Conceptual Design Development of Coal-to-Methanol Process with Carbon Capture and Utilization
Abstract
:1. Introduction
2. Simulation Methodology
3. Process Description
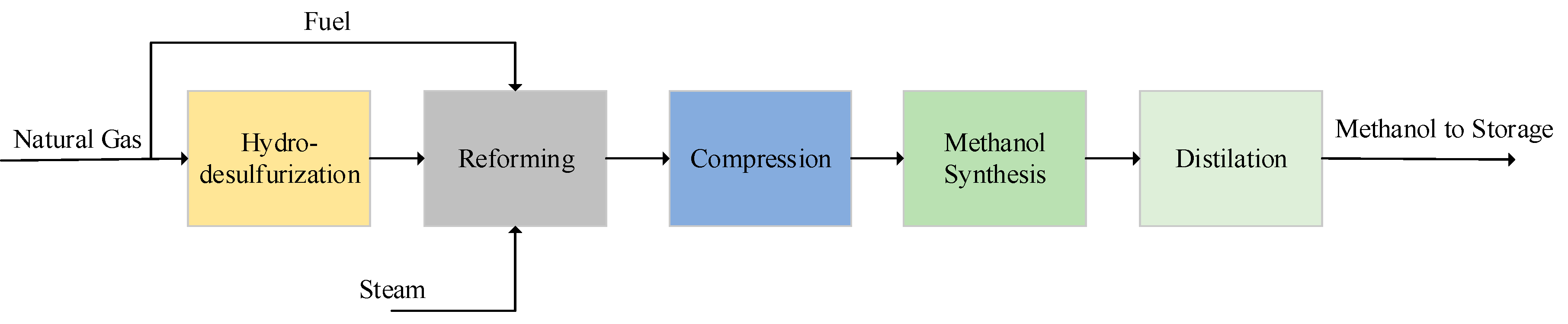
3.1. Base Case Design
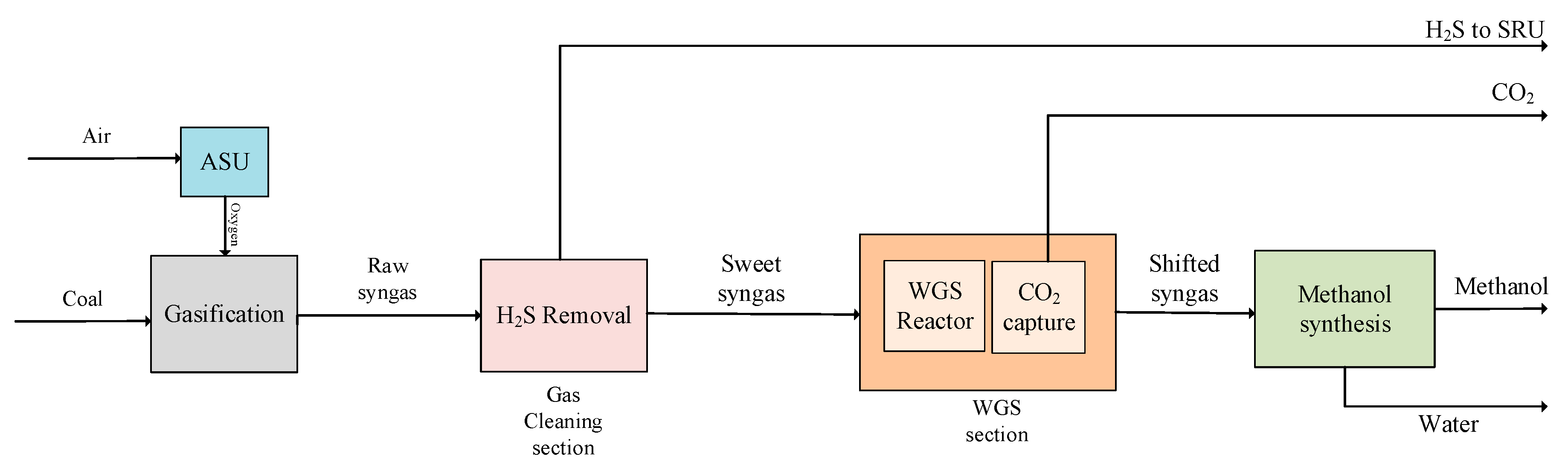
3.2. Modified Design 1 (MD1)
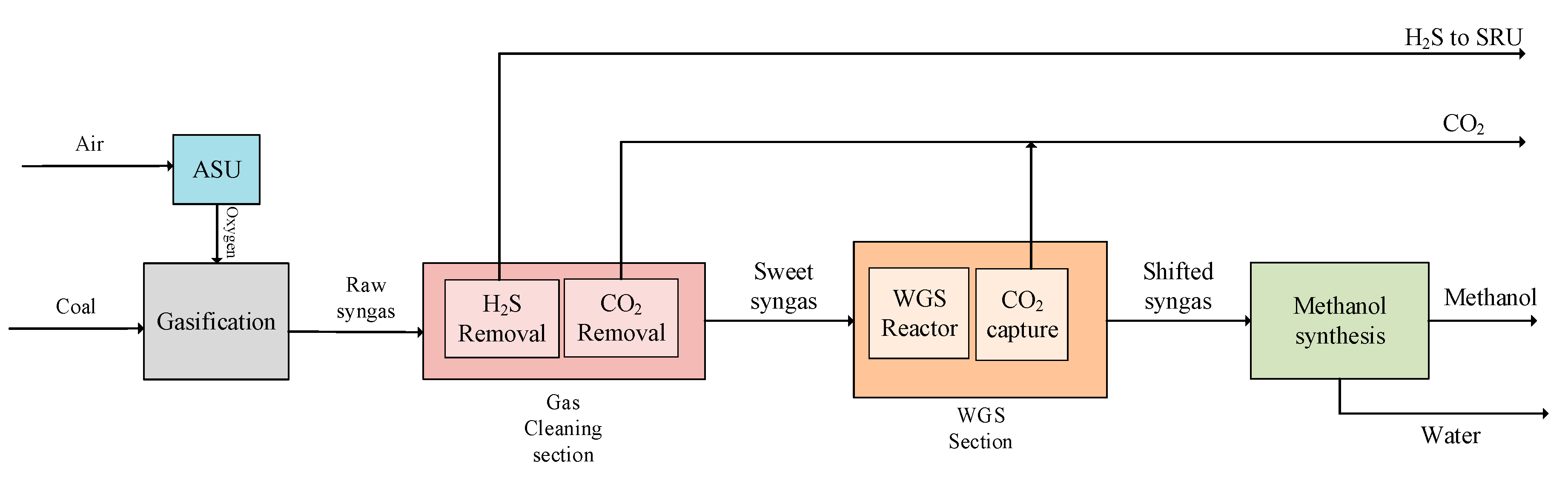
3.3. Modified Design 2 (MD2)
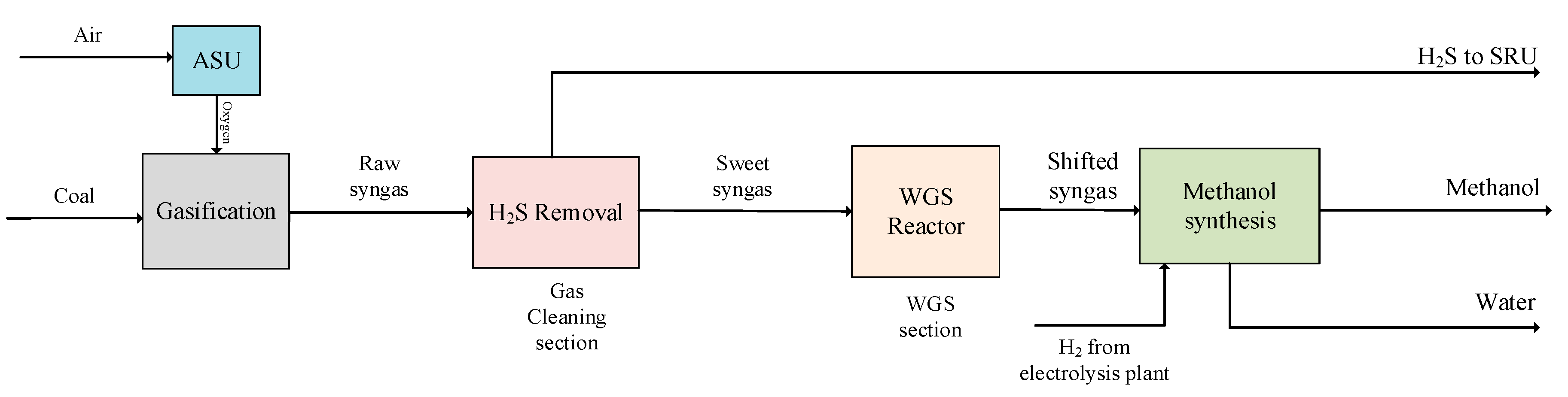
3.4. Modified Design 3 (MD3)
4. Results and Discussions
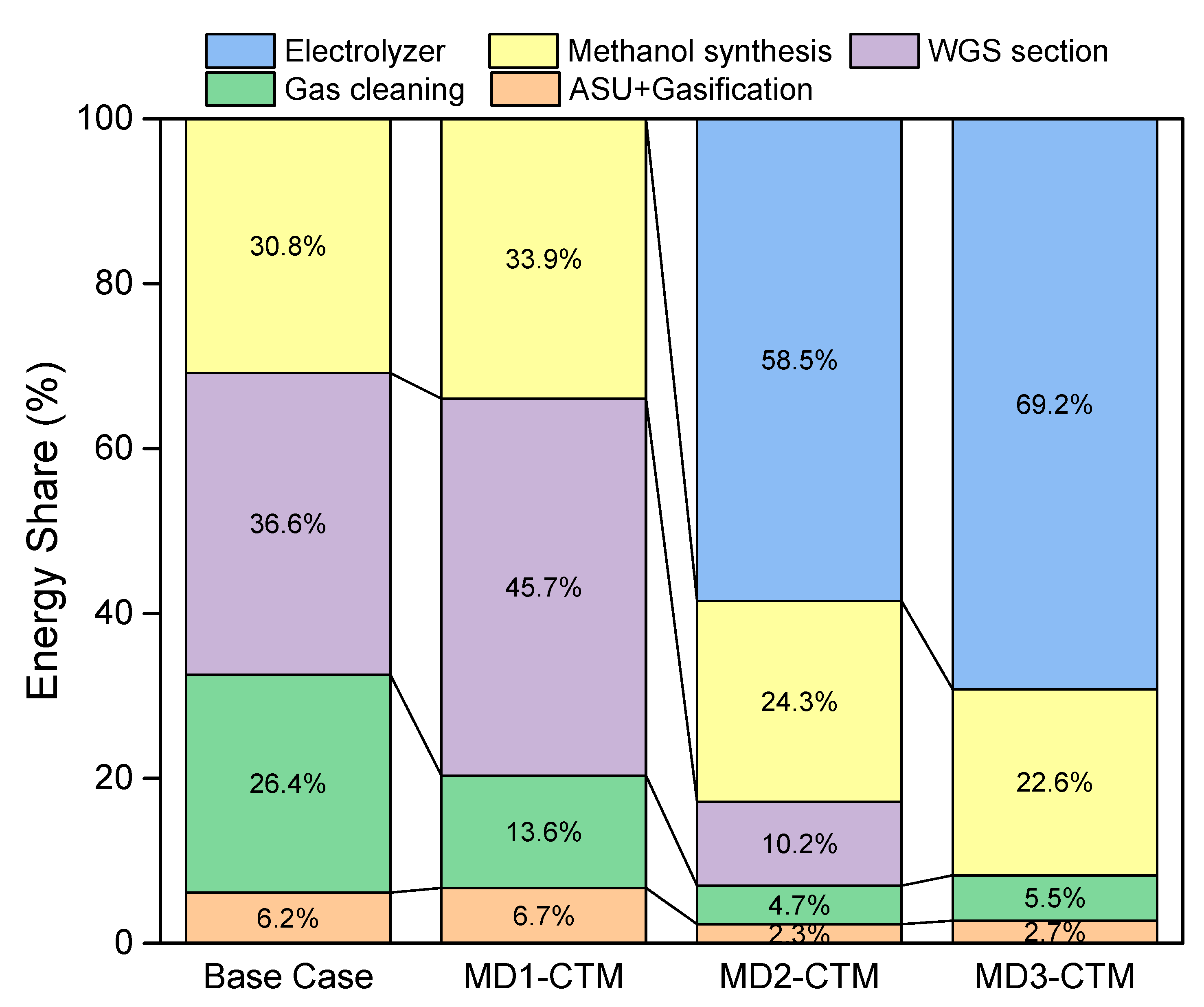
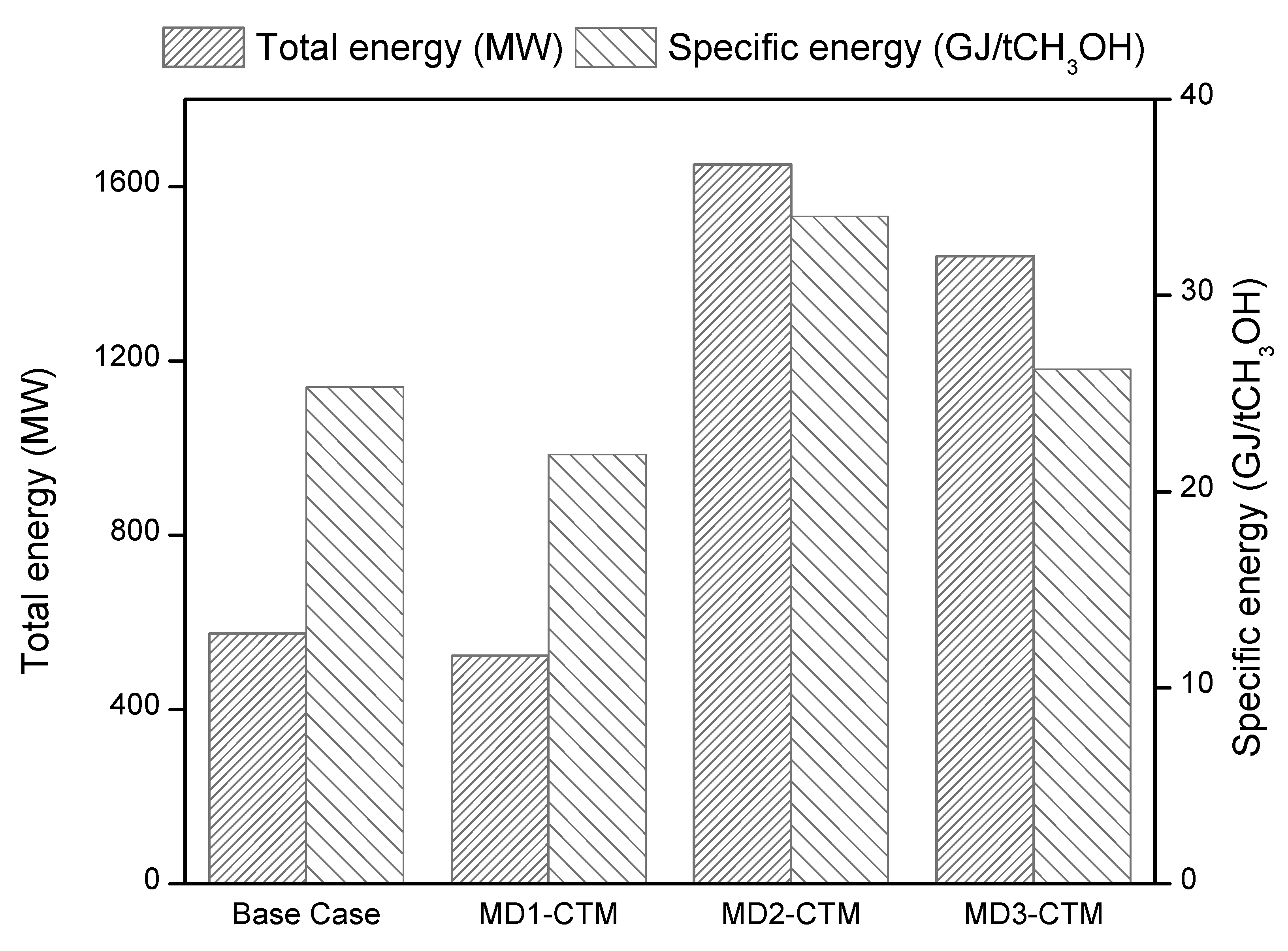
4.1. Energy Analysis
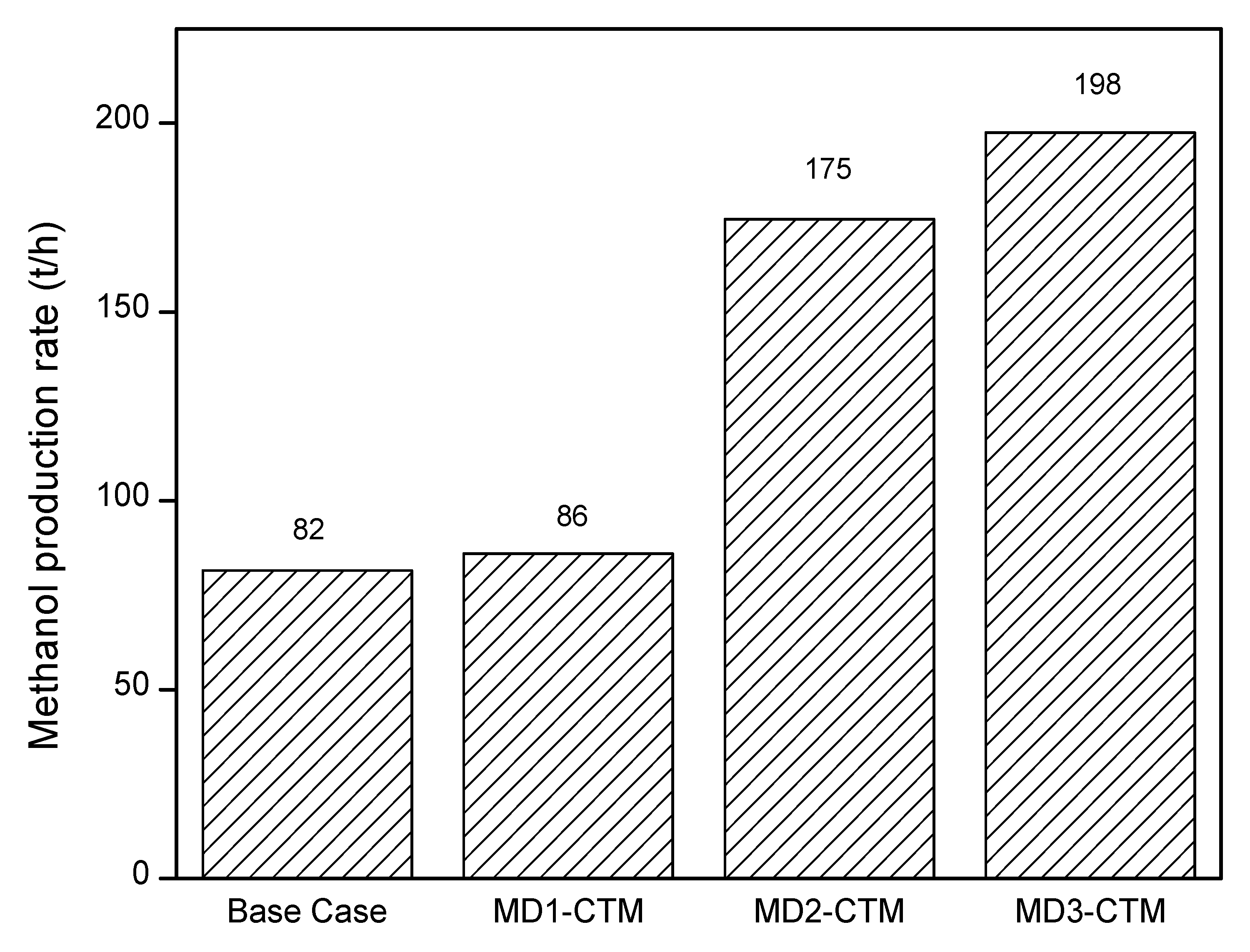
4.2. Methanol Production Rate
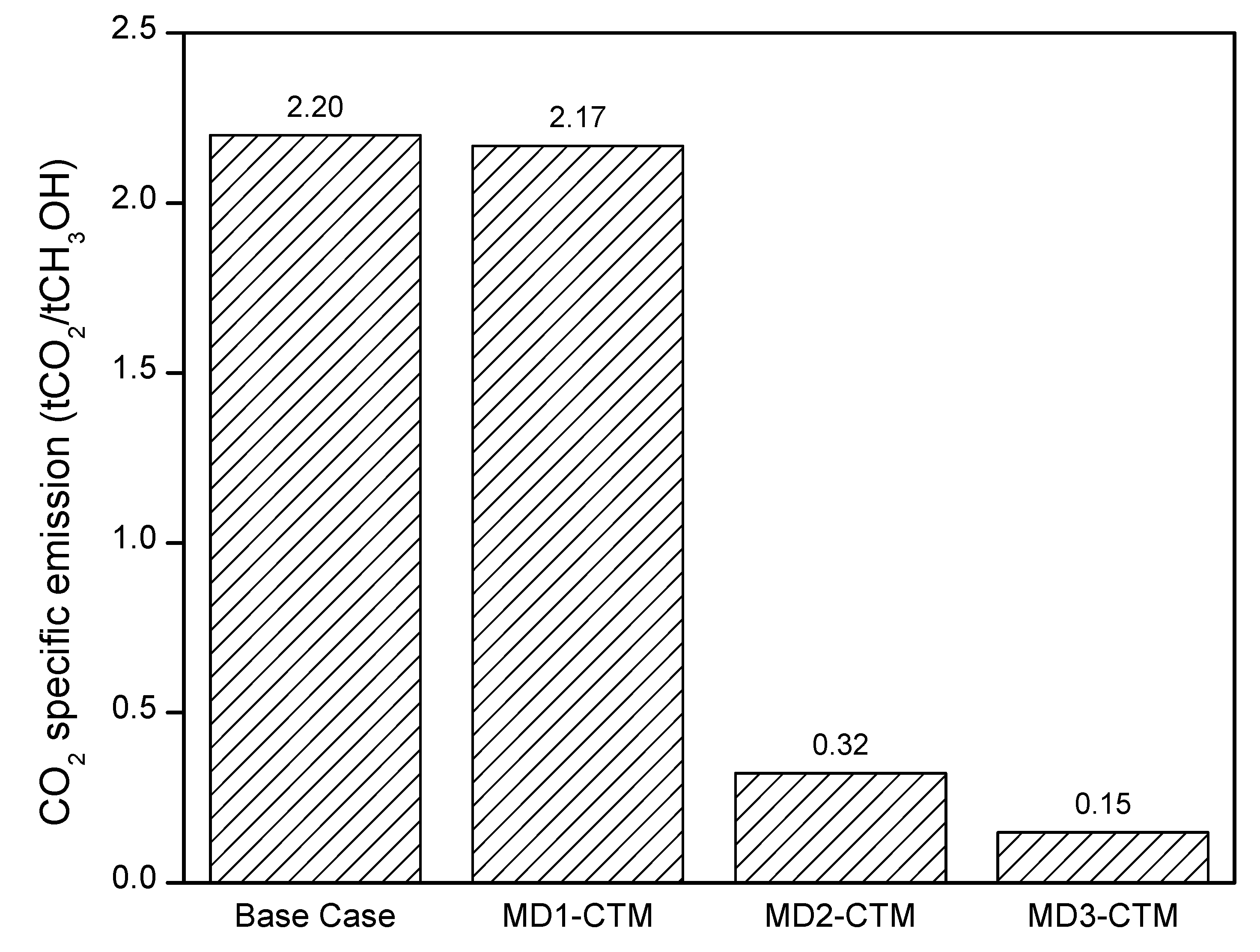
4.3. Carbon Emissions
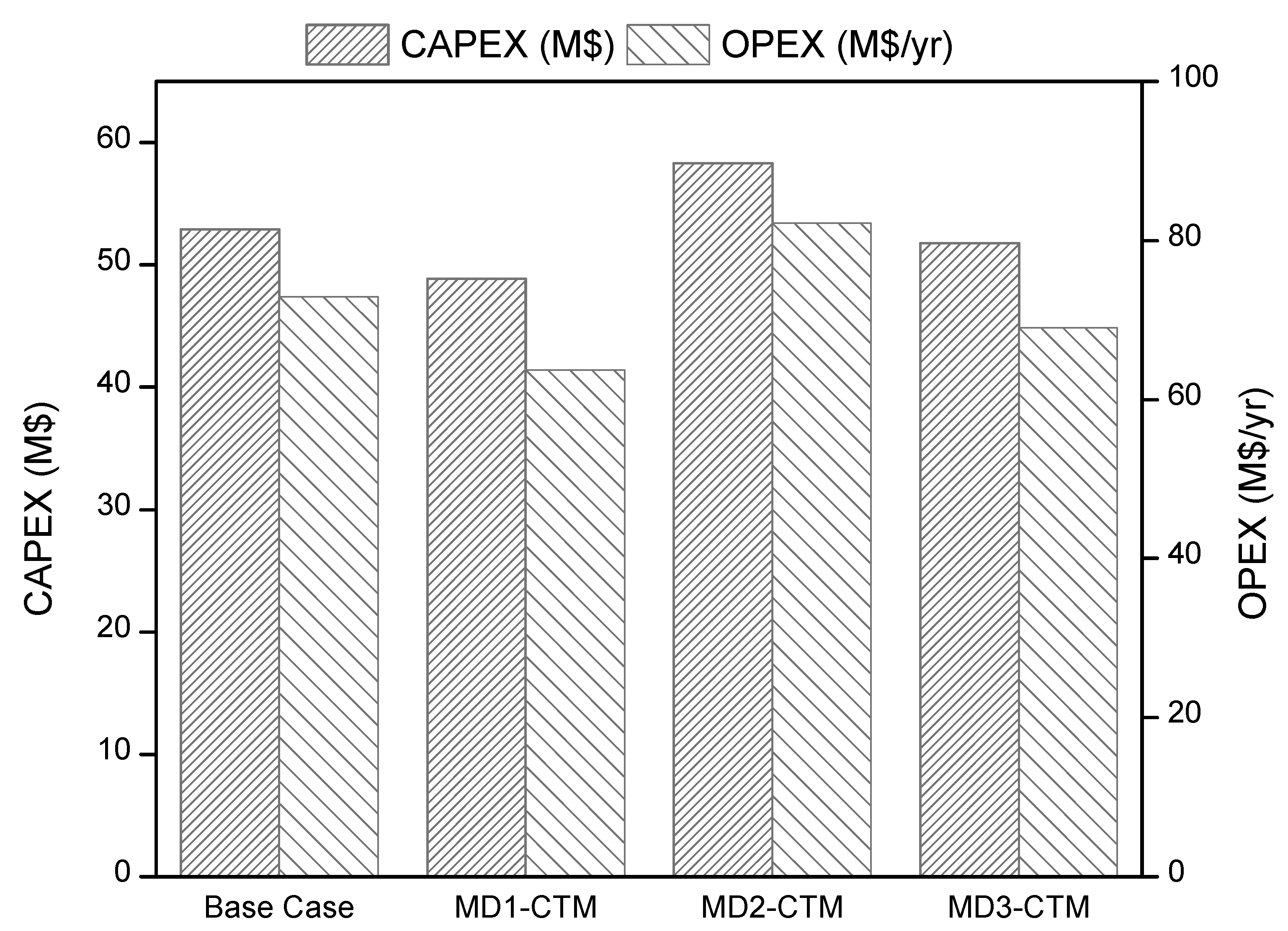
4.4. Economic Analysis
4.5. Comparative Analysis with the Literature
5. Conclusions and Recommendations
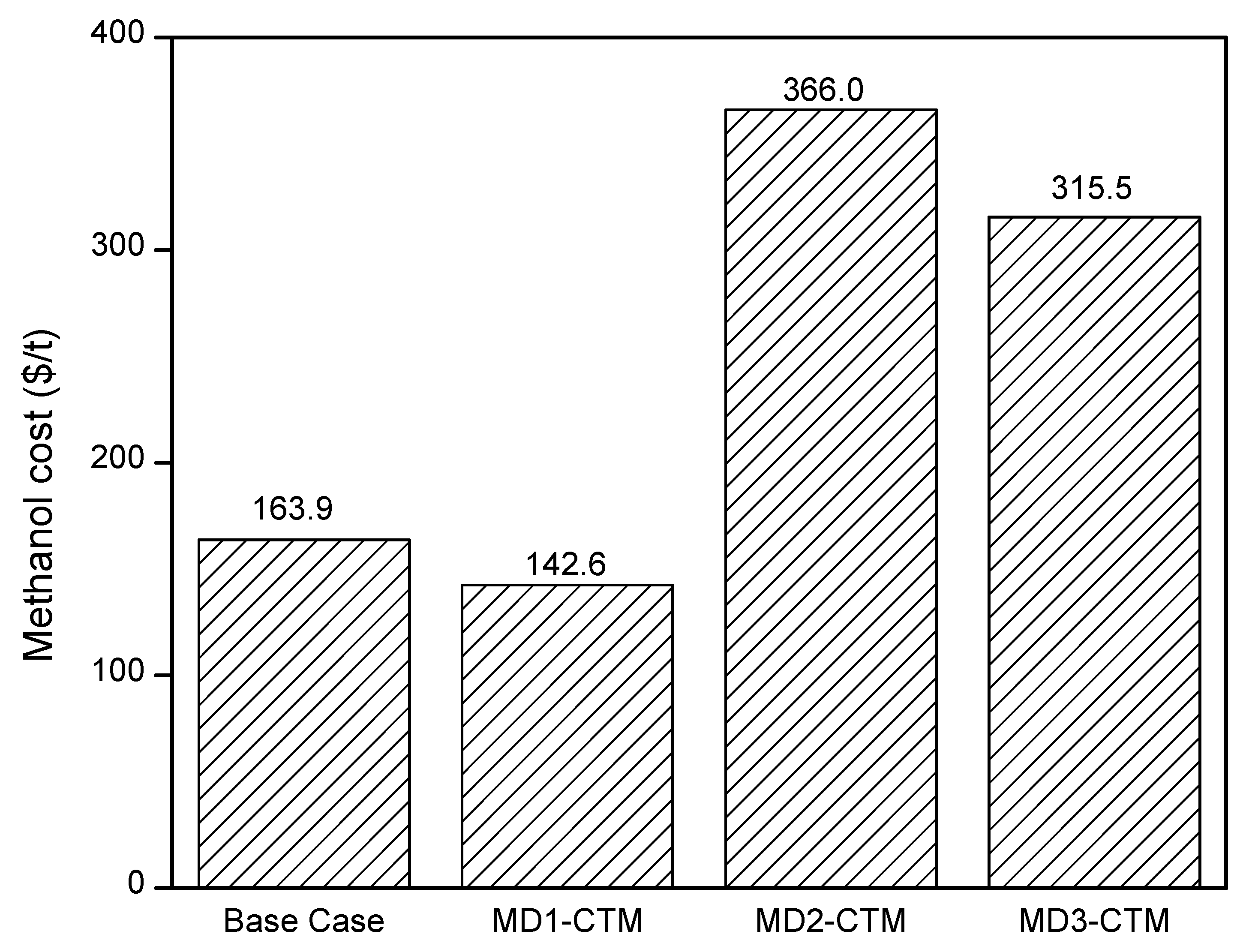
- CTM production with CCUS is technically and economically feasible process. The MD1-CTM offers the best results with an energy requirement of 21.9 GJ/tCH3OH and a product cost of 142.5 $/tCH3OH.
- CTM process is complex process with many energy intensive equipment including gasification section, gas cleaning section, WGS section and methanol synthesis section. However, the process development based on intensification concepts can simplify the process, leading to significant CAPEX and OPEX savings.
- CO2 emissions from the CTM process can be curtailed up to a certain limit. In order to further minimize the CO2 emissions from the process, H2 supply from an external source is required. In the case of CTM process integration with an electrolysis plant for the supply of clean H2, the overall economics are not feasible.
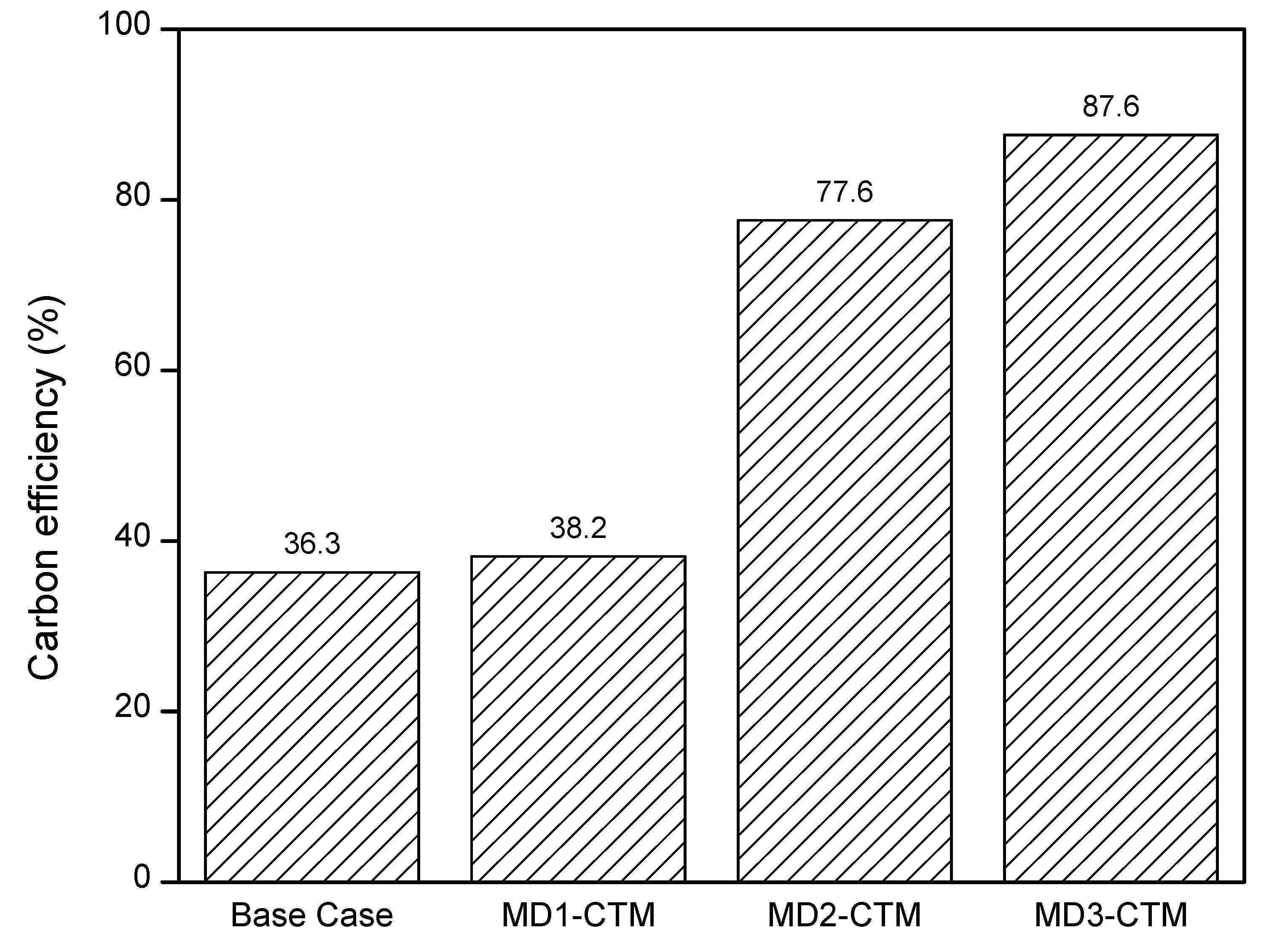
- The proposed MD2 and MD3 configurations have 8.6% and 14.6% higher process efficiency respectively compared to the base case design. However, the economics of these designs are not feasible. In particular, MD3 can become economically attractive if, (a) the carbon tax is implemented due to its low emission benefits; (b) technological advancement in the electrolysis process are made for the supply of clean and cheap H2.
Author Contributions
Funding
Conflicts of Interest
Nomenclature
| ASU | Air separation unit |
| CAGR | Compound annual growth rate |
| CAPEX | Capital expenditure |
| CCUS | Carbon capture, utilization and storage |
| DMR | Dry methane reforming |
| EOS | Equation of state |
| GHG | Greenhouse gas |
| IGCC | Integrated gasification combined cycle |
| IPCC | Intergovernmental panel on climate change |
| IRR | Internal rate of return |
| LNG | Liquefied natural gas |
| MD1 | Modified Design 1 |
| MD2 | Modified Design 2 |
| MD3 | Modified Design 3 |
| MOFs | Metal-organic frameworks |
| MTO | Methanol to olefins |
| NETL | National energy technology laboratory |
| OPEX | Operating expense |
| PR-BM | Peng-Robinson with Boston Mathias |
| SN | Stoichiometric number |
| SRU | Sulfur recovery unit |
| TAC | Total annual cost |
| TCI | Total capital investment |
| WGS | Water gas shift |
References
- Hulme, M. Is it too late (to stop dangerous climate change)? An editorial. Wiley Interdiscip. Rev. Clim. Chang. 2020, 11, e619. [Google Scholar] [CrossRef] [Green Version]
- Helgason, R.; Cook, D.; Davíðsdóttir, B. An evaluation of the cost-competitiveness of maritime fuels—A comparison of heavy fuel oil and methanol (renewable and natural gas) in Iceland. Sustain. Prod. Consum. 2020, 23, 236–248. [Google Scholar] [CrossRef]
- Bazooyar, B.; Ghorbani, A.; Shariati, A. Combustion performance and emissions of petrodiesel and biodiesels based on various vegetable oils in a semi industrial boiler. Fuel 2011, 90, 3078–3092. [Google Scholar] [CrossRef]
- Turaga, U. Small-scale methanol technologies offer flexibility, cost effectiveness. Gas Process. News 2017. Available online: http://www.gasprocessingnews.com/features/201510/small-scale-methanol-technologies-offer-flexibility,-cost-effectiveness.aspx (accessed on 13 November 2020).
- IHS Markit. Chemical World Analysis Long-Term Chemical Market Trends; IHS Markit: London, UK, 2019. [Google Scholar]
- Unnasch, S.; Lawrence, M.; Pont, J. Kalama Manufacturing and Marine Export Facility Supplemental GHG Analysis; Life Cycle Associates Report: Washington, DC, USA, 2018. [Google Scholar]
- Nguyen, T.B.H.; Zondervan, E. Methanol production from captured CO2 using hydrogenation and reforming technologies-environmental and economic evaluation. J. CO2 Util. 2019, 34, 1–11. [Google Scholar] [CrossRef]
- Leonzio, G.; Zondervan, E.; Foscolo, P.U. Methanol production by CO2 hydrogenation: Analysis and simulation of reactor performance. Int. J. Hydrogen Energy 2019, 44, 7915–7933. [Google Scholar] [CrossRef]
- Tamnitra, R.; Jitwung, R.; Puangpetch, T.; Patthaveekongka, W.; Leeheng, K. Kinetic modeling and simulation of bio-methanol process from biogas by using aspen plus. MATEC Web Conf. 2018, 192, 4–7. [Google Scholar] [CrossRef]
- Šetinc, M.; Levec, J. On the kinetics of liquid-phase methanol synthesis over commercial Cu/ZnO/Al2O3 catalyst. Chem. Eng. Sci. 1999, 54, 3577–3586. [Google Scholar] [CrossRef]
- Lee, H.W.; Kim, K.; An, J.; Na, J.; Kim, H.; Lee, H.; Lee, U. Toward the practical application of direct CO2 hydrogenation technology for methanol production. Int. J. Energy Res. 2020. [Google Scholar] [CrossRef]
- Xiang, D.; Li, P.; Yuan, X. System optimization and performance evaluation of shale gas chemical looping reforming process for efficient and clean production of methanol and hydrogen. Energy Convers. Manag. 2020, 220, 113099. [Google Scholar] [CrossRef]
- Kim, S.; Kim, M.; Kim, Y.T.; Kwak, G.; Kim, J. Techno-economic evaluation of the integrated polygeneration system of methanol, power and heat production from coke oven gas. Energy Convers. Manag. 2019, 182, 240–250. [Google Scholar] [CrossRef]
- Ahmed, U. Techno-economic feasibility of methanol synthesis using dual fuel system in a parallel process design configuration with control on green house gas emissions. Int. J. Hydrogen Energy 2020, 45, 6278–6290. [Google Scholar] [CrossRef]
- Agarwal, R.A. Methanol Hydrogenation Synthesis Using from Metal–Organic CO2 Frameworks. CO2 Sep. Purif. Convers. Chem. Fuels 2018, 79. [Google Scholar] [CrossRef]
- Ting, K.W.; Toyao, T.; Siddiki, S.M.A.H.; Shimizu, K. Low-temperature hydrogenation of CO2 to methanol over heterogeneous TiO2-Supported Re catalysts. ACS Catal. 2019, 9, 3685–3693. [Google Scholar] [CrossRef]
- Li, S.; Guo, L.; Ishihara, T. Hydrogenation of CO2 to methanol over Cu/AlCeO catalyst. Catal. Today 2020, 339, 352–361. [Google Scholar] [CrossRef]
- Van-Dal, É.S.; Bouallou, C. Design and simulation of a methanol production plant from CO2 hydrogenation. J. Clean. Prod. 2013, 57, 38–45. [Google Scholar] [CrossRef]
- Asif, M.; Gao, X.; Lv, H.; Xi, X.; Dong, P. Catalytic hydrogenation of CO2 from 600 MW supercritical coal power plant to produce methanol: A techno-economic analysis. Int. J. Hydrogen Energy 2018, 43, 2726–2741. [Google Scholar] [CrossRef]
- Charoensuppanimit, P.; Kitsahawong, K.; Kim-Lohsoontorn, P.; Assabumrungrat, S. Incorporation of hydrogen by-product from NaOCH3 production for methanol synthesis via CO2 hydrogenation: Process analysis and economic evaluation. J. Clean. Prod. 2019, 212, 893–909. [Google Scholar] [CrossRef]
- Kiss, A.A.; Pragt, J.J.; Vos, H.J.; Bargeman, G.; de Groot, M.T. Novel efficient process for methanol synthesis by CO2 hydrogenation. Chem. Eng. J. 2016, 284, 260–269. [Google Scholar] [CrossRef] [Green Version]
- Luu, M.T.; Milani, D.; Bahadori, A.; Abbas, A. A comparative study of CO2utilization in methanol synthesis with various syngas production technologies. J. CO2 Util. 2015, 12, 62–76. [Google Scholar] [CrossRef]
- Puig-Gamero, M.; Argudo-Santamaria, J.; Valverde, J.L.; Sánchez, P.; Sanchez-Silva, L. Three integrated process simulation using aspen plus®: Pine gasification, syngas cleaning and methanol synthesis. Energy Convers. Manag. 2018, 177, 416–427. [Google Scholar] [CrossRef]
- Liu, Z. Economic analysis of methanol production from coal/biomass upgrading. Energy Sources Part B Econ. Plan. Policy 2018, 13, 66–71. [Google Scholar] [CrossRef]
- Herdem, M.S.; Mazzeo, D.; Matera, N.; Wen, J.Z.; Nathwani, J.; Hong, Z. Simulation and modeling of a combined biomass gasification-solar photovoltaic hydrogen production system for methanol synthesis via carbon dioxide hydrogenation. Energy Convers. Manag. 2020, 219, 113045. [Google Scholar] [CrossRef]
- Rivarolo, M.; Bellotti, D.; Magistri, L.; Massardo, A.F. Feasibility study of methanol production from different renewable sources and thermo-economic analysis. Int. J. Hydrogen Energy 2016, 41, 2105–2116. [Google Scholar] [CrossRef]
- Wang, X.; Demirel, Y. Feasibility of Power and Methanol Production by an Entrained-Flow Coal Gasification System. Energy Fuels 2018, 32, 7595–7610. [Google Scholar] [CrossRef]
- Ye, C.; Wang, Q.; Zheng, Y.; Li, G.; Zhang, Z.; Luo, Z. Techno-economic analysis of methanol and electricity poly-generation system based on coal partial gasification. Energy 2019, 185, 624–632. [Google Scholar] [CrossRef]
- Kler, A.M.; Tyurina, E.A.; Mednikov, A.S. A plant for methanol and electricity production: Technical-economic analysis. Energy 2018, 165, 890–899. [Google Scholar] [CrossRef]
- Gu, Y. Kokossis Modeling and Analysis of Coal-Based Lurgi Gasification for LNG and Methanol Coproduction Process. Processes 2019, 7, 688. [Google Scholar] [CrossRef] [Green Version]
- McDonell, V. Key combustion issues associated with syngas and high-hydrogen fuels. In The Gas Turbine Handbook; Gulf Professional Publishing: Oxford, UK, 2006. [Google Scholar]
- Mondal, P.; Dang, G.S.; Garg, M.O. Syngas production through gasification and cleanup for downstream applications—Recent developments. Fuel Process. Technol. 2011, 92, 1395–1410. [Google Scholar] [CrossRef]
- Summers, W. Baseline Analysis of Crude Methanol Production from Coal and Natural Gas. Natl. Energy Technol. Lab. 2014. [Google Scholar] [CrossRef]
- Aspen Technology, Inc. Aspen Physical Property System-Physical Property Methods; Aspen Technology, Inc.: Burlington, MA, USA, 2013. [Google Scholar]
- Vanden Bussche, K.M.; Froment, G.F. A steady-state kinetic model for methanol synthesis and the water gas shift reaction on a commercial Cu/ZnO/Al2O3 catalyst. J. Catal. 1996, 161, 1–10. [Google Scholar] [CrossRef]
- Bozzano, G.; Manenti, F. Efficient methanol synthesis: Perspectives, technologies and optimization strategies. Prog. Energy Combust. Sci. 2016, 56, 71–105. [Google Scholar] [CrossRef]
- Samimi, F.; Rahimpour, M.R.; Shariati, A. Development of an efficient methanol production process for direct CO2 hydrogenation over a Cu/Zno/Al2O3 catalyst. Catalysts 2017, 7, 332. [Google Scholar] [CrossRef] [Green Version]
- Santos, D.M.F.; Sequeira, C.A.C.; Figueiredo, J.L. Hydrogen production by alkaline water electrolysis. Quim. Nova 2013, 36, 1176–1193. [Google Scholar] [CrossRef]
- M. Pérez-Fortes, E.T. Techno-Economic and Environmental Evaluation of CO2 Utilisation for Fuel Production. Synthesis of Methanol and Formic Acid; JRC Science Hub: ZG Petten, The Netherlands, 2016; ISBN 9789279591334. [Google Scholar]
- Calise, F.; D’Accadia, M.D.; Santarelli, M.; Lanzini, A.; Ferrero, D. Solar Hydrogen Production: Processes, Systems and Technologies; Academic Press: Cambridge, MA, USA, 2019; ISBN 0128148543. [Google Scholar]
- Turton, R.; Bailie, R.C.; Whiting, W.B.; Shaeiwitz, J.A.; Bhattacharyya, D. Analysis, Synthesis and Design of Chemical Processes, 4th ed.; Turton, R., Ed.; Pearson Education: London, UK, 2008. [Google Scholar]
- Reed, T.B. Efficiencies of methanol production from gas, coal, waste or wood. Am. Chem. Soc. Div. Fuel Chem. Prepr. 1976, 21, 2–21. [Google Scholar]
- Chen, J.; Yang, S.; Qian, Y. A novel path for carbon-rich resource utilization with lower emission and higher efficiency: An integrated process of coal gasification and coking to methanol production. Energy 2019, 177, 304–318. [Google Scholar] [CrossRef]
- Xiang, D.; Li, P.; Yuan, X.; Cui, P.; Huang, W. Highly efficient carbon utilization of coal-to-methanol process integrated with chemical looping hydrogen and air separation technology: Process modeling and parameter optimization. J. Clean. Prod. 2020, 258, 120910. [Google Scholar] [CrossRef]
- Zhang, D.; Duan, R.; Li, H.; Yang, Q.; Zhou, H. Optimal design, thermodynamic, cost and CO2 emission analyses of coal-to-methanol process integrated with chemical looping air separation and hydrogen technology. Energy 2020, 203, 117876. [Google Scholar] [CrossRef]
- Yi, Q.; Li, W.; Feng, J.; Xie, K. Carbon cycle in advanced coal chemical engineering. Chem. Soc. Rev. 2015, 44, 5409–5445. [Google Scholar] [CrossRef]
- Qin, Z.; Zhai, G.; Wu, X.; Yu, Y.; Zhang, Z. Carbon footprint evaluation of coal-to-methanol chain with the hierarchical attribution management and life cycle assessment. Energy Convers. Manag. 2016, 124, 168–179. [Google Scholar] [CrossRef]
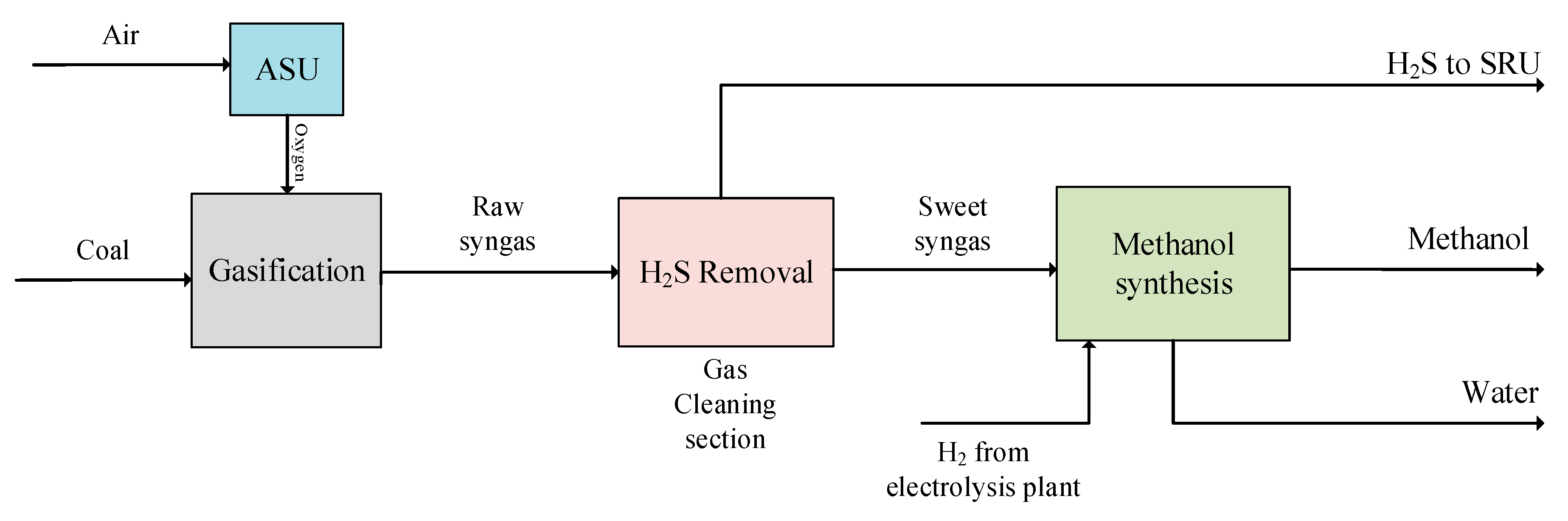



| Proximate Analysis | Ultimate Analysis | Sulfur Content | |||
|---|---|---|---|---|---|
| Element | Value | Element | Value | Element | Value |
| Moisture | 9.53 | Ash | 9.66 | Pyritic | 100 |
| Fixed Carbon | 50.91 | Carbon | 74.45 | Sulfate | 0 |
| Volatile matter | 39.45 | Hydrogen | 4.95 | Organic | 0 |
| Ash | 9.64 | Nitrogen | 1.58 | ||
| Chlorine | 0.065 | ||||
| Sulfur | 2.44 | ||||
| Oxygen | 6.84 | ||||
| Equipment | Description | Aspen Model |
|---|---|---|
| Coal flowrate | Coal = 34.95 kg/s (65% coal, 35% water) | - |
| Gasifier | Temperature = 1300 °C Pressure = 50 bar Carbon conversion = 99% | RYield, RGibbs |
| Water gas shift | Reactor configuration = 2 stage Overall CO conversion = 99.5% | REquil |
| Methanol reactor | Temperature = 210 °C Pressure = 76 bar Cu-based catalyst Catalyst density = 1725 kg/m3 Bed voidage = 0.4 | RPlug |
| Methanol distillation | Product purity ≥ 99 mol.% Reflux ratio = 1.5 | Radfrac |
| Coal Feed | Raw Syngas | H2S to SRU | CO2 | Sweet Syngas | Shifted Syngas | Ext. H2 | Methanol | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BC/MD1/MD2/MD3 | BC/MD1/MD2/MD3 | BC/MD1/MD2/MD3 | BC | MD1 | BC | MD1/MD2/MD3 | BC | MD1 | MD2 | MD2/MD3 | BC | MD1 | MD2 | MD3 | ||
| Temp. | °C | 55 | 152.5 | −9.5 | −31.6 | −27.6 | 93.3 | 93.3 | −1.2 | −2.4 | 0.0 | 25.0 | 52.5 | 52.3 | 52.5 | 33.1 |
| Pressure | bar | 50 | 27.6 | 1.1 | 1.0 | 1.0 | 27.6 | 27.6 | 24.1 | 24.1 | 24.1 | 30.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Mass flowrate | t/h | 193.6 | 284.7 | 13.0 | 148.1 | 157.3 | 169.5 | 224.3 | 165.2 | 165.7 | 322.4 | 21.5 | 81.6 | 86.0 | 174.6 | 197.6 |
| Mole flowrate | kmol/h | 13,924 | 314 | 3387 | 3588 | 9714 | 10,970 | 12,815 | 12,850 | 16,420 | 10,660 | 2552 | 2693 | 5463 | 6168 | |
| Comp. | mole.fr | |||||||||||||||
| N2 | - | 0.009 | 0.002 | 0.001 | 0.000 | 0.012 | 0.011 | 0.009 | 0.009 | 0.007 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | |
| Ar | - | 0.009 | 0.002 | 0.001 | 0.001 | 0.012 | 0.011 | 0.009 | 0.009 | 0.007 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | |
| H2O | - | 0.190 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.008 | 0.008 | 0.008 | 0.005 | |
| CO | - | 0.394 | 0.025 | 0.006 | 0.000 | 0.561 | 0.499 | 0.002 | 0.002 | 0.002 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | |
| CO2 | - | 0.107 | 0.783 | 0.985 | 0.992 | 0.002 | 0.113 | 0.244 | 0.244 | 0.407 | 0.000 | 0.002 | 0.002 | 0.002 | 0.005 | |
| H2S | - | 0.004 | 0.162 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | |
| H2 | - | 0.288 | 0.005 | 0.003 | 0.003 | 0.413 | 0.366 | 0.736 | 0.736 | 0.576 | 1.000 | 0.000 | 0.000 | 0.000 | 0.000 | |
| CH3OH | - | 0.000 | 0.016 | 0.003 | 0.004 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.990 | 0.989 | 0.990 | 0.990 | |
| Parameter | Value |
|---|---|
| Project life | 30 year |
| Tax rate | 25% |
| Interest rate | 10% |
| Stream factor | 0.95 |
| Salvage value | 10% of TCI |
| Depreciation | Straight line method |
| Coal price | 35 $/t |
| External Hydrogen price | 2.3 $/kg |
| Operating labor rate | $20/operator/h |
| Electricity cost | $0.0775/kWh |
| LP steam cost | $14.05/GJ |
| MP steam cost | $14.83/GJ |
| HP steam cost | $17.70/GJ |
| Refrigeration cost | $13.11/GJ |
| Base Case | MD-1 | MD-2 | MD-3 | Literature | |
|---|---|---|---|---|---|
| Energy efficiency (%) | 43.3 | 45.7 | 51.9 | 45.7 | 41–55 [42] |
| 51.0 [43] | |||||
| 45.5 [45] | |||||
| Carbon efficiency (%) | 36.3 | 38.2 | 77.6 | 87.6 | 37.3 [43] |
| 38.4 [44] | |||||
| 37.0 [45] | |||||
| CO2 emissions (t/t CH3OH) | 2.20 | 2.17 | 0.32 | 0.15 | 2.6 [43,46] |
| 2.3 [44] | |||||
| 5.31 [45] | |||||
| 2.97 [47] | |||||
| Methanol cost (US $/t) | 163.9 | 142.6 | 366.0 | 315.5 | 267.0 [43] |
| 290.0 [44] | |||||
| 272.6 [45] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalafalla, S.S.; Zahid, U.; Abdul Jameel, A.G.; Ahmed, U.; Alenazey, F.S.; Lee, C.-J. Conceptual Design Development of Coal-to-Methanol Process with Carbon Capture and Utilization. Energies 2020, 13, 6421. https://doi.org/10.3390/en13236421
Khalafalla SS, Zahid U, Abdul Jameel AG, Ahmed U, Alenazey FS, Lee C-J. Conceptual Design Development of Coal-to-Methanol Process with Carbon Capture and Utilization. Energies. 2020; 13(23):6421. https://doi.org/10.3390/en13236421
Chicago/Turabian StyleKhalafalla, Siddig S., Umer Zahid, Abdul Gani Abdul Jameel, Usama Ahmed, Feraih S. Alenazey, and Chul-Jin Lee. 2020. "Conceptual Design Development of Coal-to-Methanol Process with Carbon Capture and Utilization" Energies 13, no. 23: 6421. https://doi.org/10.3390/en13236421
APA StyleKhalafalla, S. S., Zahid, U., Abdul Jameel, A. G., Ahmed, U., Alenazey, F. S., & Lee, C.-J. (2020). Conceptual Design Development of Coal-to-Methanol Process with Carbon Capture and Utilization. Energies, 13(23), 6421. https://doi.org/10.3390/en13236421








