1. Introduction
In 2017, approximately 29.4 million Mg of municipal solid waste incineration bottom ash (MSWIBA) was produced in Europe, from 492 million tons of municipal solid waste. The biggest producer of MSWIBA in Europe was Germany, which created 8.04 million Mg. In Poland, 0.24 million tons of MSWIBA was produced in six incineration plants.
Figure 1 depicts the number of incineration plants and the amount of MSWIBA across European countries.
If MSWIBA is subject to valorization, it can be used in the construction industry, e.g., during road building as an aggregate or concrete components. There are a few options for slag preparation: valorization, NaOH pre-treatment, Na
2CO
3 pre-treatment, CaOH
2 pre-treatment, as well as combinations of Na
2SiO
3/NaOH pre-treatment [
2,
3,
4].
MSWIBA in the construction industry is part of sustainable development and the circular economy. Concrete is exceptionally good for the environment, as it immobilizes contaminants and pollutants. Growing production and consumerism causes an increase in the amount of produced solid municipal waste, which then increases the amount of MSWIBA in Poland. In order to operate in line with the concept of a circular economy, it is a necessity to pre-recycle secondary waste.
Figure 2 depicts the amount of municipal solid waste in Poland.
1.1. Methods of Dealing with Secondary Waste
Immobilization of contaminants is necessary to obtain a product from secondary waste. First of all, secondary waste should be properly prepared, in order to change its parameters. The better the parameters, the greater the mechanical strength of the materials, lower absorbability, and better frost resistance. Different methods of valorization are used to achieve better parameters of ash [
10,
11]:
- –
Valorization, cementation;
- –
Bituminization;
- –
Vitrification;
- –
Alkali pre-treatment (NaOH, CaOH2, NA2SiO3 + NaOH, Na2CO3 + NaOH pre-treatment);
- –
Solidification, stabilization;
- –
Other technologies (Synrock, Geodur).
The primary method of secondary waste management in the incineration plant is bottom ash valorization (
Figure 3). The valorized slag can be used in construction. Applying appropriate treatment is essential in order to achieve better product, which could be used in the industry [
12].
1.2. Valorization Process
The slag trap is equipped with a water lock. The water is kept constant. This prevents false air from entering the chamber, as well as exhaust fumes and dust from the chamber leaking outside the system. MSWIBA is cooled to a temperature of 80 °C–90 °C. Appropriate hydration prevents secondary dusting. MSWIBA is transported using a conveyor belt to the MSWIBA area, which has to be roofed, paved, sealed, and equipped with a leachate management system. MSWIBA should be stored for 15 days. Then, the loader transports it to the installation responsible for sorting and mechanical treatment of MSWIBA (
Figure 4).
MSWIBA is crushed on a <150 mm fraction; then, a rotary drum screen separates the 0–40 mm and 0–150 mm fraction. These fractions then reach the magnetic separators, where ferrous metals are isolated. The 0–40 mm fraction is directed to the vibrating screen, in which a separation into 0–8 mm and 8–40 mm fractions occur. Smaller fraction—without ferrous metals—is heaped into a pile. The 8–40 mm fraction is directed towards a non-ferrous metal separator, and then it is also heaped into a pile. After isolating ferrous and non-ferrous metals from the 40–150 mm fraction, the MSWIBA is transported to manual sorting. Combustible waste is returned to the chamber.
The slag with 0–8 mm and 8–40 mm diameter is seasoned in order to achieve the right properties. This fraction mainly consists of non-combustible substances (silicates, as well as aluminum and iron oxides). The MSWIBA seasoning consists in penetration of moisture from the air into the slag, followed by the hydration processes. The seasoning process improves the MSWIBA properties and limits the leaching of heavy metals. After the valorization process, MSWIBA may be used in the construction industry [
13].
1.3. NaOH Pre-Treatment
NaOH pre-treatment can be used for different secondary waste, e.g., MSWIBA after slag valorization, hazardous fly ash, and granulated blast furnace slag. It improves physical and chemical properties, reduces leaching of pollutants and prepares the industrial byproduct.
To increase reactivity, one should grind a sample by using—for example—a grinder, crusher, roll crusher, hammer crusher, tumbling mill, cracker, ring mill machine, but it is not a necessity. On the other hand, it is much easier to remove pre-treatment liquid from the greater fraction, if there is a need.
Using NaOH pre-treatment reduces leaching of pollutants, such as, among others, heavy metals (As, Ba, Cr, Cu, Pb, Ni, Se, Zn) Al, and Al/Zn [
4]. Due to the reduction of Al, the concrete does not swell. Reduction of heavy metals leaching allows to achieve the standard requirement, and has environmental benefits.
Table 1 shows leaching of heavy metals from MSWIBA after NaOH pre-treatment. It also displays (by percentage) how much the leachability of the presented elements has decreased.
The quality of pre-treatment affects the molar concentration, length of time (from hours to weeks), temperature, ratio, sample fraction, and chemical composition. The longer the treatment, the higher temperature, and higher ratio, the better the effects [
4].
2. Materials and Methods
The research part covered municipal solid waste incineration bottom ash after valorization (MSWIBABV) and municipal solid waste incineration bottom ash after valorization (MSWIBAAV) analysis.
Table 2 shows the standards and norms, according to which the tests were carried out. The study included physicochemical analysis of MSWIBABV and MSWIBAAV, leaching of MSWIBAAV and MSWIBAAV impurities, as well as qualitative examination of cement beams. Samples for unification were taken from various parts of the storage.
The leaching from waste was prepared for analysis and assessment of the degree of environmental nuisance. The water extract was made in a 1:10 proportion of waste to distilled water, shaken for 24 h, and then filtered.
The LCA (Life cycle assessment) method, which can be used in order to analyze the environmental results of the work, is based on analysis from cradle-to-grave. In order to compare the environmental impact of using the sub-products in concrete production, the LCA analysis was conducted in the fossil depletion and climate change impact categories, as a representative of the problem for resource depletion and the main environmental problem linked with this topic. The methodology has 18 different impact categories, but those two are the most important in the area of cement production. The analysis was conducted using SimaPro Analyst 9.0 software and ReCiPe midpoint (H) methodology, which is one of the most popular in this type of analysis presented by independent software:
https://www.ipoint-systems.com/blog/lcia-indicator/. The Ecoinvent 3.0 database was used, while the data taken refers to an average impact of 1 kg of production of different types of cement. The analysis includes production of cement from raw materials and/or additional components, taking into account all processes of production, extraction, and transportation to the final consumer. The analysis does not include the final disposal, because concrete is a specific type of waste. It is too complicated to correctly assess the disposal of ingredients of concrete in our scenario. The analysis is focused on the positive impact of managing the waste from thermal treatment in the incineration plant.
Figure 5 depicts secondary waste from an incineration plant. From 1000 kg of burning waste, approximately 300 kg of slag is produced in the incinerator plant.
Figure 5a depicts MSWIBABV and
Figure 5b depicts MSWIBAAV. Slag valorization is carried out on the incinerator premises.
MSWIBABV and MSWIBAAV were collected from the same Polish incinerator plant. MSWIBABV has 0–10 mm fraction. MSWIBAAV has 0–8 fraction. MSWIBABV contains inclusions and impurities: glass, porcelain, ferrous, and non-ferrous metals. MSWIBAAV contains less water, ferrous, and non-ferrous metals, but still contains glass and porcelain. MSWIBABV is divided into different fractions after the valorization process, which depends on the recipient. The 0–8 mm MSWIBAAV fraction is one of the few produced at the Polish incineration plant. Both wastes are odorless, do not dust, and are not hazardous waste.
3. Results
In both slags, physicochemical properties were studied.
Table 3 shows moisture (M), Decomposable Organic Substance (DOS), Undecomposable Organic Substance (UOS), and Mineral Substance (MS), crucial for concrete mixture and bulk density (ρ), which, in turn, is important for transport and storage.
Both slags are characterized by high humidity, which negatively affects pozzolana activity. Usually, the humidity should be in the range of 1–3%; therefore, the slag should be dried before it is used in the concrete or cement mix [
16].
Table 4 depicts the contents of macroelements in slags. It is important to study the secondary waste before using it in concrete, as chemical changes may occur in the concrete at the molecular level.
Both slags are characterized by a low TOC content (MSWIBABV 0.64% and MSWIBAAV 0.65%), which leads to less water demand, better frost resistance of mortar and concrete, as well as a lighter color of the mixture.
The low sulfur content in MSWIBAAV (0.20%) does not affect the formation of sulfate corrosion, the formation of ettringite in the free spaces of concrete and swelling. The chlorine content is also low (MSWIBABV 0.41% and MSWIBAAV 0.12%), which means that the reinforcements are not exposed to corrosion and the concrete is not cracked [
24]. In terms of pH, both slags are alkaline, which means they can be used in concrete, as low pH would cause reinforcement corrosion.
Table 5 shows the leachability of pH, which should be alkaline for use in concrete and other selected contaminants.
Table 6 depicts the leachability of selected macroelements of MSWIBABV and MSWIBAAV. Studies of leachability from waste are particularly important for the environment. Norms and standards are defined by law.
The parameters of leachability specified by the regulations relate to chlorides, sulfates, phosphorus, sodium, potassium, and barium. Only barium is surpassed in both slags. After using the slag in the concrete mix, it is necessary to check the leachability of this element from the concrete. The barium will likely be immobilized; however, this is a necessity to make sure leachability is within normal limits.
Figure 6 depicts loss on ignition (LOI) in 600 °C, 815 °C, and 950 °C in MSWIBABV and MSWIBAAV.
In
Figure 6, the 5% limit value for waste in Category A was marked. In terms of LOI, MSWIBABV cannot be used in Category A concrete, because the LOI at 950 °C is 7.26%, but MSWIBAAV can be used in Category A (
Table 7). Both slags can be landfilled, because the value does not exceed 8% at 600 °C.
Table 7 presents the use of waste in three categories of concrete. MSWIBAAV can be used in Category A, while MSWIBABV can be used in Category C.
Heavy metals are some of the most dangerous pollutants for the environment. They occur in large quantities in secondary waste, but mainly in fly ash. MSWIBAAV is waste that can be used as a raw material in construction, because environmental standards are met.
Table 8 shows the amount of heavy metals in MSWIBABV and MSWIBAAV.
Table 9 shows strength tests of 30% slag and CEM I 42.5 R and 30% slag and CEM I 42.5 SR. There was no swelling, cracking, or rupture occurring in the tested slags. However, this is not the rule, as the content of swelling compounds (e.g., Al, sulfides) is related to the properties of the waste being processed. Flexural and compressive strengths of mortar are comparable and similar. The 42 mm mortar beam has a strength of 2.75 MPa. The 44 mm mortar beam has a strength of 2.63 MPa. Slightly more compressive strength has a 42 mm mortar beam (70.86 MPa), in comparison to a 44 mm mortar beam (69.94 MPa), which is adequate to the strength of mortar.
The physical and chemical properties of this aggregate (slag) should fulfil the following requirements:
Grain content below 0.125 mm to 10% by weight;
Content of foreign impurities up to 1% by weight;
Content of SO3 sulfur compounds up to 2%;
LOI must not exceed 6%, if the slag is used only as aggregate for concrete, and 12% if it is used as a component of a fine mixture with other aggregate.
Concrete made of slag with unknown properties (or those that do not meet standard conditions) may undergo unfavorable processes. The content of sulfur, aluminum, burnt coal, slag age, or LOI compounds greater than the permissible values may cause swelling of concrete, but that is not a rule. Older slag causes less swelling in the concrete.
A negative characteristic of slag concrete is its high bulk density with low compressive strength, slow drying, and high humidity, which adversely affects the thermal conductivity. The low quality of slag concrete leads to its limited range of use. It should not be used for reinforced concrete or concretes exposed to permanent moisture above 75%.
According to PN-60/B-23011, the acceptable coal content in the slag found by roasting is up to 30%; however, for reinforced concrete, it is recommended to use aggregate with a carbon content below 5%.
Soluble sulfur salts are a very harmful admixture of slag. They react with cement components and form compounds that increase in volume and, thereby, break down concrete [
34]. The most harmful of these compounds are highly hydrated calcium sulfate-aluminate-3CaO • A1
2O
3 • 3CaSO
4 (30–31) H
2O. Moreover, sulfur compounds, in particular hydrogen sulfide formed from them, promote the development of fungi in wooden elements, if they are in direct contact with slag concrete. The sulfur content of the slag also affects the rusting of steel parts placed in slag concrete. Therefore, these parts should be previously protected against corrosion.
Calcium oxide in slag up to 8% is harmless, if it occurs in the form of fine grains smaller than 2 mm, evenly distributed in the slag mass, and easily quenched with water. Slag cannot be used when containing more than 8% calcium oxide, when it is in a form of coarse grains, has uneven fraction or over-burnt lime, which is hard to stuff out.
Ash and dust in lightweight concrete are undesirable, because they increase the volumetric weight of concrete, and also contain the most sulfur admixtures. There is the possibility to get rid of them by sifting slag through a 0.5 mm sieve, or by shoveling the slag in a strong wind that blows away the ash (which is less accurate) [
35,
36,
37,
38].
4. Environmental Analysis
The important aspect of the implementation of Circular Economy (CE) assumptions is a plan for reduction of MSWIBA weight deposited at landfills and increase of the share of waste, which can be returned or reused in the system [
39,
40,
41]. This is a huge challenge—doing it without any cost for the environment. Some methods, which can be used for those purposes, are identified in the manuscript. However, before each implementation, the method should be tested and analyzed, in terms of the environment. In order to assess the impact, the comparison between the current stage and new model should be made. The best approach to assess the real impact is the LCA analysis, but it requires a lot of data and specific information. The most important facts can help in order to realize the general direction.
Basically, the environmental analysis can help to identify, in full life cycle, the real impact on the environment. Those tools are also important in comparing different scenarios of the production, use, or disposal phase [
41,
42]. Thanks to the environmental approach, one can assess the real impact and choose the best possible scenario. The circular economy strives to develop waste management in the most efficient way, from reuse and recycle, and finally to landfill. However, each case needs to be analyzed separately, because, during the recycling and treatment, the environmental cost is also present [
43,
44,
45].
Regarding the treatment of MSWIBA, an assessment of three scenarios can be conducted. The first scenario states that the MSWIBA, after necessary treatment, is directed to the landfill. The second scenario consists of using valorized MSWIBAAV as a replacement of raw material in concrete production. The third scenario shows the potential of using the more advance methods of treatment, in order to achieve better product at the end of the life.
Figure 7 shows the scenarios, which have positive and negative impact on the environment.
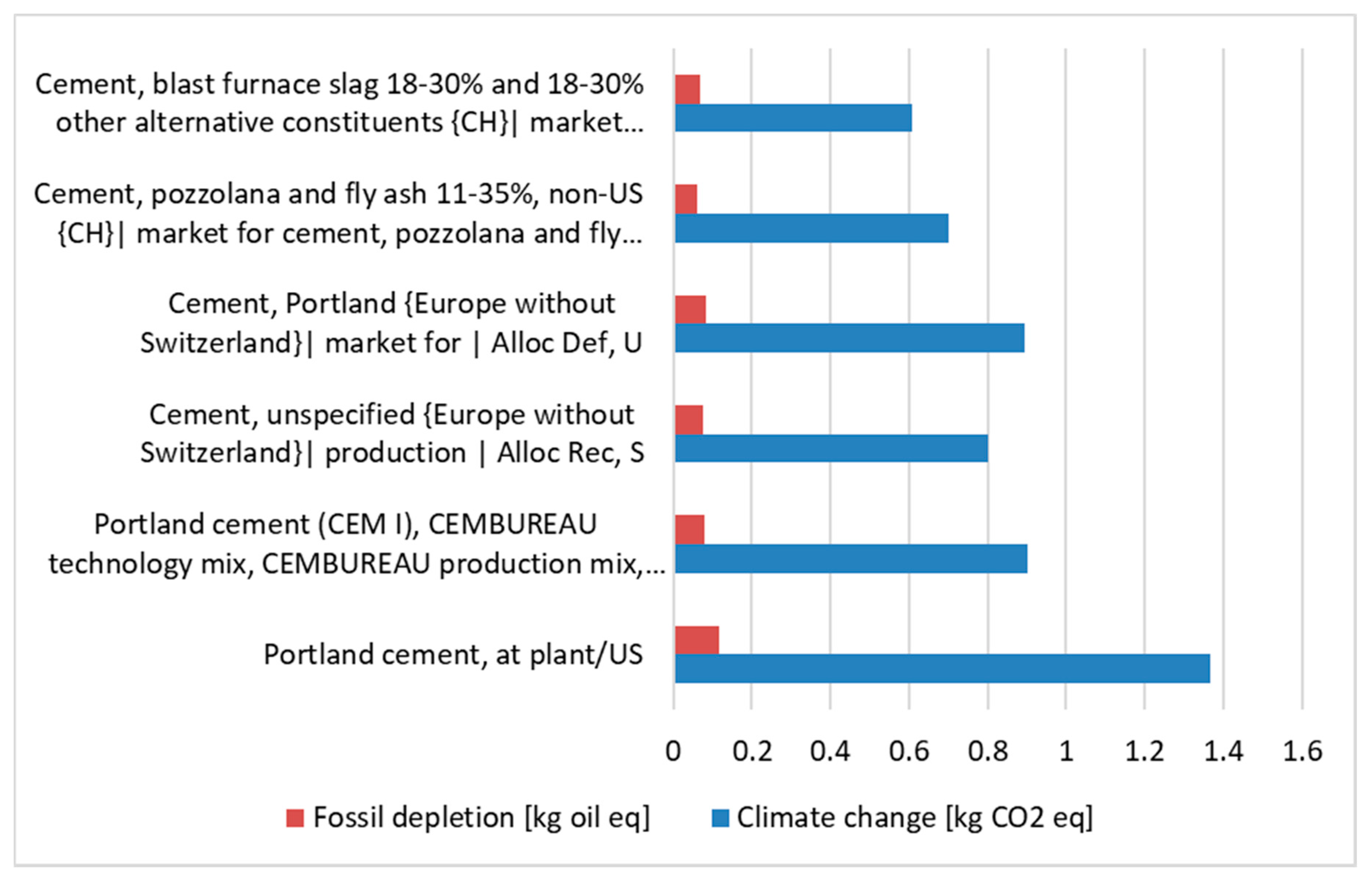
The sub-product, which can be replaced in concrete production, is cement, which can be created from the raw material, fly ash, or slag as a supplement. The results are presented in the unit of oil equivalent per 1 kg of product and CO
2 equivalent per 1 kg of product, in
Figure 8.
When analyzing a particular result, it can be observed that, in the fossil depletion category, the cement from raw material production has the biggest impact on the environment in each case—CEM I, Portland cement, and unspecified—average in EU (Europe). This is related to the depletion of the raw material in relation to the total reserves that are available and using fossil fuels in order to extract the product. By using secondary waste, which has the smallest impact on the environment, this valuable raw material can be saved, while additionally utilizing waste that is difficult to manage (cement with slag and ash). The only downside is that this waste must be prepared and processed before use (like in any process, chemicals and energy are used). However, when considering the overall environmental impact, especially the emissions to water and soil avoided during possible landfill, the process has a lower environmental impact in the fossil depletion category than the use of raw material in cement production. The same result can be observed in the case of the climate change category. The biggest impact can be seen in case of cement production from raw sources. The results for cement production using fly ash or slag are lower than basic ones. It is also linked to the process of production and utilization of fly ash as a secondary waste.
The result shows the basic advantages of the analyzing method of MSWIBA utilization and points the potential link with environmental benefits. Additionally, the concept is strongly associated with the principles of a circular economy, and can help in achieving the final assumptions for difficult secondary type waste, such as MSWIBA. In order to see a full picture, the LCA should be conducted based on real data on real examples [
46].
5. Discussion
The variability of physicochemical composition of slag is the critical factor of slag as a potential component of mortar and concrete. Seasons also have a direct impact, which means that the slag has a different composition in different seasons. Waste collection also affects the quality of secondary waste. A changeable stream of municipal waste can cause different quality of slag, which means that the recipient is forced to conduct ongoing analyses and recognize changes in concrete mixes.
Slag from an incineration plant does not have radioactive isotopes, because municipal waste is checked at the entrance gate, equipped with a radioactivity sensor, so there is no need to check it. Slag meeting the PN-EN 12620: 2004 standard may become valuable raw material and an alternative to aggregates [
47,
48]. Slag placed in the concrete mix delays the setting of the structure. Initial compressive strength is low, but after 28 days, ternary concrete containing 20–25% slag and 3–5% silica dust exceeds the strength of the control test. The compressive strength of the concrete mix is optimal when combining dust and slag. The combination of this secondary waste also affects the increased immobilization of pollution. Concrete with slag composition (instead of a smaller fraction component, e.g., cement) has a higher porosity and, hence, less thermal conductivity; however, the concrete matrix structure can be compacted by adding fly dust from the incineration plant. Due to the consolidation of various wastes, a synergy effect occurs [
49,
50,
51,
52,
53].
When neutralizing secondary waste from combustion, the phenomenon of solidification and stabilization is also used in the case of stabilization, while the chemical nature of the element changes. As a result of sorption, substitution, and precipitation, the pollution changes to an insoluble form, preventing leaching into the environment. Solidification makes it possible to change physical characteristics by obtaining waste materials with a relatively low liquid phase content. Efficiency of the solidification/stabilization process is described by matrix permanency and leaching of heavy metals. The precise indication of solidifying composite is complicated, because s/s is described by many different parameters, among others, strength, water absorption, water penetration depth, and porosity [
54,
55].
Comparing the interdisciplinary literature, it is stated that the technology of recycling secondary waste should be developed. Valorization mainly concerns slag, which can be used in some branches of the construction industry. The next (e.g., in the case of slag) or the only one (e.g., in the case of fly ah) step is NaOH pre-treatment. Literature sources indicate that, depending on the temperature, time, and concentration of the NaOH [
53], the properties of the waste can be improved, e.g., immobilization of heavy metals [
4], strength of the produced building material, with the addition of activated waste [
3].
6. Conclusions
Research on the alkaline activation of copper slag shows that, as a result of chemical reactions between ground copper slag and the alkaline activator (NaOH or sodium water glass), the following are formed:
Low-basic hydrosilicates of the C-S-H type;
Hydrated low-basic aluminates and aluminosilicates of the hydrogranate type;
Calcite;
Magnesium hydrosilicates;
Mixed sodium-potassium compounds;
Alkaline hydrated aluminosilicates of the hydronefeline, analcime, and natrolite type.
The resulting products are different from products in traditional cement. Choosing the right type and amount of activator is a complex task and depends mainly on the chemical composition and specific surface of the slag. Along with the decreasing CaO content, the content of C-S-H and C-A-H phases, formed as a result of hydration, decreases, and the content of zeolite-like phases increases [
56,
57,
58].
The basis of waste management is the minimization of their generation (according to Directive 2008/98/EC on waste). Despite adhering to the waste management hierarchy and the recommendations of a circular economy, there will always be a waste stream that cannot be recycled. That is why it is important to look for new and more effective solutions. Waste management processes should follow best available technology (BAT).
Alkaline pre-treatment gives a chance in the management of secondary waste. Alkaline pre-treated waste should always be tested, because of the heterogeneity of the composition. Improving secondary waste quality is in line with the principles of sustainable development and a circular economy [
59,
60,
61].
The use of municipal solid waste incineration bottom ash saves raw material in the production of concrete and, thus, has a positive effect on the environment, especially in the abiotic depletion category. In addition, it has a positive impact, because we do not store it in a landfill, which does not fit into a circular economy. According to the idea of a circular economy, secondary waste is the biggest problem, because, almost always, it cannot be directly reused or recycled. The proposed form of municipal solid waste incineration bottom ash management gives this waste a second life, and minimizes its negative impact on the environment.