1. Introduction
The burning of fossil fuels by power plants and other process industries contributes around half of the world’s CO
2 emissions [
1]. These emissions’ adverse effects are evident: the melting of glaziers, deforestation, and droughts in several places [
2,
3]. With the projected growth of the world’s population, there will be a corresponding increase in the amount of CO
2 emissions. Consequently, human intervention is required for the mitigation of climate change. According to the Intergovernmental Panel on Climate Change (IPCC), the United States Environmental Protection Agency (EPA), and the International Energy Agency (IEA), carbon capture and storage (CCS) is necessary to achieve the 2-degree goal and the 1.5-degree goal of the Paris Agreement [
4,
5,
6].
Several CO
2 capture technologies and methods have been identified. They are based on chemical absorption and desorption using solvents [
3], adsorption using solid adsorbent [
7], and cryogenic separation that involves the separation of CO
2 by refrigerating and condensing the flue gas consecutively at different condensation temperatures [
3,
7]. They are also based on membrane separation technology ([
3,
7], and the direct injection of flue gas into reservoirs of naturally existing methane hydrate to displace methane and form a new CO
2 hydrate [
8]. Among these, the amine solvent-based CO
2 absorption and desorption process is the most technologically and commercially matured option [
9,
10,
11,
12,
13,
14,
15,
16]. However, its industrial deployment requires large investments and an enormous energy supply for desorption [
17]. Therefore, it is essential to look into the primary units contributing the most to the capital cost for cost-saving potential. The most expensive units in a standard solvent-based CO
2 capture process are mostly the absorption column and the lean/rich heat exchanger (LRHX), also referred to as the main or cross exchanger. This study focuses on the latter equipment.
Even though the shell and tube heat exchangers (STHX) are the most robust, especially the floating head type [
18], and are the most common heat exchangers [
18,
19,
20] in the process industry, there are other types of heat exchangers that are also popular [
18,
21]. Examples are the plate and frame heat exchangers (PHE) and double-pipe heat exchangers (including finned double-pipe heat exchanger (FDP-HX)). The majority of available CO
2 capture cost studies either do not disclose or merely state the broad classification and not the exact type of heat exchanger employed in the process [
1,
12,
21,
22,
23]. Examples of broad classifications are shell and tube heat exchangers (STHX) and plate and frame heat exchangers (PHE). However, there are different heat exchangers within these broad classifications, and each has a different cost [
18,
20,
24]. The STHXs have different types of designs and, by implication, different costs and technical advantages [
18,
20]. Therefore, there is a need to study how each of the popular heat exchangers within their collective classifications affects the process’s cost.
No study has been found where different designs of heat exchangers for CO
2 capture have been examined. This work aims to overview the cost implications of selecting any of six different heat exchangers as the LRHX of the CO
2 capture process. Six different CO
2 capture plant scenarios, each with one of the following heat exchangers as the LRHX, are examined: U-tube shell and tube heat exchangers (UT-STHX) [
18,
20], fixed tube-sheets shell and tube heat exchangers (FTS-STHX) [
18,
20], floating head shell and tube heat exchangers (FH-STHX) [
18,
20], finned double-pipe heat exchangers (FDP-HX) [
18,
20], and gasketed-plate heat exchangers (G-PHE) and welded-plate heat exchangers (W-PHE) as the LRHX [
18,
19,
20,
25]. The technical strength and limitations of these heat exchangers are also reviewed.
Our study could only focus on solvent-based post-combustion CO
2 absorption and desorption process where the lean/rich heat exchanger is an important integral part of the process for heat recovery. Monoethanolamine (MEA) with ~30 wt.% concentration is the standard CO
2 absorption solvent and the most extensively studied solvent [
1,
12,
26,
27]. Therefore, it was the solvent used in this study. A typical cement plant flue gas without NOx and Sox is used in this work. This is because the scope of this study does not cover flue gas pre-treatment. It is not necessary because this work focuses on the lean/rich heat exchanger. Cement manufacturing processes, including the combustion of fuel in the manufacturing process, account for 8 percent of global CO
2 emissions, primarily responsible for global warming. Thus, much attention is currently being given to CO
2 capture from the cement industry [
12,
28,
29,
30,
31,
32,
33,
34,
35,
36]. The process specifications, including the flue gas composition, are obtained from [
11,
37] and are given in Table 1. The flue gas contains 25.2 mole% of CO
2, typical of the CO
2 concentrations (22–29%) of flue gas from the clinker production loop [
12]. Some previous works on CO
2 capture from cement industry flue gas also primarily focused on these high concentrations of CO
2 from the clinker production loop as done in this work [
11,
31,
38,
39,
40,
41]. Reference [
12] covered exhaust gas from both the clinker production loop and fuel combustion. Since this study’s focus is on the main heat exchanger units, the source of flue gas is not important. Another objective is the comprehensive application of the enhanced detailed factor (EDF) method for capital cost estimation, established in Reference [
11]. Relevant details of this method are given in
Section 2.3 and more comprehensively in Reference [
11].
2. Methodology
2.1. Materials and Methods
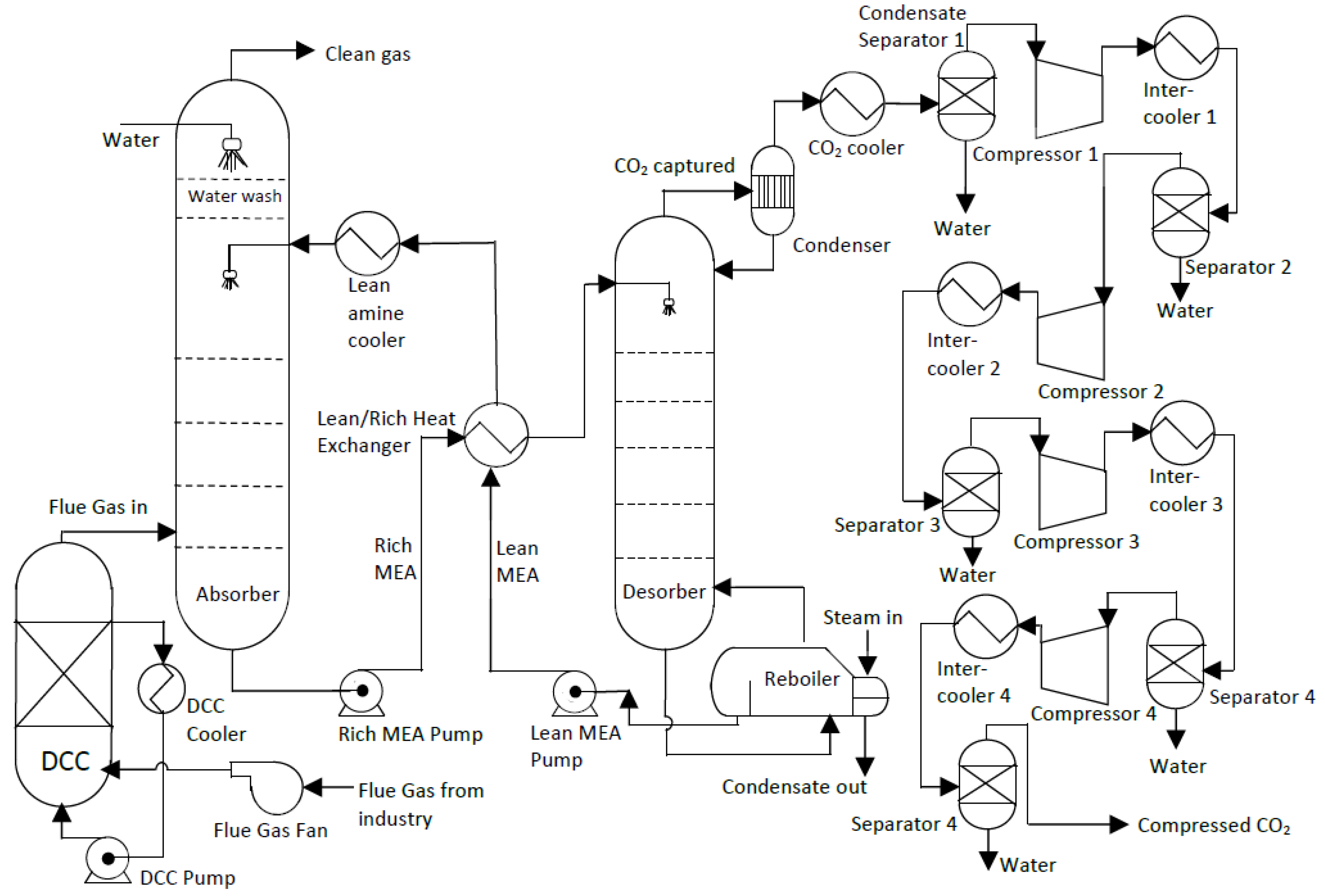
This study is based on the standard or conventional amine-based CO
2 absorption and desorption process. Its simplified process flow diagram (PFD) is shown in
Figure 1, and the Aspen HYSYS PFD can be found in
Figure A1 in
Appendix B. The process can be divided into three parts.
Pre-capture process and simulation: This is the part of the process before the main CO
2 absorption in the absorber. In order to minimize the complexity of process simulations, flue gas pre-treatment equipment such as a selective catalytic reduction (SCR) unit, flue gas desulfurization (FGD), or baghouse are not included in this work [
6]. The equipment considered in this first part of the process is therefore limited to the flue gas fan for the transport of the flue gas to the absorber, through the direct contact cooler (DCC) unit where the flue gas temperature is reduced to 40 °C; the DCC pump for pumping cooling water into the DCC unit; and the DCC cooler to cool the water down to the required temperature.
Capture process: The relevant equipment includes a simple absorption column and desorption column (stripper) with a condenser and a reboiler, a main heat exchanger (LRHX), two pumps (lean pump and rich pump), and a lean amine cooler. The flue gas from the DCC unit enters the absorber at the bottom of the column where the CO
2 in the flue gas is absorbed into a counter-current flowing amine solvent, which is monoethanolamine (MEA) in this work. An amine solution rich in CO
2 leaves from the bottom of the absorber. The rich pump then pumps it through the LRHX, where it is heated before it flows into the desorber for regeneration. The CO
2 is stripped off the amine solution and leaves through the top of the column and through the condenser. The regenerated solvent, the lean amine, is pumped by the lean pump back to the absorber, but first, through the LRHX to heat the CO
2-rich stream. It is further cooled to 40 °C by a cooler before entering the absorber at the top to continue another absorption cycle. Even though the water wash section is shown in
Figure 1, it is not included in this study for simplicity.
Post capture process: This part of the process involves the compression of the CO
2 to the required utilization pressure. In this study, transport and storage of the compressed CO
2 are not considered. The equipment included are 4 stage-compressors with inter-stage coolers, a CO
2 cooler, and separators [
11,
12].
2.2. Base Case Process Specifications, Assumptions and Simulation
The mass and energy balances on which equipment sizing was based, as well as utilities consumption used for estimation of variable operating costs, were made available from process simulations. The simulations were performed within the scope of the equipment in
Figure 1. The simulations are based on the flue gas specifications given in
Table 1. They represent a flue gas of a typical cement plant and are obtained from References [
11,
37]. The simulation method used in this work was the same as that used in References [
42,
43]. The difference was that in this work, the simulations were performed using Aspen HYSYS Version 10, where the acid gas property package replaced the Amine property package in previous versions. CO
2 capture of 85% was assumed [
28,
32,
44].
The absorption column and the desorption column were both simulated as equilibrium stages with stage efficiencies (Murphree efficiencies). Each equilibrium stage was assumed to be 1 m high for both columns [
11,
45]. The industry’s flue gas had a temperature of 80 °C, and it was cooled to 40 °C before entering the absorption column at the bottom at 1.21 bar. The absorber was simulated with 15 packing stages, which was the cost optimum in Reference [
17]. Murphree efficiencies of 11–21% were specified from the bottom to the top of the absorption column as in Reference [
11]. The desorber was simulated with 10 packing stages, which was also the cost optimum in Reference [
17]. For the desorber, a constant Murphree efficiency of 50% for each stage was assumed [
11]. The modified HYSIM inside-out algorithm was selected in the columns because it improves convergence [
43].
Adiabatic efficiency of 75% was specified for all the pumps and the flue gas fan. The lean pump raised the lean amine stream pressure by 3 bar before passing through the LRHX. Similarly, the rich pump increased the pressure of the CO
2-rich amine solution stream by 2 bar. The minimum approach temperature (
) in the LRHX was specified as 10 °C [
42]. The lean amine cooler further reduced the lean amine stream’s temperature to 40 °C before flowing back into the absorber.
Table 1.
Specifications and assumptions for simulation.
Table 1.
Specifications and assumptions for simulation.
| Parameter | Value | Source |
|---|
| CO2 capture efficiency (%) | 85 | [44] |
| Flue gas |
| Temperature (°C) | 80 | [37] |
| Pressure (kPa) | 121 | [11] |
| CO2 mole-fraction | 0.2520 | [37] |
| H2O mole-fraction | 0.0910 | [37] |
| N2 mole-fraction | 0.5865 | [37] |
| O2 mole-fraction | 0.0705 | [37] |
| Molar flow rate (kmol/h) | 11,472 | [37] |
| Temperature of flue gas into absorber (°C) | 40 | [43] |
| Pressure of flue gas into absorber (kPa) | 121 | [11] |
| Lean MEA |
| Temperature (°C) | 40 | [42] |
| Pressure (kPa) | 121 | [11] |
| Molar flow rate (kmol/h) | 96,850 | Calculated |
| Mass fraction of MEA (%) | 29 | [42] |
| Mass fraction of CO2 (%) | 5.35 | [42] |
| Absorber |
| No. of absorber stages | 15 | [17] |
| Absorber Murphree efficiency (%) | 11–21 | [11] |
| ΔTmin lean/rich heat exchanger (°C) | 10 | [12,42] |
| Desorber |
| Number of stages | 10 | [17] |
| Desorber Murphree efficiency (%) | 50 | [11] |
| Pressure (kPa) | 200 | [42] |
| Reflux ratio in the desorber | 0.3 | [42] |
| Temperature into desorber (°C) | 104.6 | [43] |
| Reboiler |
| Reboiler temperature (°C) | 120 | [42] |
| Saturated steam temperature (°C) | 160 | [46] |
| Exit temperature of steam (°C) | 151.8 | [46] |
| CO2 compression final pressure (kPa) | 15,100 | [47,48] |
The captured CO
2 undergoes a four stages compression with inter-stage cooling [
11,
12]. The final pressure is 151 bar with a purity of 99.8%, which is 0.17% less than [
47] with 90% CO
2 capture. This was consistent with the requirements for enhanced oil recovery (EOR) and/or offshore geological sequestration [
12,
31,
38,
48,
49]. The major uses of the compressed CO
2 include EOR, coalbed CH
4 recovery, and injection into un-minable coal seams or deep saline formation [
48]. A potential future application is the injection of the CO
2 (especially in mixture with nitrogen) into naturally existing methane hydrate reservoirs for simultaneous CH
4 production and storage of CO
2 in the form of hydrate [
48,
50,
51,
52,
53]. The compressed CO
2 pressure is expected to be 110–152 bar [
48,
49,
54]. A similar assumption (150 bar) was made by References [
12,
21,
31,
47]. The following studies assumed 110 bar [
28,
29,
55]. The final pressure depends on the transport distance between the CO
2 capture plant and the sequestration site/utilization point.
2.3. Capital Cost Estimation Method
When equipment cost data are available, a factorial method of capital cost estimation can be applied. Various forms of factors or factorial schemes are available, from Lang Factors [
56], Hand Factors [
57], to more detailed factors found in References [
58,
59], and more recently in References [
60] and in [
11]. Most of the factorial methods are based on the work of References [
58,
59]. The most popular of them is the methodology documented by the National Energy Technology Laboratory (NETL) [
61], which is used for capital cost estimation in [
62].
In this work, the enhanced detailed factor (EDF) method, which is comprehensively documented in Reference [
11], was applied to estimate the total capital cost/CAPEX. This has the same strategy as the individual factor and subfactor estimating method in Reference [
60]. However, the EDF method’s installation factors are more detailed [
11]. The EDF cost estimation scheme developed by Nils Eldrups at SINTEF Tel-Tek and the University of South-Eastern Norway (USN) has been used extensively in these Norwegian institutions for several years.
The EDF method was chosen for this work because the installation factors for all equipment pieces, irrespective of their sizes and cost, cannot be the same [
60] as they are treated in most of the other methods already mentioned. Applying individual installation factors to an individual piece of equipment improves capital cost estimates [
60]. Therefore, the EDF method’s merits include higher accuracy of cost estimates in the early stage, highlighting an individual piece of equipment for optimization [
11]. Individual installation factors are applied to each separate unit of equipment, thereby handling each individual equipment as a separate project. This eventually improves the accuracy of capital cost estimates. The EDF cost estimation method gives a high level of accuracy in the early-stage chemical plant cost estimates. In addition, it can easily and straightforwardly be employed to implement cost engineering studies of new technologies or retrofits (extension) or modifications of projects for an existing chemical plant [
11].
The total installed cost (CAPEX) estimated using the EDF scheme corresponds to the total plant costs (TPC) using the methodology of NETL. It is important to emphasize that the EDF method does not consider the cost escalations and interest accrual during the construction period, the costs for land purchase and preparation, long pipelines, long belt conveyors, office buildings, and workshops, and other costs incurred by the owner.
2.4. Scope of Capital Cost Estimation
The equipment considered in this work included the (1) flue gas fan, (2) direct contact cooler (DCC Unit), (3) DCC pump, (4) DCC cooler, (5) absorber, (6) desorber, (7) condenser, (8) reboiler, (9) lean/rich heat exchanger (LRHX), (10) lean MEA cooler, (11) lean pump, (12) rich pump, (13) condensate cooler, (14) compressors (×4), (15) inter-stage cooler (×4), and (15) Separators (×4). Some other types of equipment that were not included in this study but are vital for the operation and performance of this type of plant were (1) water wash section, (2) MEA reclaimer, (3) equipment for conditioning of make-up MEA and make-up water, and (4) cooling water pumps for DCC cooler, condensate cooler and inter-stage coolers. In addition, the cost of acquiring the site (land), preparing the site, and service buildings are not included.
2.5. Equipment Dimensioning and Assumptions
Mass and energy balances from the process simulations are used for sizing the equipment listed above. The dimensioning approach is the same for previous studies at USN [
11,
17,
55]. The dimensioning factors and assumptions are summarised in
Table 2. Since CO
2 is an acid gas with a risk of corrosion, stainless steel SS316 is assumed for all equipment except the flue gas fan and compressor casings, which are assumed to be manufactured from carbon steel.
Even though the water wash section is not included in the cost estimate, the tangent-to-tangent height (TT) of the absorber is assessed to cover the water-wash requirements, demister, packing, liquid distributors, gas inlet and outlet, and sump [
11]. Similarly, the packing requirements, liquid distributor, gas inlet, inlet for the condenser, and sump are accounted for in evaluating the tangent-to-tangent height (TT) of the desorption column [
11].
The heat transfer area required is the key design parameter in the initial cost estimate of the general heat transfer equipment. These include the LRHX, reboiler, condenser, and coolers. The heat transfer area is computed from the heat duty (heat transfer from hot to cold stream), overall heat transfer coefficient, and the log-mean temperature difference (LMTD) [
63]. The overall heat transfer coefficient (U-value) assumed for the lean/rich heat exchanger scenarios with the STHXs and FDP-HX is 500 W/m
2K [
45]. This value is close to the 550 W/m
2K used by SINTEF (a research organization in Norway) [
64]. The overall heat transfer coefficient for the PHEs was conservatively assumed to be 1000 W/m
2K. This is because the overall heat transfer coefficient of the PHEs is much higher than that of other exchangers like the STHXs, thereby having an order of magnitude higher surface area per unit volume compared to the STHXs [
18,
19]. According to Reference [
65], the U-value for the PHEs is 2–4 times the STHXs. The welded-plate heat exchanger was assumed to cost 25% more than the gasketed-plate heat exchanger based on information from Reference [
18].
The flowrate and power (duty) of each pump, compressor, and flue gas fan were obtained directly from the Aspen HYSYS simulation. The separators were sized from gas flowrate, mass densities of the gas and liquid phases, using the Souders-Brown Equation with a k-factor of 0.101 m/s [
66,
67]. The wall thickness determination followed the typical format, with joint efficiency of 0.8, corrosion allowance of 0.001 m, and stress of 2.15 × 10
8 Pa. The design pressure was obtained from Aspen HYSYS. After evaluating the vessel’s outer diameters (D
o), the TT was estimated from the assumption of TT = 3D
o. The dimensions and purchase costs of all the equipment are given in
Table A2,
Table A3,
Table A4 and
Table A5 in
Appendix C.
Table 2.
Equipment dimensioning factors and assumptions.
Table 2.
Equipment dimensioning factors and assumptions.
| Equipment | Basis/Assumptions | Sizing Factors |
|---|
| DCC Unit | Velocity using Souders-Brown equation with a k-factor of 0.15 m/s [66]. TT =15 m, 1 m packing height/stage (4 stages) [11] | All columns: Tangent-to-tangent height (TT), Packing height, internal and outer diameters (all in (m)) |
| Absorber | Superficial velocity of 2 m/s, TT=40 m, 1 m packing height/stage (15 stages) [11,17] |
| Desorber | Superficial velocity of 1 m/s, TT=22 m, 1 m packing height/stage (10 stages) [11,17]. |
| Packings | Structured packing: SS316 Mellapak 250YB | See DCC Unit, absorber and desorber |
| Lean/rich heat exchanger | U = 0.5 kW/m2K [45] | Heat transfer area, A (m2) |
| Reboiler | U = 0.8 kW/m2K [45] |
| Condenser | U = 1.0 kW/m2K [45] |
| Coolers | U = 0.8 kW/m2K [45] |
| Intercooler pressure drop | 0.5 bar [20] | U-tube HX |
| Pumps | Centrifugal | Flowrate (L/s) and power (kW) |
| Flue gas fan | Centrifugal | Flow rate (m3/h) |
| Compressors | Centrifugal; 4-stages [11,12,54]; Final pressure = 151 bar [48,49]; pressure ratio = 3.2 | Power (kW) and flowrate (m3/h) |
| Separators | Vertical vessels; vessel diameter using Souders-Brown equation, a k-factor of 0.101 m/s [66,67]; corrosion allowance of 0.001 m; joint efficiency of 0.8; stress of 2.15 × 108 Pa [45]; TT = 3Do [67] | Outer diameters (Do); tangent-to-tangent height (TT), (all in (m)) |
2.6. Source of Equipment Purchase Costs
The best source of the purchase cost of a piece of equipment is from quotations directly from equipment vendors or equipment manufacturers. This is usually not easy to obtain, and they may also require comprehensive design details. The next best option to this is cost data of the same equipment recently purchased. This kind of cost data may never be accessible to research cost engineers. Therefore, they must rely on either in-house cost data that may not be necessarily very recent or on commercial databases such as Aspen In-plant Cost Estimator from AspenTech, which is the most popular. This AspenTech database was developed by a team of cost engineers based on data obtained from equipment manufacturers and EPC companies. These cost data are updated every year; thus, they are recent and reliable [
20]. This study’s cost data were obtained from the most recent Aspen In-plant Cost Estimator Version 11 with the cost year of 2018 January. Therefore, the purchase costs of equipment in this course are updated.
When cost engineers or researchers do not have access to licenses of such databases that are regularly updated, they can use data in the open literature. Some of these data are available as cost correlations in the form of tables or graphs for several types of equipment, some of which can be found in References [
18,
20,
62]. A free internet equipment cost database with a cost period of January 2002 is available in Reference [
68].
2.7. Capital Cost Estimation Assumptions
The economic assumptions used for estimating the total capital cost (CAPEX) and annualized CAPEX in the EDF method [
11] are given in
Table 3. Equipment costs are obtained in Euros (€) and are converted to Norwegian kroner (NOK) to use the installation factors developed in NOK. A brownfield project is assumed. The installation factors are provided in CS; therefore, Equation (1) is applied to convert equipment purchase costs from stainless steel (SS) to CS. The list of installation factors is attached in
Appendix A.
When the appropriate detailed installation factors for each piece of equipment is obtained, Equations (2) and (3) can be used to calculate the installed cost of any equipment in CS (flue gas fan and compressors in this work):
Installed costs of equipment manufactured from SS are obtained from Equations (4) and (5):
The individual piece of equipment’s installed costs is then converted back to €. The installed costs are escalated to January 2020 using the Norwegian Statistisk Sentralbyrå industrial cost index (2018 = 106; 2020 = 111.3) [
40].
The sum of all the equipment installed costs in 2020 is the plant total installed cost (CAPEX). The annualized CAPEX is evaluated using Equations (7) and (8):
The annualized factor is calculated, as shown in Equation (8):
where
n represents operational years and
r is discount/interest rate for a 2-year construction period and 23 years of operation.
2.8. Operating and Maintenance Costs (O&M or OPEX) and Assumptions
The operating and maintenance costs (O&M) are mainly referred to as OPEX, operating expenses. These costs are usually divided into fixed operating costs and variable operating costs. Fixed operating costs are operating costs that do not vary in the short term and do not depend on the units of materials consumed or produced. Fixed operating costs do not depend on how much CO
2 is captured. The fixed operating costs in this work followed the assumptions used in Reference [
11]. They only include:
Maintenance cost in this study is estimated as follows [
33]:
Variable operating costs are the operating expenses that vary with either the units of materials consumed or produced. These are mainly utilities and raw materials. In this study, they are limited to:
Cost of electricity consumed by the flue gas fan, pumps, and compressors
Cost of steam consumption in the reboiler.
Cost of cooling water required by the coolers.
Cost of process (demineralized) water in the amine solution solvent and make-up water.
Cost of solvent.
Each variable operating cost is estimated using Equation (10):
where the unit for electricity and steam consumption is kWh. The unit for all water and solvent is m
3. The assumptions for the fixed operating costs and unit prices of the variable operating costs are given in
Table 4. These costs are updated to 2020 from the references. The total annual operating expenses (annual OPEX) is the sum of all the annual costs of fixed operating costs and variable operating costs. Costs for CO
2 transport and storage, pre-production costs, insurance, taxes, first fill cost, and administrative costs are not included in the OPEX.
2.9. Total Annual Cost and CO2 Captured Cost
Total Annual Cost and CO
2 capture Cost are the bases for techno-economic analysis in this work. The total annual cost is simply the sum of annualized CAPEX and yearly total operating cost as given in Equation (11):
Most literature reports their results in CO
2 avoided cost [
72,
73], and CO
2 captured cost [
74]. Therefore, it was important to perform our estimates with similar cost metrics for comparison with other works. In this study, results are reported in CO
2 capture cost and total annual cost. In this work, different scenarios of CO
2 capture plants with different heat exchanger types were compared. CO
2 captured cost is the annual cost per ton or per
kmol of CO
2 captured as expressed in Equations (12) and (13):