1. Introduction
The demand of electric vehicles (EV) has increased, hence lithium batteries are used enormously, and in this situation enormous heat is also generated from the battery [
1]. Batteries are very sensitive to frequent temperature changes and it affects battery life, hence it is important to design effective battery thermal management systems to enhance heat dissipation. Until now, many researchers developed various battery thermal management systems (BTMS) to meet the requirements such as high power, fast charging rates, and improved driving performance. These rapid changes of the batteries had to be monitored carefully and managed to avoid safety and thermal related issues. From the existing literature the BTMS had been broadly divided into two categories, they are active and passive cooling. The active cooling system is the forced circulation of air and water cooling systems [
2]. Even air cooling can meet the requirement of vehicles during normal operating conditions, but battery temperature will be high with high charging and discharging conditions. The modified battery design such as such as changing the battery position, adding the guide plate, and entrance angle modification, but still this air cooling does not meet the requirements of electric vehicles [
3,
4]. The liquid or water cooling is better than the air cooling but includes complicated setup for circulation and that will increase the complexity and weight of the vehicle [
5,
6,
7].
The PCM (phase change materials) cooling system is the best substitute for the above traditional thermal management methods [
8,
9,
10]. The pump or blower is not required in this PCM cooling system, hence, it is cost effective and simple in design and construction. This PCMs cooling method is known as passive thermal management system. The excess heat generated by the battery of the electric vehicle can be absorbed by the PCMs close to the battery module. If the temperature of the battery reaches the PCM’s melting temperature, then heat will be stored in the form of latent heat and the temperature increase will be minimized.
PCMs undergo the phase change phenomenon by absorbing or releasing the abundant heat from the battery. This passive PCM cooling technology maintain the operating temperature of batteries at a relatively constant temperature range. In this way PCMs are the best solution for an effective thermal management system of an electric vehicle battery by maintaining a uniform temperature distribution even in any weather conditions. Zaho et al. [
11] summarized different methods of thermal management system out of all those methods, concluding that PCMs are very effective technology for battery thermal management systems. Karimi et al. [
12] experimentally studied on a cylindrical lithium ion battery thermal management system using composite PCMs and obtained the solution that metal matrix-PCM composites decreases the maximum ΔT between the battery surface and composite PCM up to 70%. Azizi and Sadrameli [
13] proposed a thermal management system for a LiFePO
4 battery pack with composite PCM and aluminum wire mesh plates and found the maximum temperature of the battery surface had been reduced by 19%, 21%, and 26% at 1C, 2C, and 3C discharge rates. 1C means the battery will discharge completely in 1 h, similarly, 2C means the battery will discharge completely in 2 h and vice-versa. Organic PCMs are widely used due to significant advantages such as high latent heat, high melting point range, effective thermal performance, adaptable molding during phase change, low corrosion, nontoxic, inflammable, and low cost. Moreover, low thermal conductivity restricts the use of PCM based thermal management systems in required applications [
14].
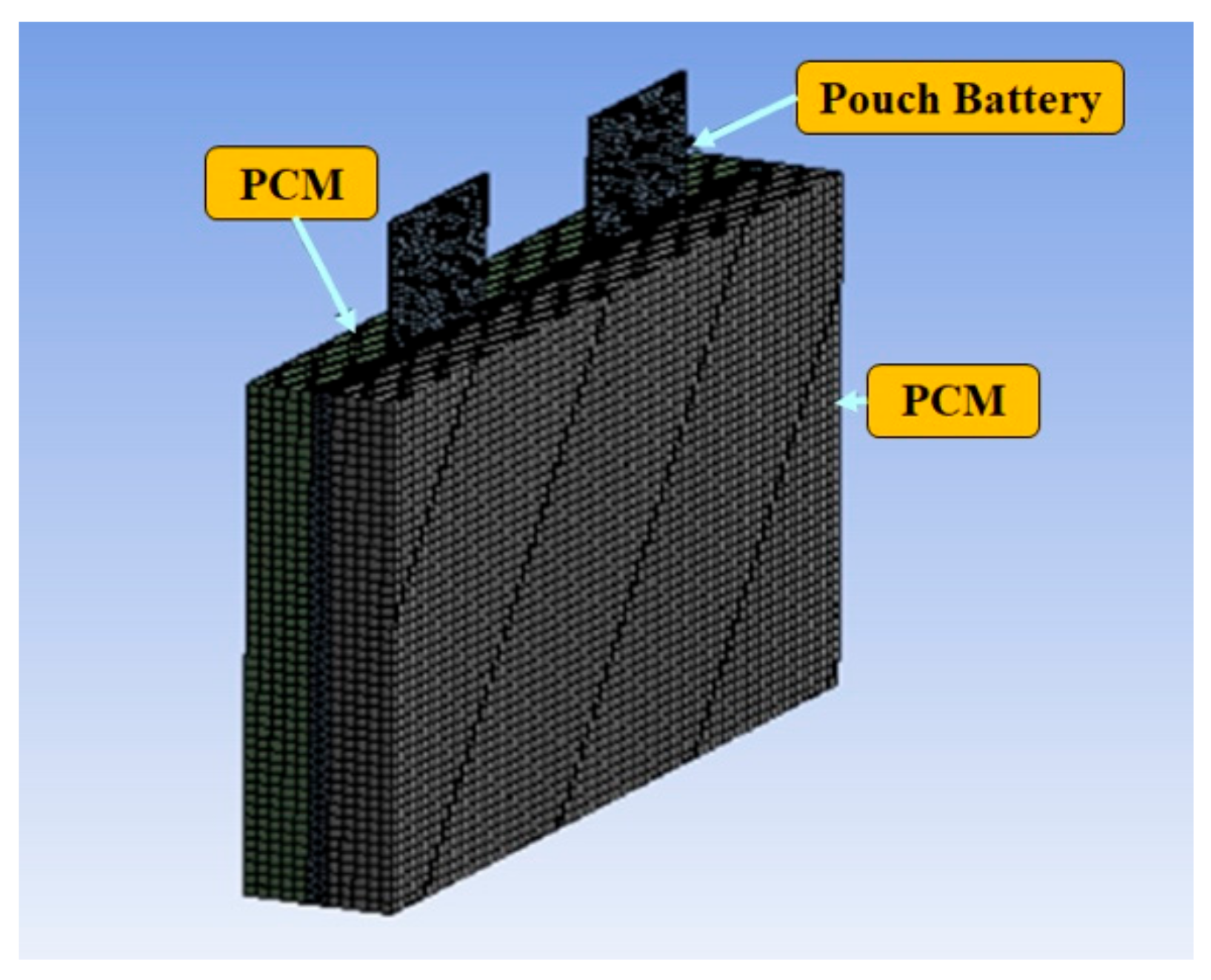
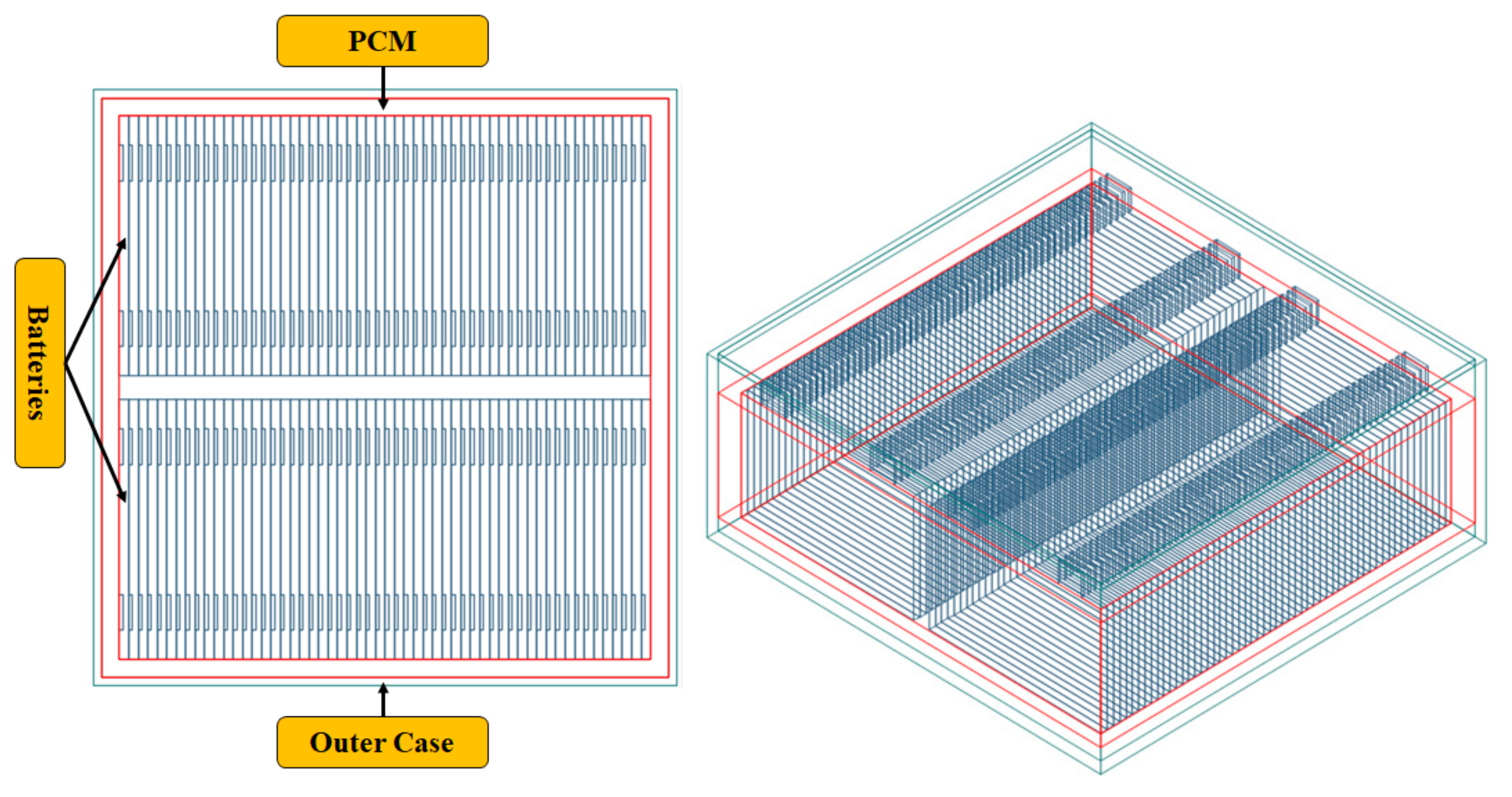
In the present paper, design and simulation of 6 kW battery pack was carried out based on the previous experimental and simulation results on a single lithium polymer pouch battery. The experiment and simulation showed that expanded graphite phase change material is the best PCM due to enhanced thermal properties to control the battery temperature within the safe operating temperatures. Important parameters that were considered to support expanded graphite PCM as the best PCM are due to its thermal conductivity, heat energy storage capacity, liquid leakage, and mass of PCM that effects the heat transfer rate. Liquid leakage was also tested in this experiment. Hence, from the experiment, we observed that during the energy storage process, no leakage was observed in the expanded graphite PCM and leakage was observed in the non-expanded graphite materials like RT15, RT31. The mass of PCM required to enhance heat transfer rate was also considered in this experiment.
From previous literature study, expanded graphite materials with high thermal conductivities and melting points were selected and show enhancement of heat transfer in the thermal management system of lithium batteries of electric vehicle. The key parameters for selection of a proper PCM for passive BTMS, depend not only thermal conductivity but also melting point of PCM is very crucial. At the same time, high melting points over the operating range are also not preferred because beyond melting point PCM acts as an insulating material which is not useful. The important parameters considered while selecting the PCMs are listed below [
15]:
High thermal conductivity and high specific heat.
Melting point in the desired operating range.
Less or no sub cooling during freezing.
Volume changes are negligible during solidifying or during melting.
Non-poisonous, non-flammable, non-explosive, and should be stable.
Low cost and should be available in large quantities.
The RT44HC/EG PCM in [
16], has a high thermal conductivity of 7.85 W/m K, the battery temperature reached 46.3 °C without PCM, but with PCM the battery temperature was controlled up to 36.3 °C, and hence the maximum temperature difference obtained was ΔT is 7.0 °C. From the result in [
17], with PCMs of thermal conductivity range from 1 to 3 W/m K, there is good decrease in the temperature observed as ΔT is 6.7 °C, but if thermal conductivities increased from 7 to 15 W/m K, they observed no significant changes in temperature drop, only temperature change of 0.31–0.66 °C is seen.
Hence in this research we selected expanded graphite materials with low melting points between the range of 5–26 °C for better results. This expanded graphite PCMs we selected were firstly used for BTMS. The specific objectives are shown below:
Design and propose the 6-kW Li polymer pouch battery with a PCM thermal management system based on single battery experimental results.
Maintain the battery temperature in the safe operating condition.
Simulate the proposed model of the battery module with different PCMS.
With the simulation result, realize the experiment setup for cold and hot soaking and run the experiment with proposed PCMs at different temperatures of the battery.
Compare results and identify the best PCM that satisfies the design specifications and maintains the battery under same operating temperature limits.
Apply the best PCM on the proposed 6-kW battery pack.
5. Results and Discussion
From analysis result, we conducted experiments with the proposed PCMs on the single pouch battery. The initial battery temperature is 26.1 °C before starting the discharge, the initial voltage of the battery is 14 V and for 1C, 1/2C and 1/4 battery discharge rates. During this condition the battery temperature was raised without PCM up to 35.9 °C, whereas with PCMs the temperatures were controlled to 32.6, 31.3, 30.6, and 30.7 °C for RT16, RT31, EG26, and EG6, respectively. From this experimental result, we observed expanded graphite PCMs are controlling the temperature below 30.5 °C when compared to other PCMs. Thermal performance of the proposed PCMs during experiment and simulation on single lithium polymer pouch battery is given in
Table 4.
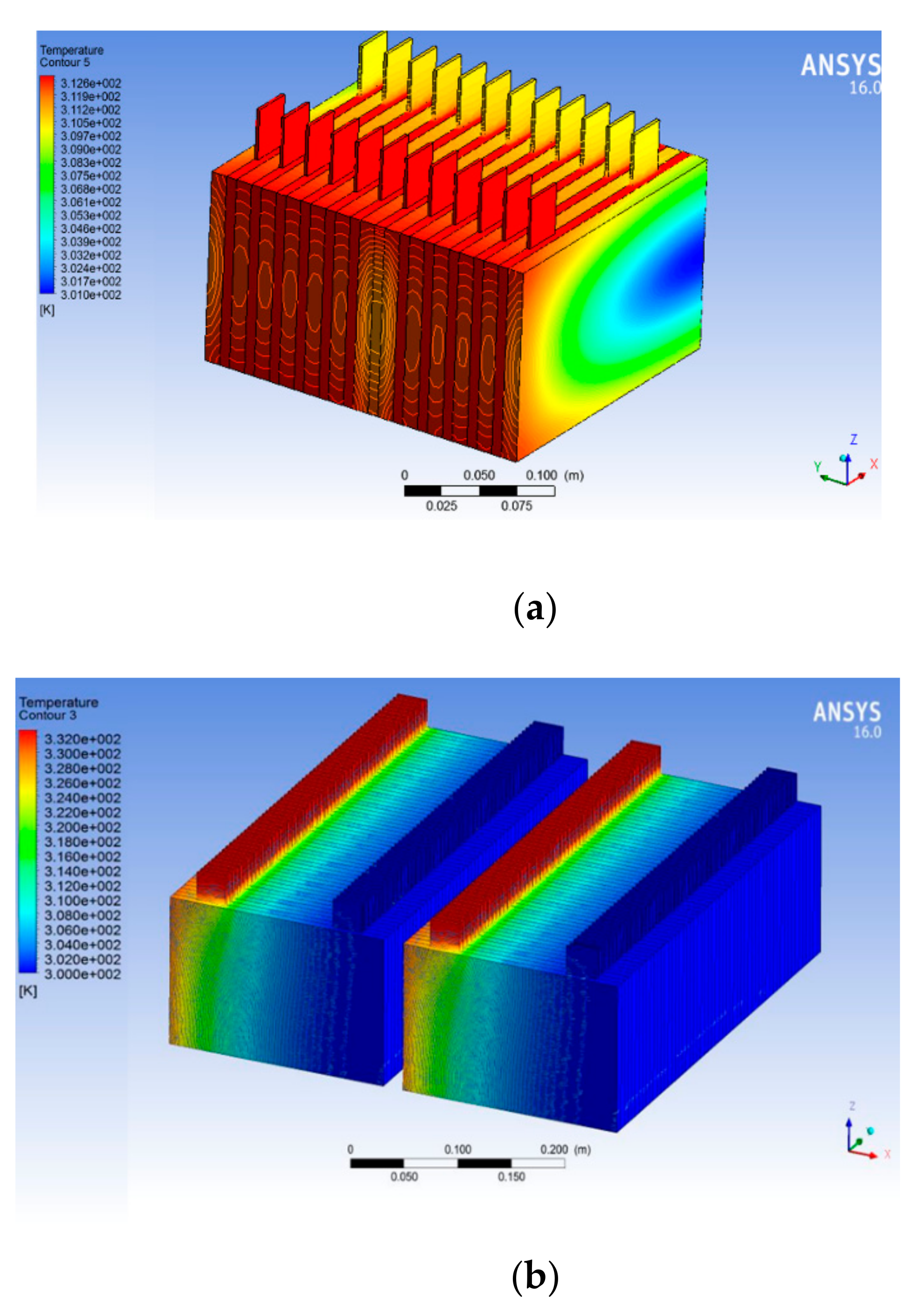
The analysis results show battery temperature with expanded graphite PCM shows less temperature contours, whereas without PCM around the battery yields a greater temperature than with PCM. In future we will analyze the thermal management system of 6 kW with hybrid cooling of PCM with air cooling and water cooling.
The mass of PCM required to store thermal energy is given as in Equation (3), and
Table 5 gives the heat transfer rate with PCM mass of 1000 gm. In addition, from the obtained result it is observed that maximum heat transfer rate is observed with expanded graphite PCM and least heat transfer rate is with the battery without PCM. To improve heat transfer rate in other batteries such as 1, 2, and 5 the quantity of PCM required also increased 4.2, 2.5, and 8.8 times when compared with expanded graphite PCMs. The maximum temperatures in the 6-kW battery module with selected PCMs is shown in
Table 6, from the analysis result battery maximum temperature during discharge without PCMs reached 36.5 °C, whereas with PCMs temperature can be maintained at 30.0 °C, meaning that ΔTmax is observed as 6.5 °C.