optimal for biogas production nor wastewater disposal and must be partially neutralized to a pH of 6–7.
Reported streams are clear non turbid liquids and contrary with Tommassen, G. [25], turbidity is not proportional with COD; however, regarding electrical conductivity, it is proportionally inverse with water content, which is coincident with Tommassen, G. [25].
5. Conclusions
Tequila vinasses are liquid waste from tequila distillation process that have been treated as a single stream; however, there are six residual streams (residual must, first stage heads and tails, second stage heads, tails and non-evaporated fraction) that could be treated separately instead of conforming a single stream; this perspective will simplify their destination. Second stage heads can be used as biofuel in a biomass boiler or even in vehicles with proper mixing with chemical fuels. First and second stage heads can be directed as industrial alcoholic mixtures as chemical solvents or other industrial uses. Concentrating effluents for bioreactors is also an option. Streams with high water content, first stage tails, second stage tails and non-evaporated fraction could lead to biogas production, aiming for biohydrogen with low pH (3–5), or methane by increasing pH to the 6–8 range. There is also room for water recovery. Proposed treatmets will cover an approximate of 30% of tequila distillation waste which reflects near 990 × 106 L annually, avoiding pollution of soil and water bodies. There is also a potential for biogas production.
Energy consumption is high for double distillation process, CO2 production reaches 1.3 kg CO2 per tequila liter. One of the challenges ahead is to recover water to convert tequila factories into a non-consuming water factory. This could be made using recovered purified water for steam production and makeup water for the fermentation process without affecting product quality.
This scope could serve for new studies to propose treatments for still distillation tequila process residual streams converting them from waste to biocompounds or bioenergy production.
Author Contributions
Conceptualization, E.M.-O., P.G.-M., and J.N.-A.; methodology, E.M.-O., P.G.-M., N.S.-O., R.G.U.-M., and J.N.-A.; literature review and data search revision P.G.-M., R.G.U.-M., L.A.L.-S., L.H.A.-V., and E.R.M.-E.; investigation, E.M.-O., and N.S.-O.; resources and samples, E.M.-O., and N.S.-O.; data curation, J.N.-A., L.A.L.-S., R.G.U.-M., and C.D.l.M.-O.; writing—Original draft preparation, E.M.-O., P.G.-M., N.S.-O., and C.D.l.M.-O.; writing—Review and editing, E.M.-O., P.G.-M., J.N.-A., N.S.-O., C.D.l.M.-O., and L.H.A.-V.; supervision, P.G.-M., R.G.U.-M., L.A.L.-S., L.H.A.-V., and E.R.M.-E. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
This research was done with support from SEP-SES-PRODEP Program folio 511-6/2019.-7840, the Technological Institute of Sonora, and the Technological Institute José Mario Molina Pasquel y Henríquez. We thank the following tequila factories: Destilería Morales S.A. de C.V., Feliciano Vivanco y Asociados S.A. de C.V., and Productos Finos de Agave S.A. de C.V. for the samples provided.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cámara Nacional de la Industria Tequilera 2020. El Tequila. Available online: https://www.tequileros.org/el-tequila/ (accessed on 7 July 2020).
- Secretaría de Energía. NOM-006-SCFI-2012 Alcoholic Beverages-Tequila-Specifications, Diario Oficial de la Federación 13/12/2012. Available online: http://www.dof.gob.mx/nota_detalle.php?codigo=5282165&fecha=13/12/2012 (accessed on 7 July 2020).
- Cedeño, M.C. Tequila Production. Crit. Rev. Biotechnol. 1995, 15, 1–11. [Google Scholar] [CrossRef]
- Bautista-Justo, M.; García-Oropeza, L.; Barboza-Corona, J.E.; Parra-Negrete, L.A. EL Agave tequilana Weber Y La Producción De Tequila. Acta Univ. 2001, 11, 26–34. [Google Scholar] [CrossRef]
- Secretaría de Energía. NOM-142-SSA1/SCFI-2014 Alcoholic Beverages-Sanitary Specifications-Sanitary and Commercial Labeling, Diario Oficial de la Federación, 23 March 2015. Available online: https://www.dof.gob.mx/nota_detalle.php?codigo=5386313&fecha=23/03/2015 (accessed on 7 July 2020).
- Consejo Regulador Del Tequila. Información Estadística; CRT: Madrid, Spain, 2020. Available online: https://www.crt.org.mx/EstadisticasCRTweb/ (accessed on 14 July 2020).
- Huitrón, C.; Pérez, R.; Gutiérrez, L.; Lappe, P.; Petrosyan, P.; Villegas, J.; Aguilar, C.; Rocha-Zavaleta, L.; Blancas, A. Bioconversion of Agave tequilana fructans by exo-inulinases from indigenous Aspergillus niger CH-A-2010 enhances ethanol production from raw Agave tequilana juice. J. Ind. Microbiol. Biotechnol. 2013, 40, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Murugan, C.S.; Rajendran, S. Bioethanol Production from Agave Leaves Using Saccharomyces cerevisiae (MTCC 173) and Zymomonas mobilis (MTCC 2427). Int. J. Microbiol. Res. 2013, 4, 24223–24226. Available online: http://idosi.org/ijmr/ijmr4(1)13/4.pdf (accessed on 14 July 2020).
- Chembid. Market Intelligence Platform Search Engine for Chemical Prices; Chembid: Oldenburg, Germany, 2020. Available online: https://www.chembid.com/en/ (accessed on 28 September 2020).
- Alibaba. Manufacturers, Suppliers, Exporters and Importers; Alibaba: Hangzhou, China, 2020. Available online: https://www.alibaba.com/ (accessed on 14 November 2020).
- López-López, A.; Davila-Vazquez, G.; León-Becerril, E.; Villegas-García, E.; Gallardo-Valdez, J. Tequila vinasses: Generation and full scale treatment processes. Rev. Environ. Sci. Biotechnol. 2010, 9, 109–116. [Google Scholar] [CrossRef]
- López-López, A.; Contreras-Ramos, S.M. Tratamiento de Efluentes y Aprovechamiento de Residuos. In Ciencia y Tecnología Del Tequila: Avances y Perspectivas, 2nd ed.; CIATEJ Guadalajara: Jalisco, México, 2015; pp. 343–378. Available online: https://ciatej.repositorioinstitucional.mx/jspui/handle/1023/453 (accessed on 14 July 2020).
- Alemán-Nava, G.S.; Gattia, I.A.; Parra-Saldivar, R.; Dallemand, J.F.; Rittmann, B.E.; Iqbal, H.M.N. Biotechnological Revalorization of Tequila Waste and By-Product Streams for Cleaner Production—A Review from Bio-Refinery Perspective. J. Clean. Prod. 2018, 172, 3713–3720. [Google Scholar] [CrossRef]
- Nava-Cruz, N.Y.; Medina-Morales, N.A.; Martinez, J.L.; Rodriguez, R.; Aguilar, C.N. Agave Biotechnology: An Overview. Crit. Rev. Biotechnol. 2014, 35, 546–559. [Google Scholar] [CrossRef] [PubMed]
- García-Depraect, O.; Osuna-Laveaga, D.R.; León-Becerril, E. A Comprehensive Overview of the Potential of Tequila Industry By-Products for Biohydrogen and Biomethane Production: Current Status and Future Perspectives. In New Advances on Fermentation Processes; Martínez-Espinoza, R.M., Ed.; Intechopen: London, UK, 2019. [Google Scholar] [CrossRef]
- Sanchez, A.; Sanchez, S.; Dueñas, P.; Hernández-Sánchez, P.; Guadalajara, Y. The Role of Sustainability Analysis in the Revalorization of Tequila Residues and Wastes Using Biorefineries. Waste Biomass Valorization 2020, 11, 701–713. [Google Scholar] [CrossRef]
- Méndez-Acosta, H.O.; Snell-Castro, R.; Alcaraz-González, V.; González-Álvarez, V.; Pelayo-Ortiz, C. Anaerobic treatment of Tequila vinasses in a CSTR-type digester. Biodegradation 2010, 21, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Lizárraga-Palazuelos, E.; García-Sandoval, J.P.; Méndez-Acosta, H.O.; González-Álvarez, V. Regulation of methane production in a Tequila vinasses anaerobic digestion pilot plant. In Proceedings of the IWA Conferences, Narbonne, France, 19 September 2013; Available online: https://www.researchgate.net/publication/236943626 (accessed on 28 September 2020).
- Arreola-Vargas, J.; Jaramillo-Gante, N.E.; Celis, L.B.; Corona-González, R.I.; González-Álvarez, V.; Méndez-Acosta, H.O. Biogas Production in an Anaerobic Sequencing Batch Reactor by Using Tequila Vinasses: Effect of PH and Temperature. Water Sci. Technol. 2016, 73, 550–556. [Google Scholar] [CrossRef] [PubMed]
- Buitrón, G.; Carvajal, C. Biohydrogen Production from Tequila Vinasses in an Anaerobic Sequencing Batch Reactor: Effect of Initial Substrate Concentration, Temperature and Hydraulic Retention Time. Bioresour. Technol. 2010, 101, 9071–9077. [Google Scholar] [CrossRef] [PubMed]
- Buitrón, G.; Gopalakrishnan, K.; Martinez-Arce, G.; Moreno, G. Hydrogen and Methane Production via a Two-Stage Processes (H2-SBR + CH4-UASB) Using Tequila Vinasses. Int. J. Hydrogen Energy 2014, 39, 19249–19255. [Google Scholar] [CrossRef]
- Buitrón, G.; Prato-Garcia, D.; Zhang, A. Biohydrogen Production from Tequila Vinasses Using a Fixed Bed Reactor. Water Sci. Technol. 2014, 70, 1919–1925. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Félix, E.; Contreras-Ramos, S.M.; Davila-Vazquez, G.; Rodríguez-Campos, J.; Marino-Marmolejo, E.N. Identification and quantification of volatile compounds found in vinasses from two different processes of Tequila production. Energies 2018, 11, 490. [Google Scholar] [CrossRef]
- Darıcı, M.; Bergama, D.; Cabaroglu, T. Effect of triple pot still distillation on the volatile compositions during the Rakı production. J. Food Process. Preserv. 2019, 43, e13864. [Google Scholar] [CrossRef]
- Tommassen, G. On the Correlation between Turbidity, Conductivity and COD. Master’s Thesis, Delft University of Technology, Delft, The Netherlands, 30 July 2014. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).