Investigation of the Physico-Chemical Properties of the Products Obtained after Mixed Organic-Inorganic Leaching of Spent Li-Ion Batteries
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Mechanical Treatment
3.2. Quantitative and Qualitative Analysis of Battery Powder before Leaching
3.3. Acidic Reductive Leaching
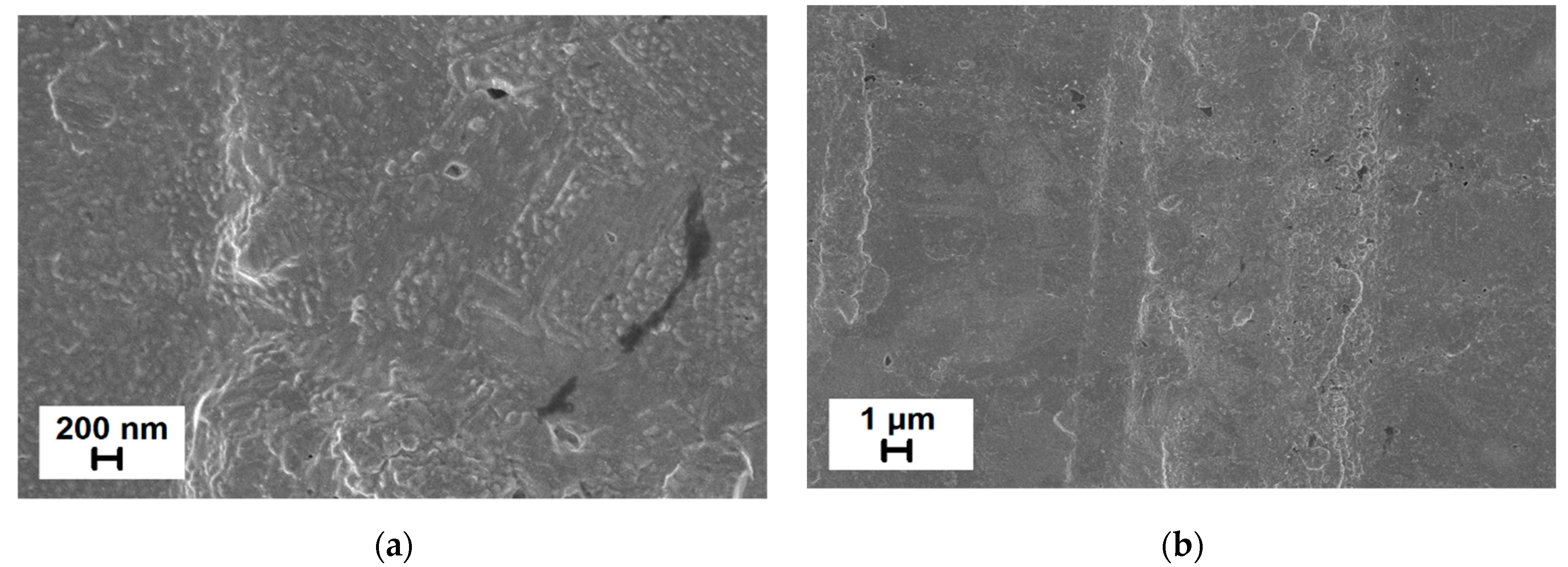
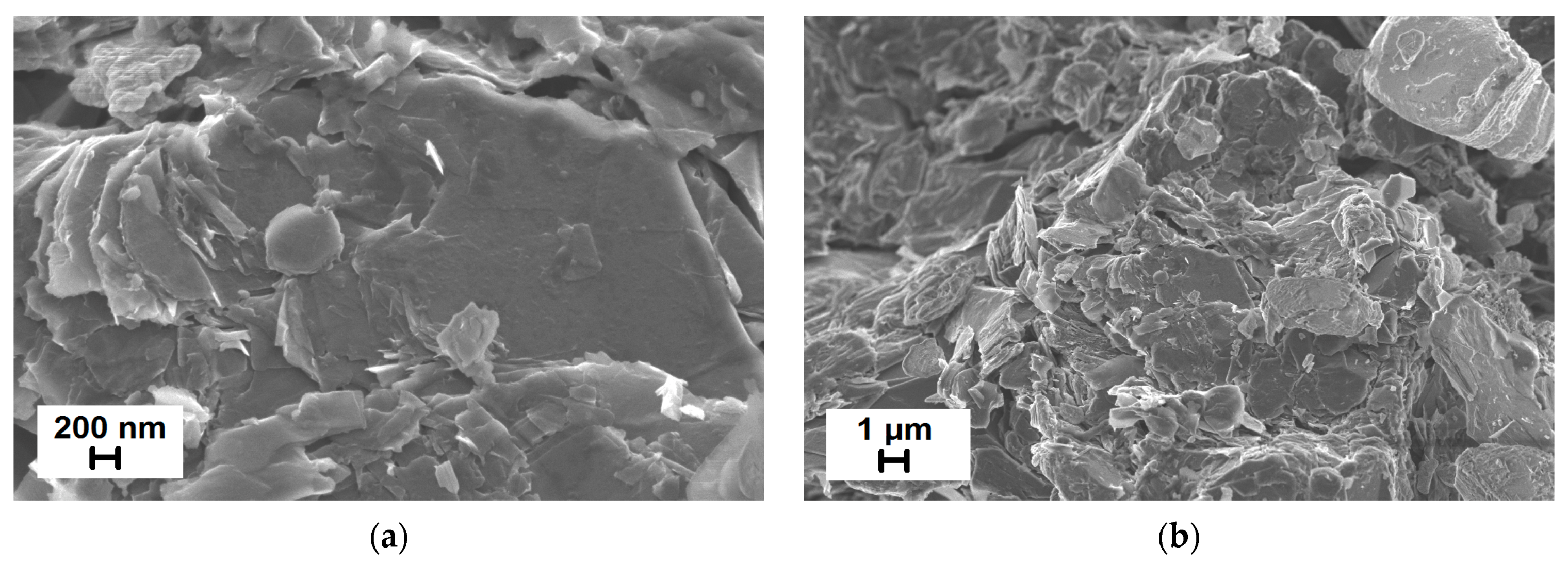
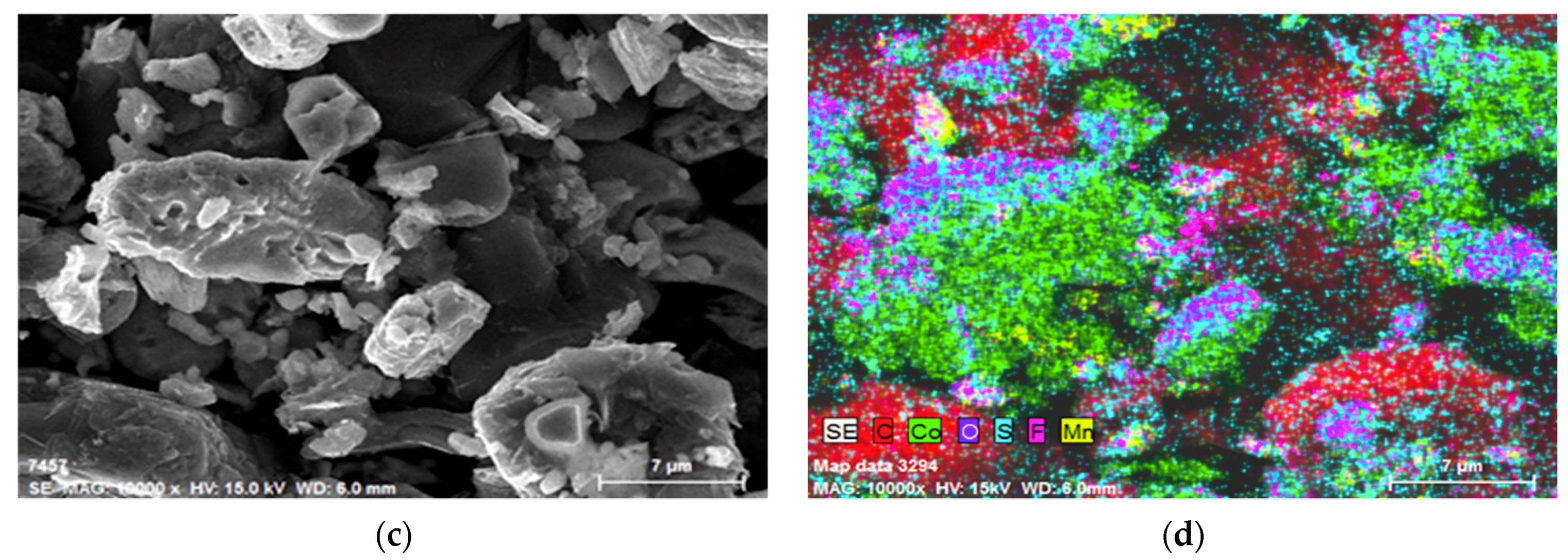
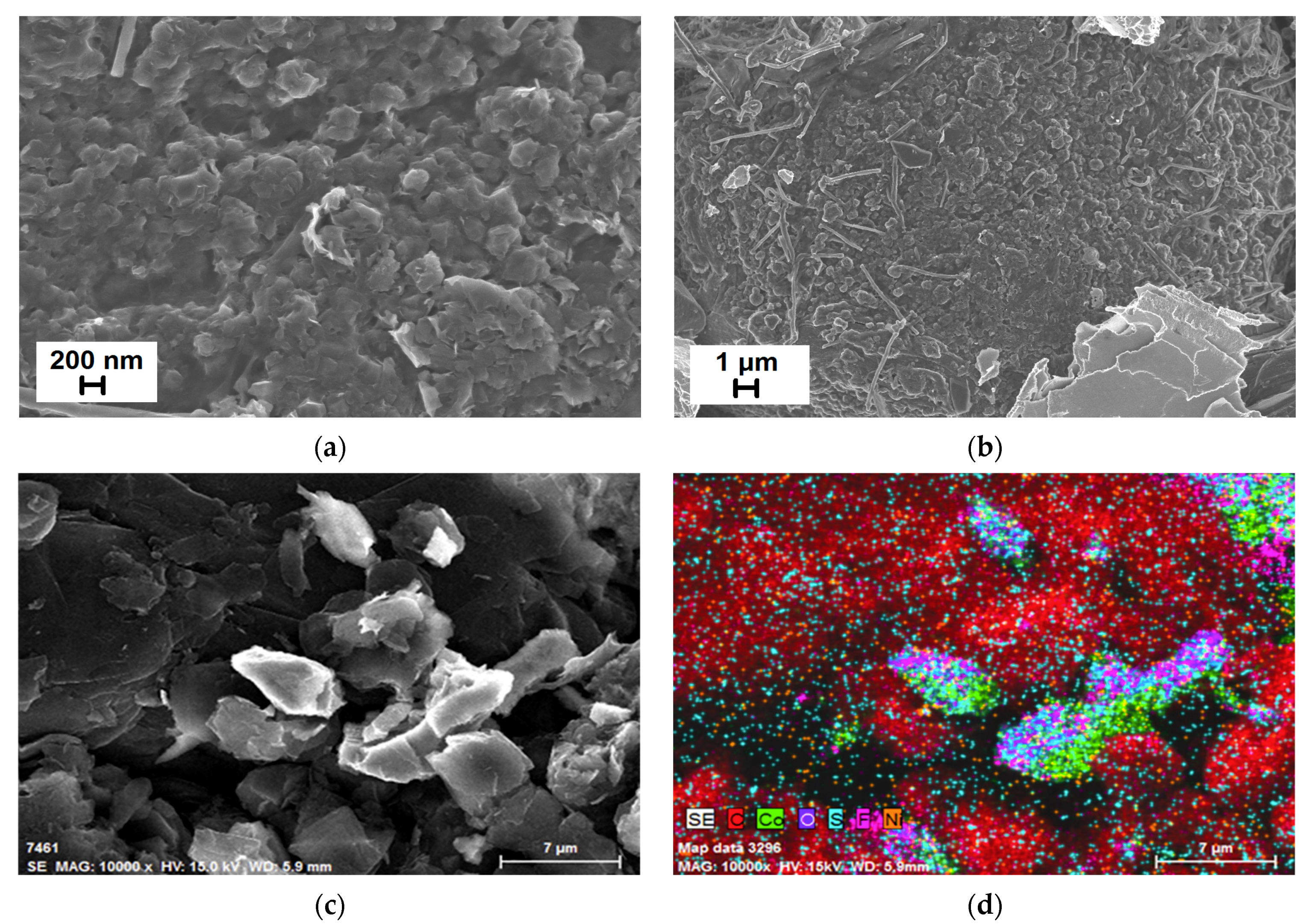
3.4. Morphological Studies
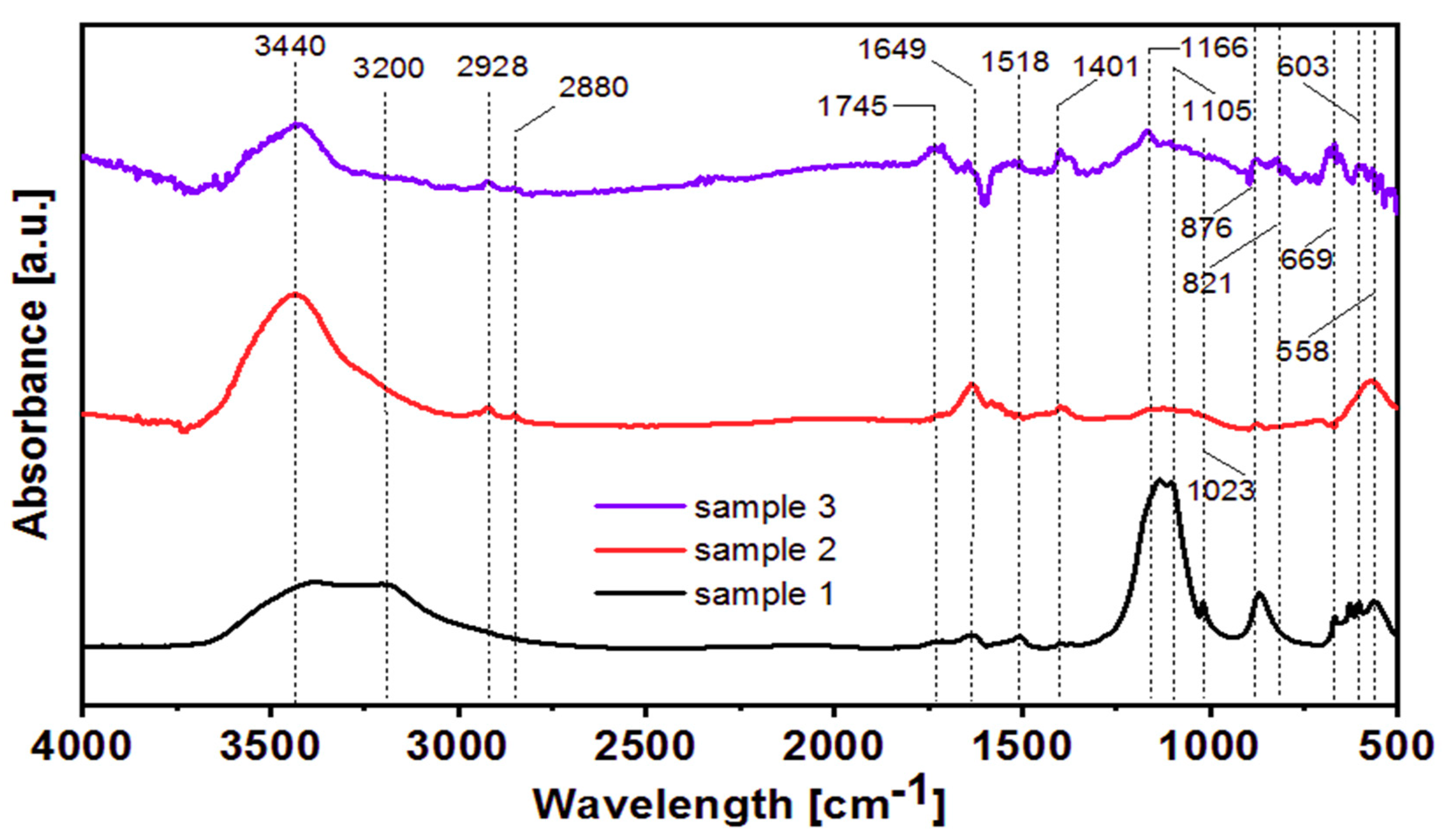
3.5. FT-IR Analysis
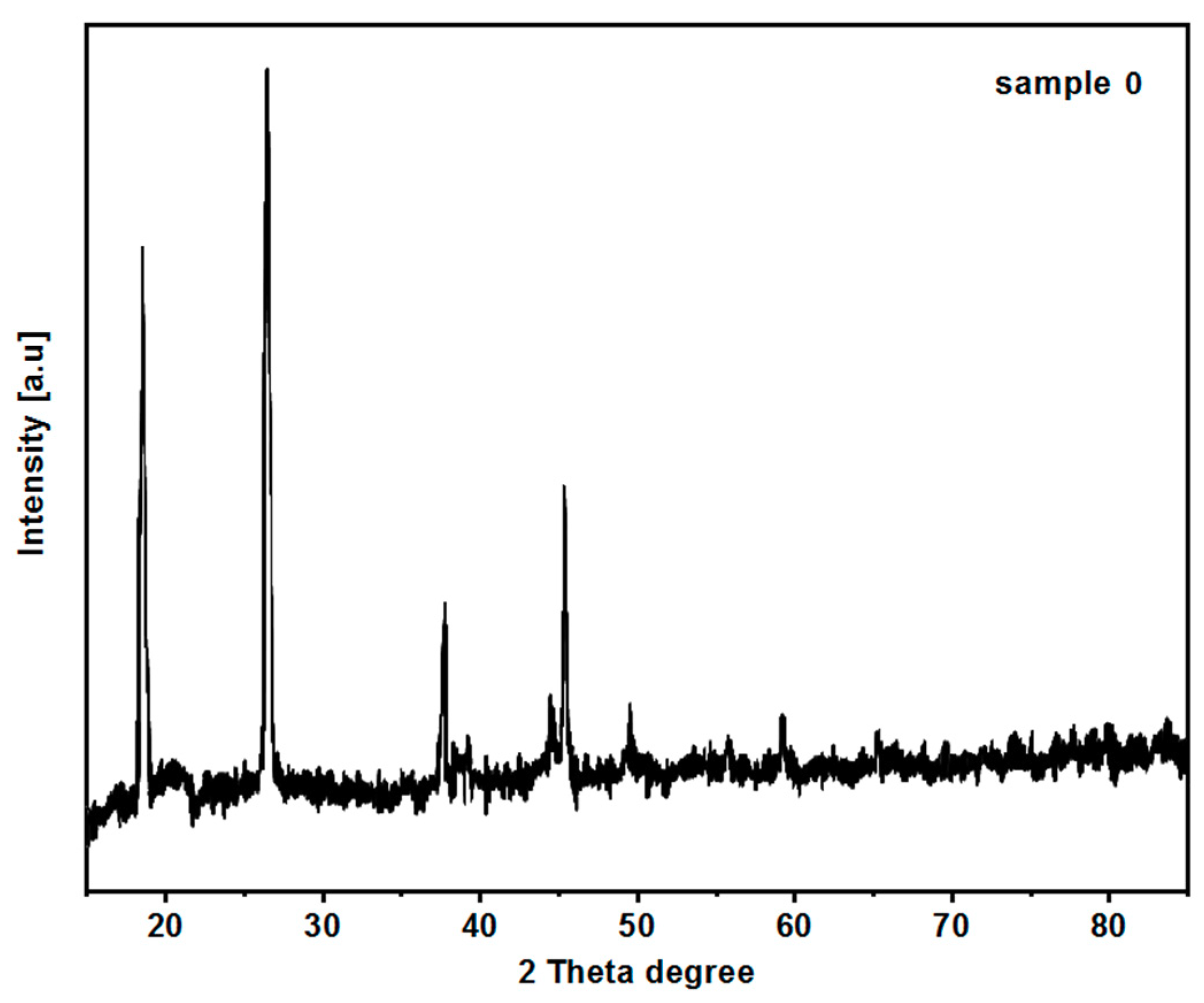
3.6. Crystallographic Structure
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Czerwiński, A. Akumulatory, Baterie, Ogniwa; WKŁ: Warszawa, Poland, 2018. (In Polish) [Google Scholar]
- Moćko, W.; Szmidt, E. Recovery Technologies of Co and Li from spent lithium-ion cells. Arch. Waste Manag. Environ. Prot. 2012, 14, 1–10. [Google Scholar]
- Scrosati, B.; Garche, J. Lithium batteries: Status, prospects and future. J. Power Sources 2010, 195, 2419–2430. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Park, K.-S. The Li-Ion Rechargeable Battery: A Perspective. J. Am. Chem. Soc. 2013, 135, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Tarascon, J.M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Sirnoval, V.; Scagliarini, V.; Murugadoss, S.; Tomatis, M.; Yakoub, Y.; Turci, F.; Hoet, P.; Lison, D.; van den Brule, S. LiCoO2 particles used in Li-ion batteries induce primary mutagenicity in lung cells via their capacity to generate hydroxyl radicals. Part. Fibre. Toxicol. 2020, 17, 6. [Google Scholar] [CrossRef] [PubMed]
- De Boeck, M.; Lison, D.; Kirsch-Volders, M. Evaluation of the in vitro direct and indirect genotoxic effects of cobalt compounds using the alkaline comet assay. Influence of interdonor and interexperimental variability. Carcinogenesis 1998, 19, 2021–2029. [Google Scholar] [CrossRef] [Green Version]
- Kang, D.H.P.; Chen, M.; Ogunseitan, O.A. Potential Environmental and Human Health Impacts of Rechargeable Lithium Batteries in Electronic Waste. Environ. Sci. Technol. 2013, 47, 10–5495. [Google Scholar] [CrossRef]
- Lee, C.K.; Rhee, K.I. Preparation of LiCoO2 from spent lithium-ion batteries. J. Power Sources 2002, 109, 17–21. [Google Scholar] [CrossRef]
- Kang, J.; Senanayake, G.; Sohn, J.; Shin, S.M. Recovery of cobalt sulfate from spent lithium ion batteries by reductive leaching and solvent extraction with Cyanex 272. Hydrometallurgy 2010, 100, 168–171. [Google Scholar] [CrossRef] [Green Version]
- Joulié, M.; Lacournet, R.; Billy, E. Hydrometallurgical process for the recovery of high value metals from spent lithium nickel cobalt aluminum oxide based lithium-ion batteries. J. Power Sources 2014, 247, 551–555. [Google Scholar] [CrossRef]
- Zhuang, L.; Sun, C.; Zhou, T.; Li, H.; Dai, A. Recovery of valuable metals from LiNi0.5Co0.2Mn0.3O2 cathode materials of spent Li-ion batteries using mild mixed acid as leachant. Waste Manag. 2019, 85, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Golmohammadzadeh, R.; Rashchi, F.; Vahidi, E. Recovery of lithium and cobalt from spent lithium-ion batteries using organic acids: Process optimization and kinetic aspects. Waste Manag. 2017, 64, 244–254. [Google Scholar] [CrossRef]
- Li, L.; Qu, W.; Zhang, X.; Lu, J.; Chen, R.; Wu, F.; Amine, K. Succinic acid-based leaching system: A sustainable process of recovery of valuable metals from spent Li-ion batteries. J. Power Sources 2015, 282, 443 544–551. [Google Scholar] [CrossRef]
- Gao, W.; Song, J.; Cao, H.; Lin, X.; Zhang, X.; Zheng, X.; Zhang, Y.; Sun, Z. Selective recovery of valuable metals from spent lithium-ion batteries—Process development and kinetics evaluation. J. Clean. Prod. 2018, 178, 833–845. [Google Scholar] [CrossRef]
- He, L.P.; Sun, S.Y.; Mu, Y.Y.; Song, X.F.; Yu, J.G. Recovery of lithium, nickel, cobalt and manganese from spent lithium-ion batteries using L-tartaric acid as leachant. ACS Sustain. Chem. Eng. 2017, 5, 714–721. [Google Scholar] [CrossRef]
- Yao, Y.; Zhu, M.; Zhao, Z.; Tong, B.; Fan, Y.; Hua, Z. Hydrometallurgical processes for recycling spent lithium-ion batteries: A critical review. ACS Sustain. Chem. Eng. 2018, 6, 13611–13627. [Google Scholar] [CrossRef]
- Meshram, P.; Abhilash; Pandey, B.D.; Mankhand, T.R.; Deveci, H. Comparison of Different Reductants in Leaching of Spent Lithium Ion Batteries. J. Met. 2016, 68, 2613–2623. [Google Scholar] [CrossRef]
- Bertuol, D.A.; Machado, C.M.; Silva, M.L.; Calgaro, C.O.; Dotto, G.L.; Tanabe, E.H. Recovery of cobalt from spent lithium-ion batteries using supercritical carbon dioxide extraction. Waste Manage. 2016, 51, 245–251. [Google Scholar] [CrossRef]
- Zheng, Y.; Long, H.L.; Zhou, L.; Wu, Z.S.; Zhou, X.; You, L.; Yang, Y.; Liu, J.W. Leaching procedure and kinetic studies of cobalt in cathode materials from spent lithium ion batteries using organic citric acid as leachant. Int. J. Environ. Res. 2016, 10, 159–168. [Google Scholar] [CrossRef]
- Fan, B.; Chen, X.; Zhou, T.; Zhang, J.; Xu, B. A sustainable process for the recovery of valuable metals from spent lithium-ion batteries. Waste Manag. Res. 2016, 34, 474–481. [Google Scholar] [CrossRef]
- Santana, I.L.; Moreira, T.F.M.; Lelis, M.F.F.; Freitas, M.B.J.G. Photocatalytic properties of Co3O4/LiCoO2 recycled from spent lithium-ion batteries using citric acid as leaching agent. Mater. Chem. Phys. 2017, 190, 38–44. [Google Scholar] [CrossRef]
- Peng, C.; Hamuyuni, J.; Wilson, B.P.; Lundstrom, M. Selective reductive leaching of cobalt and lithium from industrially crushed waste Li-ion batteries in sulfuric acid system. Waste Manag. 2018, 76, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Nayaka, G.P.; Pai, K.V.; Manjanna, J.; Keny, S.J. Use of mild organic acid reagents to recover the Co and Li from spent Li-ion batteries. Waste Manag. 2016, 51, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Nayaka, G.P.; Zhang, Y.; Dong, P.; Wang, D.; Zhiu, Z.; Duan, J.; Li, X.; Lin, Y.; Meng, Q.; Pai, K.V.; et al. An environmental friendly attempt to recycle the spent Li-ion battery cathode through organic acid leaching. J. Environ. Chem. Eng. 2019, 7, 102854. [Google Scholar] [CrossRef]
- Aaltonen, M.; Peng, C.; Wilson, B.P.; Lundstrom, M. Leaching of Metals from Spent Lithium-Ion Batteries. Recycling 2017, 2, 20. [Google Scholar] [CrossRef] [Green Version]
- Nayaka, G.P.; Manjanna, J.; Pai, K.V.; Vadavi, R.; Keny, S.J.; Tripathi, V.S. Recovery of valuable metal ions form the spent lithium-ion battery using aqueous mixture of mild organic acid as alternative to mineral acids. Hydrometallurgy 2015, 151, 73–77. [Google Scholar] [CrossRef]
- Nayaka, G.P.; Pai, K.V.; Santhosh, G.; Manjanna, J. Dissolution of cathode active material of spent Li-ion batteries using tartaric acid and ascorbic acid mixture to recover Co. Hydrometallurgy 2016, 161, 54–57. [Google Scholar] [CrossRef]
- Dorella, G.; Mansur, M.B. A study of the separation of cobalt from spent Li-ion battery residues. J. Power Sources 2007, 170, 210–215. [Google Scholar] [CrossRef]
- Nayl, A.A.; Elkhashab, R.A.; Badawy, S.M.; El-Khateeb, M.A. Acid leaching of mixed spent Li-ion batteries. Arab. J. Chem. 2017, 10, 3632–3693. [Google Scholar] [CrossRef] [Green Version]
- Mantuano, D.P.; Dorella, G.; Elias, R.C.V.; Mansur, M.B. Analysis of a hydrometallurgical route to recover base metals from spent rechargeable batteries by liquid–liquid extraction with Cyanex 272. J. Power Sources 2006, 159, 1510–1518. [Google Scholar] [CrossRef]
- Urbańska, W. Recovery of Co, Li, and Ni from Spent Li-Ion Batteries by the Inorganic and/or Organic Reducer Assisted Leaching Method. Minerals 2020, 10, 555. [Google Scholar] [CrossRef]
- Heydarian, A.; Mousavi, S.M.; Vakilchap, F.; Baniasadi, M. Application of a mixed culture of adapted acidophilic bacteria in two-step bioleaching of spent lithium-ion laptop batteries. J. Power Sources 2018, 378, 19–30. [Google Scholar] [CrossRef]
- Meshram, P.; Pandey, B.D.; Mankhand, T.R. Recovery of valuable metals from cathodic active material of spent lithium ion batteries: Leaching and kinetic aspects. Waste Manag. 2015, 45, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Lv, W.; Wang, Z.; Cao, H.; Zheng, X.; Jin, W.; Zhang, Y.; Sun, Z. A sustainable process for metal recycling from spent lithium-ion batteries using amonnium chloride. Waste Manag. 2018, 79, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Guo, C.; Ma, H.; Li, J.; Zhou, T.; Cao, L.; Kang, D. Organic reductants based leaching: A sustainable process for the recovery of valuable metals from spent lithium ion batteries. Waste Manag. 2018, 75, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Pinto, P.S.; Lanza, G.D.; Ardisson, J.D.; Lago, R.M. Controlled Dehydration of Fe(OH)3 to Fe2O3: Developing Mesopores with Complexing Iron Species for the Adsorption of β-Lactam Antibiotics. J. Braz. Chem. Soc. 2019, 30, 310–317. [Google Scholar] [CrossRef]
- Xia, X.; Tu, J.; Zhang, Y.; Mai, Y.; Wang, X.; Gu, C.; Zhao, X. Freestanding Co3O4 nanowire array for high performance supercapacitors. RSC Adv. 2012, 2, 1835–1841. [Google Scholar] [CrossRef]
- Allaedini, G.; Muhammad, A. Study of influential factors in synthesis and characterization of cobalt oxide nanoparticles. J. Nanostructure Chem. 2013, 3, 77. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; He, Y.; Yang, Y.; Xie, W.; Ling, X. Research on quantifying the hydrophilicity of leached coals by ftir spectroscopy. Physicochem. Probls. Miner. Process. 2017, 53, 227–239. [Google Scholar] [CrossRef]
- Tannenbaum, R.; Zubris, M.; David, K.; Ciprari, D.; Jacob, K.; Jasiuk, I.; Dan, N. FTIR Characterization of the Reactive Interface of Cobalt Oxide Nanoparticles Embedded in Polymeric Matrices. J. Phys. Chem. B 2006, 110, 2227–2232. [Google Scholar] [CrossRef]
- Noriega, S.; Subramanian, A. Consequences of Neutralization on the Proliferation and Cytoskeletal Organization of Chondrocytes on Chitosan-Based Matrices. Int. J. Carbohydr. Chem. 2011, 809743. [Google Scholar] [CrossRef]
- Uznanski, P.; Zakrzewska, J.; Favier, F.; Kazmierski, S.; Bryszewska, E. Synthesis and characterization of silver nanoparticlesfrom (bis)alkylamine silver carboxylate precursors. J. Nanopart. Res. 2017, 19, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Funda, S.; Ohki, T.; Liu, Q.; Hossain, J.; Ishimaru, Y.; Ueno, K.; Shirai, H. Correlation between the fine structure of spin-coated PEDOT:PSS and the photovoltaic performance of organic/crystalline-silicon heterojunction solar cells. J. Appl. Phys. 2016, 120, 033103. [Google Scholar] [CrossRef]
- Fila, D.; Hubicki, Z.; Kołodyńska, D. Recovery of metals from waste nickel-metal hydride batteries using multifunctional Diphonix resin. Adsorption 2019, 25, 367–382. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Wang, Y.; Xie, X.; Huang, C.; Yang, S. Dehydration of fructose, sucrose and inulin to 5-hydroxymethylfurfural over yeast-derived carbonaceous microspheres at low temperatures. RSC Adv. 2019, 9, 9041–9048. [Google Scholar] [CrossRef] [Green Version]
- Bounaas, M.; Bouguettoucha, A.; Chebli, D.; Reffas, A.; Harizi, I.; Rouabah, F.; Amrane, A. High efficiency of methylene blue removal using a novel low-cost acid treated forest wastes, Cupressus semperirens cones: Experimental results and modeling. Particul. Sci. Technol. 2019, 37, 504–513. [Google Scholar] [CrossRef]
- Zhang, F.; Yuan, C.; Lu, X.; Zhang, L.; Zhang, X.; Che, Q. Facile growth of mesoporous Co3O4 nanowire arrays on Ni foam for high performance electrochemical capacitors. J. Power Sources 2012, 203, 250–256. [Google Scholar] [CrossRef]
- Freitas, B.G.A.; Siqueira, J.M., Jr.; da Costa, L.M.; Ferreira, G.B.; Resende, J.A.L.C. Synthesis and Characterization of LiCoO2 from Different Precursors by Sol-Gel Method. J. Braz. Chem. Soc. 2017, 28, 2254–2266. [Google Scholar] [CrossRef]
- Lakshmanan, R.; Gangulibabu; Bhuvaneswari, D.; Kalaiselvi, N. Temperature Dependent Surface Morphology and Lithium Diffusion Kinetics of LiCoO2 Cathode. Met. Mater. Int. 2012, 18, 249–255. [Google Scholar] [CrossRef]
- Xie, J.; Cao, H.; Jiang, H.; Chen, Y.; Shi, W.; Zheng, H.; Huang, Y. Co3O4-reduced graphene oxide nanocomposite as an effective peroxidase mimetic and its application in visual biosensing of glucose. Anal. Chim. Acta 2013, 796, 92–100. [Google Scholar] [CrossRef]
- Qi, C.; Koenig, G.M. High-Performance LiCoO2 Sub-Micrometer Materials from Scalable Microparticle Template Processing. ChemistrySelect 2016, 1, 3992–3999. [Google Scholar] [CrossRef]
- Santiago, E.I.; Andrade, A.V.C.; Paiva-Santos, C.O.; Bulhoes, L.O.S. Structural and electrochemical properties of LiCoO2 prepared by combustion synthesis. Solid State Ion. 2003, 158, 91–102. [Google Scholar] [CrossRef]
- Zeng, X.; Li, J.; Shen, B. Novel approach to recover cobalt and lithium from spent lithium-ion battery using oxalic acid. J. Hazard. Mater. 2015, 295, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Jha, A.K.; Jha, M.K.; Kumari, A.; Sahu, S.K.; Kumar, V.; Pandey, B.D. Selective separation and recovery of cobalt from leach liquor of discarded Li-ion batteries using thiophosphinic extractant. Sep. Purif. Technol. 2013, 104, 160–166. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, K.; Shi, Z.; Zhang, S. LiF as an Artificial SEI Layer to Enhance the High-Temperature Cycle Performance of Li4Ti5O12. Langmuir 2017, 33, 11164–11169. [Google Scholar] [CrossRef]
- Babkair, S.S.; Azam, A. Color Centers Formation in Lithium Fluoride Nanocubes Doped with Different Elements. J. Nanomater. 2013, 872074, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Yu, X.Q.; Sun, J.P.; Tang, K.; Li, H.; Huang, X.J.; Dupont, L.; Maier, J. Reversible lithium storage in LiF/Ti nanocomposites. Phys. Chem. Chem. Phys. 2009, 11, 9497–9503. [Google Scholar] [CrossRef]
- Ungár, T.; Gubicza, J.; Ribárik, G.; Pantea, C.; Zerda, T.W. Microstructure of carbon blacks determined by X-ray diffraction profile analysis. Carbon 2002, 40, 929–937. [Google Scholar] [CrossRef] [Green Version]
- Tao, S.; Zhan, Z.; Wang, P.; Meng, G. Chemical stability study of Li2SO4 on the operation condition of a H2/O2 fuel cell. Solid State Ion. 1999, 116, 29–33. [Google Scholar] [CrossRef]
- Kohl, M.; Bruckner, J.; Bauer, I.; Althues, H.; Kaskel, S. Synthesis of highly electrochemically active Li2S nanoparticles for Lithium-sulfur-Batteries. J. Mater. Chem. A 2015, 3, 16307–16312. [Google Scholar] [CrossRef] [Green Version]
- Pan, A.; Wang, Y.; Xu, W.; Nie, Z.; Liang, S.; Nie, Z.; Wang, C.; Cao, G.; Zhang, J. High-performance anode based on porous Co3O4 nanodiscs. J. Power Sources 2014, 255, 125–129. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, J.; Zeng, S.; Zhou, L.; Xu, W.; Zheng, D.; Liu, J.; Zeng, Y.; Lu, X. An ultrathin defect-rich Co3O4 nanosheet cathode for high-energy and durable aqueous zinc ion batteries. J. Mater. Chem. A 2019, 7, 21678–21683. [Google Scholar] [CrossRef]
- Rahman, M.M.; Wang, J.Z.; Deng, X.L.; Li, Y.; Liu, H.K. Hydrothermal synthesis of nanostructured Co3O4 materials under pulsed magnetic field and with an aging technique, and their electrochemical performance as anode for lithium-ion battery. Electrochim. Acta 2009, 55, 504–510. [Google Scholar] [CrossRef]
- Zhou, H.; Lv, B.; Wu, D.; Xu, Y. Synthesis of the polycrystalline Co3O4 nanowires with excellent ammonium perchrolate catalytic decomposition property. Mater. Res. Bull. 2014, 60, 492–497. [Google Scholar] [CrossRef]



| Sample | Volume of 1.5 M H2SO4 | Concentration of Reducing Agent | Time (h) | Slurry Density (w/v) | Temp. (°C) | Rotation Rate (rpm) |
|---|---|---|---|---|---|---|
| Sample 1 | 100 mL | 0.9% H2O2 (v/v) | 2 | 10/100 | 90 | 500 |
| Sample 2 | 100 mL | 5% C5H8O4 (w/v) | 2 | 10/100 | 90 | 500 |
| Sample 3 | 100 mL | 0.9% H2O2 (v/v) + 5% C5H8O4 (w/v) | 2 | 10/100 | 90 | 500 |
| Sample 4 | 100 mL | - | 2 | 10/100 | 90 | 500 |
| Metal | Al | Ca | Co | Cr | Cu | Fe | Li | Mn | Ni | Si | Zn |
|---|---|---|---|---|---|---|---|---|---|---|---|
| C (g/kg) | 0.93 | 0.40 | 256.0 | 0.005 | 3.59 | 0.27 | 33.20 | 0.560 | 14.40 | 3.76 | 0.07 |
| Metal | Al | Ca | Co | Cr | Cu | Fe | Li | Mn | Ni | Si | Zn | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Recovery, % | Sample 1 | 71.61 | 48.73 | 1.95 | 67.49 | 83.53 | 87.35 | 84.19 | 56.42 | 80.25 | 71.68 | 87.58 |
| Sample 2 | 10.64 | 24.58 | 32.30 | 66.25 | 82.12 | 99.45 | 75.86 | 31.22 | 38.77 | 43.30 | 47.66 | |
| Sample 3 | 53.66 | 35.98 | 59.37 | 84.60 | 97.36 | 100.00 | 79.81 | 62.39 | 81.75 | 70.25 | 93.93 | |
| Sample 4 | 49.76 | 34.09 | 30.94 | 47.67 | 79.96 | 93.44 | 72.32 | 31.10 | 37.61 | 58.52 | 50.55 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urbańska, W.; Osial, M. Investigation of the Physico-Chemical Properties of the Products Obtained after Mixed Organic-Inorganic Leaching of Spent Li-Ion Batteries. Energies 2020, 13, 6732. https://doi.org/10.3390/en13246732
Urbańska W, Osial M. Investigation of the Physico-Chemical Properties of the Products Obtained after Mixed Organic-Inorganic Leaching of Spent Li-Ion Batteries. Energies. 2020; 13(24):6732. https://doi.org/10.3390/en13246732
Chicago/Turabian StyleUrbańska, Weronika, and Magdalena Osial. 2020. "Investigation of the Physico-Chemical Properties of the Products Obtained after Mixed Organic-Inorganic Leaching of Spent Li-Ion Batteries" Energies 13, no. 24: 6732. https://doi.org/10.3390/en13246732






