1. Introduction
CO
2 capture and storage (CCS) is among the key low-carbon technologies required to reduce power plant-related CO
2 emissions in an economically sustainable way [
1]. To accelerate the pace of deployment, several R&D institutions, industrial technology providers and operators are currently pursuing R&D programs and activities aimed at reducing the CO
2 avoidance cost, while demonstrating and improving the commercial maturity of various CCS technologies [
2,
3].
One of the most established programs in this field is the CO
2 Capture Project (CCP) [
4,
5], a partnership of major energy companies, which has now entered the fourth phase, CCP4. Within the framework of CCP4, the post-combustion capture of CO
2 from natural-gas-fired combined cycles (NGCCs) is a key R&D area. This paper presents the outcome of a preliminary study aimed at assessing the potential techno-economic advantages of novel post-combustion technologies, and benchmarking their performance against conventional technology, namely post-combustion capture by monoethanolamine (MEA). In addition to this, to give a broader perspective to the analysis, a comparison against an advanced solvent technology, aqueous piperazine (PZ), which could be considered as a prospective benchmark, is also made.
Even though CO
2 capture technologies based on amine solvents have been recently demonstrated in full or relevant-scale power plants [
6], their high CO
2 avoidance cost still remains a significant limitation to the largescale adoption of CO
2 capture and storage (CCS) in the field of power generation [
7]. This has spurred the development of different innovative technologies with the objective of improving performance and/or reducing costs [
8]. Examples of such technologies are supersonic, flow-driven anti-sublimation [
9], membranes [
10], capture from pressurized combustion [
11] and molten carbonate fuel cells (MCFCs) [
12,
13].
Balepin and Castrogiovanni [
14] and Castrogiovanni [
9] proposed a process aimed at exploiting the supersonic acceleration and expansion of flue gas to separate the CO
2 as a solid phase. This concept is not completely new in the field of gas purification, since a similar system has been recently designed and installed for natural gas conditioning [
15]. Nevertheless, its application as a post-combustion CO
2 capture technique is new, and entails different design and operating conditions. According to the patent of Balepin et al. [
16], flue gas can be derived either from natural gas or coal combustion, and CO
2 is captured via anti-sublimation, followed by inertial separation, both resulting from the supersonic expansion of the gas mixture. The core equipment unit of the whole process is named as the “Inertial CO
2 Extraction System”, and consists of a De Laval nozzle followed by a diffuser [
9]. The system is currently being tested and developed, at the bench scale, by Orbital ATK and ACENT Laboratories. Dehydrated flue gas, previously pressurized, enters the convergent tube section (subsonic nozzle) of the CO
2 extraction system, and passes through a throat designed to achieve sonic conditions. This is followed by a divergent tube section (supersonic nozzle), where the expansion occurs, and supersonic velocities and very low temperatures and pressures are achieved. As a result, the CO
2 freezes into solid particles, which are collected towards the internal wall of the tube, as a result of the combination of swirl (i.e., the non-negligible tangential velocity component provided at the tube entrance through vanes) and higher density of the solid phase. Then a cyclone removes the collected particles, such that the remaining gas stream is decarbonized. At the end of this section, another convergent tube (supersonic diffuser) followed by a sonic throat and a divergent tube (subsonic diffuser) discharge the CO
2-depleted flue gas at nearly atmospheric pressure. In both the diffusers, deceleration provides pressure recovery and temperature increase. According to the developers [
9], the main advantages of the technology are: (i) the very reduced footprint compared to an amine plant of similar capacity (with potentially less than half of the area required for the installation of the whole system); (ii) the attractive energy penalties and cost of CO
2 avoided, estimated to be near to 50
$/t
CO2 avoided (t
CO2 stands for metric ton of CO
2 as in the rest of the paper) for the application of the technology to a coal-fired power plant.
Another promising technology for the post-combustion capture of CO
2 from NGCCs is selective membranes. Merkel et al. [
10] proposed the use of polymeric CO
2-permeable membranes, by introducing the concept of “selective flue gas recirculation”. The flue gas discharged from a combined cycle is cooled down to near ambient temperature, removing most of its water content, then compressed to a moderate pressure (1.1–3 bar) and fed to a first membrane separator for CO
2 capture. With a cross-flow configuration, this device produces a stream of highly concentrated CO
2, based on the selectivity of the membrane. However, the membrane does not completely remove the CO
2, so a significant amount remains in the flue gas, which is fed to a second membrane separator. This second stage utilizes the turbine combustion air as a sweep gas to achieve removal of almost all of the remaining CO
2.
After the second separation stage, the CO2-depleted flue gas, optionally expanded in a turbine, is discharged into the atmosphere. The stream of CO2-enriched air, with a CO2 content in the range 8.8–16.2% mol, is used as an oxidant in the gas turbine. The benefits of the selective flue gas recirculation are: (i) the concentration of CO2 in the flue gas is increased, which reduces the energy penalty for its capture; (ii) the excessive dilution of the air stream at the inlet of the gas turbine that occurs with conventional flue gas recirculation is avoided, since the use of a CO2-selective membrane minimizes the recycle of other species than CO2. In particular, (ii) is the crucial aspect of this approach, which makes possible recirculating a high flow rate of CO2, increasing the CO2 in the flue gas from 4% to 15–20% on a molar basis, while keeping the concentration of O2 in the CO2-enriched air, high enough to be suitable for the normal, albeit off design, operation of the gas turbine, without entailing any significant modification in its design.
Christensen et al. [
11] recently proposed a method to remove the CO
2 from NGCC flue gas while it is still at high pressure. In the proposed scheme, a multi-shaft gas turbine, namely the GE LMS100 model [
17], is split into a gas generator providing a pressurized stream of flue gas at high pressure and temperature (8 bar and 800 °C), and a final expansion stage. Normally, the hot, high pressure combustion gases are directly expanded to generate power. Instead, the hot stream is cooled by generating steam in a heat recovery steam generator (HRSG) for a recuperative steam cycle, which produces a significant portion of the electrical power of the overall plant. In an alternative configuration [
11], a pressurized natural gas-fired boiler heats up the hot gases, ahead of the heat recovery process, while increasing the CO
2 concentration up to 9% mol. In either case, a pressurized solvent-based capture unit decarbonizes the flue gas, which is then heated via waste heat recovery, and finally expanded to atmospheric pressure to generate additional electric power. The capture unit is a hot potassium carbonate solvent-based Benfield absorption scheme designed and licensed by Honeywell UOP [
18], but other solutions favored by the high CO
2 partial pressure may be envisaged. The developers report that the main advantage of this scheme are: (i) the high CO
2 partial pressure at the inlet of the CO
2 capture section, which should reduce the energy penalty due to capture; (ii) the more compact size of the HRSG and of the CO
2 capture section due to higher pressure; (iii) the maturity of the technology, which involves only conventional units, i.e., the LMS100 gas turbine, the well-known Benfield process and a Heat Recovery Steam Cycle. Compared to other solutions, this technology is readily implementable as a short-term CCS option, with limited investment required for research costs.
Molten Carbonate Fuel Cells (MCFCs) are direct fuel conversion systems, which can be efficiently exploited as active CO
2 separators and electricity generators. The concept of using MCFCs as CO
2 concentrators relies on their capability to transfer CO
2 as an oxygen carrier, in the form of carbonate ions (CO
32−), from the cathode to the anode side, where CO
2 is concentrated as a result of both this mass transfer and fuel oxidation. In more detail, at the cathode, O
2 and CO
2 molecules form carbonate ions (½O
2 + CO
2 + 2e
− = CO
32−) as a result of a catalytic reaction promoted by the nickel oxide (NiO) cathode [
19]. These ions, produced on the porous surface of the cathode, are then transferred through a selective membrane, where the electrolyte (a liquid potassium/lithium carbonate electrolyte retained in an LiAlO
2 ceramic matrix) conducts the ions to the anode. At the anode, CO
32− ions react with hydrogen, producing water and carbon dioxide (CO
32− + H
2 = CO
2 + H
2O + 2e
−). For each CO
32− ion transferred through the fuel cell membrane, two electrons flow externally in the opposite direction, generating a current flow. As a consequence of these electrochemical phenomena, a certain amount of CO
2 is removed from the cathode stream of the MCFC (the removal efficiency depends upon the CO
2 utilization factor, which is in turn related to the MCFC area and operating conditions), and is then concentrated in the gaseous effluent exiting the anode side. The anode effluent contains water and unconverted CO/H
2 syngas (produced by CH
4 reforming within the cell) in addition to CO
2. The CO
2 can easily be removed from the CO/H
2, which is recycled for fuel use, in a Gas Processing Unit (GPU). One of the most interesting advantages of MCFCs is that their operating temperature, close to 650 °C, closely matches with the exhaust conditions of heavy-duty gas turbines (GTs), making their process integration easier.
Moreover, nickel surfaces within the fuel cell catalyze the internal fuel reforming of the natural gas (NG) feed. The use of MCFCs as CO
2 capture devices has been widely studied for applications in coal power plants, IGCCs, NGCCs and other industrial applications [
12], showing the potential for high capture rates with limited energy penalty, especially in the NGCC case. The MCFC can be positioned between the gas turbine (GT) and the heat recovery steam generator (HRSG) of the modified NGCC, and the bottoming steam cycle can increase its output compared to the reference NGCC by exploiting not only the waste heat from the GT flue gases, but also the one from the MCFC anodic stream [
12]. In this configuration, the MCFC could capture up to 80%–90% of the total CO
2 released by the fuel combustion, and could increase the electric output of the power plant by around 25% [
20]. Various configurations of MCFCs combined with NGCCs for CO
2 capture purposes have been recently studied by the authors [
21], where the impact of major operating conditions, of the utilization factor and issues such as the MCFC ability, to be used in retrofit applications, are considered.
Currently, the main issues with the use of MCFCs are reliability (tests are still ongoing to prove 10 years’ operation for 1.4 MW
el stacks), their limited availability (latest installations [
22] are targeting 8000 equivalent hours per year with 95% availability), and costs which are difficult to predict, because there is not yet a mature market (Ahmed et al. [
23] report around 2000
$/kW
el expected for a 5 MW
el annual production of MCFCs, projected to reduce to 1250
$/kW
el if the annual production demand increases to 50 MW
el).
Preliminary estimates released by FCE [
24], the largest MCFC manufacturer, and focused on CCS from coal, report a cost of CO
2 avoided lower than 40
$/t
CO2 avoided.
The study reported in this paper focuses on CO
2 capture from flue gas produced from natural gas sources, is funded by the CCP and carried out by a group of LEAP (Laboratorio Energia e Ambiente Piacenza) and GECOS (Group of Energy Conversion Systems)—Politecnico di Milano researchers, together with the CCP team. This work originates from the preliminary study reported by Forsyth et al. in 2017 [
25], and focuses on the preliminary assessment of the following four promising technologies for post-combustion CO
2 capture from NGCCs:
CO2 permeable membranes (MEMs)
Molten Carbonate Fuel Cells (MCFCs)
Pressurized CO2 absorption integrated with multi-shaft gas turbine and Heat Recovery Steam Cycle (HPS)
Supersonic, flow-driven CO2 anti-sublimation and inertial separation (SSD)
Compared to the introductory work by Forsyth et al. [
25], this paper provides the following novel contributions:
the assessed technologies are described in more detail, and their potential and limitations have been highlighted;
a new configuration has been considered for the MCFC, coherently with the findings of Spinelli et al. [
21];
the whole techno-economic analysis is more comprehensive (with multiple configurations and cases reported for each technology), including a fully detailed performance assessment (based on the SPECCA index) and costing methodology.
Figure 1 depicts the CO
2 capture concept at the basis of each of the novel technologies evaluated.
The paper is structured as follows. First, the technical and economic framework for the comparative analysis is presented in
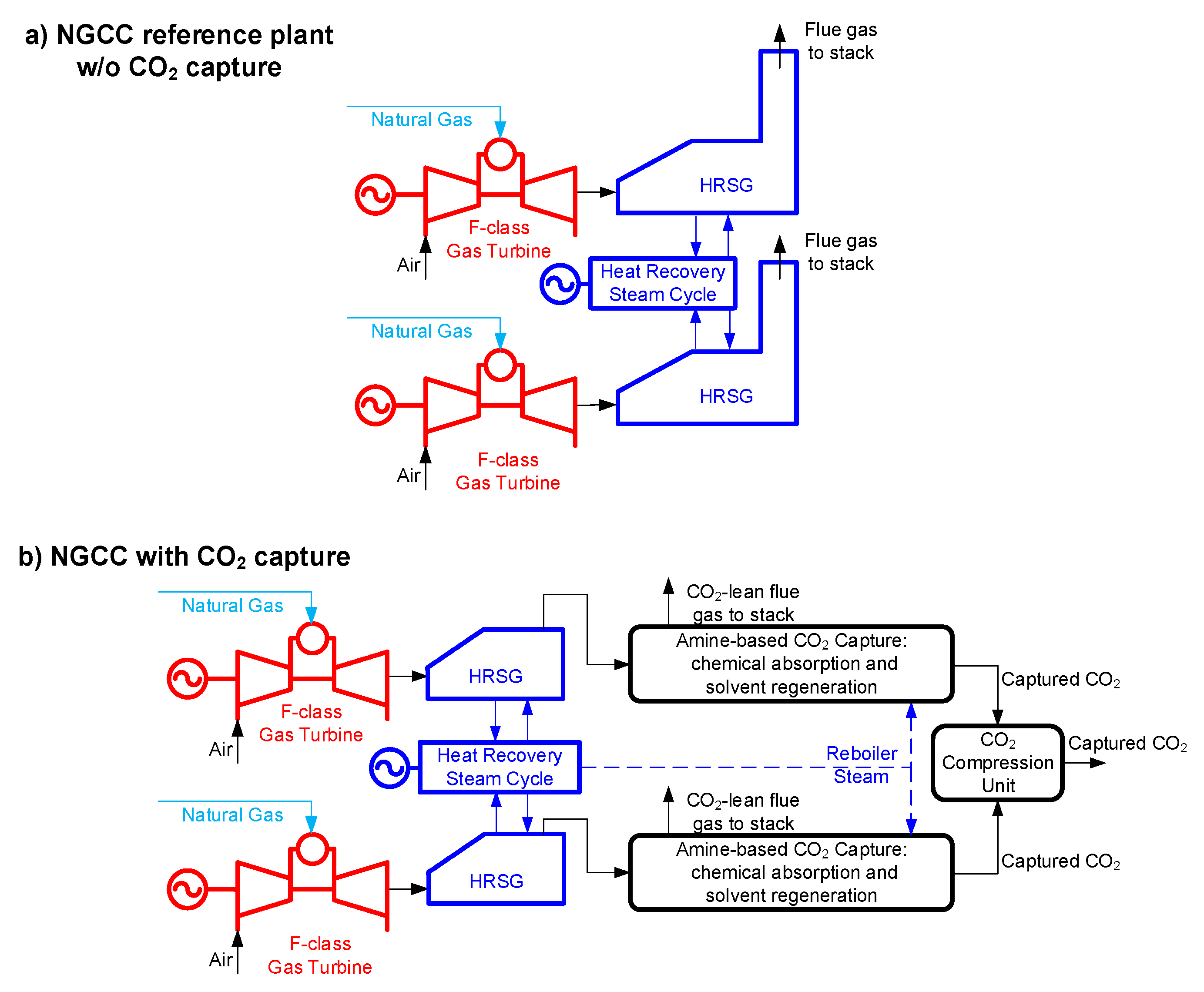
Section 2, which also reports a description, performance and costs figures for the reference NGCC without and with CCS.
Section 3,
Section 4,
Section 5 and
Section 6 describe in detail the process flow diagram and modeling approach followed to evaluate each of the four novel technologies.
Section 7 and
Section 8 outline and compare the performance and costs of all the schemes evaluated. Finally, in
Section 9, conclusions are drawn.
3. CO2 Permeable Membranes
Polymeric membrane technology has been widely employed for CO
2 separation from natural gas or biogas. Typical conditions for the application of these membranes are pressurized natural gas or biogas streams containing more than 10% CO
2 on a molar basis [
44]. By contrast, post-combustion CO
2 capture from NGCCs flue gases is more challenging compared to natural gas purification or biogas upgrading, since it involves a more CO
2-diluted stream, starting from close-to-atmospheric pressure.
The scheme proposed by Merkel et al. [
10] and shown in
Figure 3 has been assessed. In this configuration, the NGCC (reported as gas turbine and HRSC in
Figure 3) is the same as from the base case (i.e., two F-class GTs and one steam turbine), while the membranes act as a post-combustion CO
2 separation technology. CO
2-permeable membranes are used in the CO
2 capture process to pursue three goals: to carry out a selective recycle of CO
2-enriched air to the gas turbine intake; to separate a CO
2-concentrated stream from the CO
2-enriched flue gases discharged by the HRSG; and as a final CO
2 recovery step from the off-gas stream released by the CO
2 purification section. In the case considered in this paper, the pressure conditions proposed by Merkel et al. [
10] are employed for the CO
2 capture membrane: the pressure ratio is set to 10, with a feed pressure of 3 bar and a permeate pressure of 0.3 bar (i.e., sub-atmospheric) controlled by a vacuum pump.
The following process schemes have been studied and evaluated:
MEM-0: a scheme very similar to the original one published by Merkel et al. with only one minor difference: the retentate stream coming from the CO
2 purification membrane is sent downstream of the main flue gas compressor (and not upstream of the water knock-out), as shown in
Figure 3. This saves some compression power. The membrane area has been adjusted to reach a high CO
2 removal rate, i.e., 94% CCL.
MEM-1: same configuration as MEM-0 but with a smaller membrane area, adjusted to reach 90% CCL.
MEM-2: same configuration as MEM-0 scheme, but featuring lower pressure levels for both the feed (1.7 bar) and the backpressure of the capture membrane (0.2 bar). This design explores the performance and economics trade-offs between membrane feed pressure and membrane area. The membrane area has been adjusted to reach 94% CCL.
MEM-3: same as option MEM-2, but featuring a smaller membrane area, reduced so as to reach a CCL equal to 90%.
Hence, one of the goals of this analysis is to understand whether there are economic benefits (i.e., lower CO
2 avoidance costs) from increasing the CCL from the target value of 90% reported by [
10].
As a consequence of the selective recirculation of CO
2 to the GT compressor inlet, the EBTF gas turbine is set to operate in off-design via the regulation of compressor VIGV, to adapt the machine to the variation in air mass flow-rate compared to the design conditions. Hence, the GT performance change was considered and modeled in off-design operation with the GS code [
28]. The HRSG and steam cycle configuration/design, ambient temperature, cooler exit temperatures, condensing temperature of the steam cycle and the refrigerator, were changed to align the assumptions with the EBTF study. The HRSG and steam cycle are specifically redesigned for the resulting gas composition, temperature and mass flow rate. The CO
2 purification unit is based on a multistage, intercooled compressor which brings the CO
2 extracted by the vacuum pump to 27 bar, followed by a dryer and a low-temperature phase separator working at −30 °C; the phase-change temperature is reached via an external refrigerator; the liquid CO
2 produced by the separator (stream CO
2 in
Figure 3) is pumped at 110 bar for subsequent delivery, while the gas phase (stream F in
Figure 3) is recycled to the CO
2 purification membrane.
4. Molten Carbonate Fuel Cells
The MCFC power island has been integrated with the baseline NGCC, as depicted in
Figure 4. Fuel Cell Energy, a leading technology provider of MCFC technology, provided guidance on the MCFC equipment, performance, operating conditions, and costs assumed in this paper. More details can be found in [
21]. As shown in
Figure 4, the anode of the MCFC is fed with a mixture of natural gas, recycled unconverted fuel and steam, which undergoes an internal reforming process. Natural gas and the unconverted fuel are first pre-heated up to 300 °C, sent to the zinc-oxide unit for the removal of sulfur components [
45], and then heated to the inlet temperature of the MCFC-anode (450 °C) via direct mixing with superheated steam (565 °C) bled from the LP superheater of the steam cycle. In the proposed configuration, the MCFC is placed between the gas turbine and the heat recovery steam generator (HRSG), and separates the CO
2 from the high-temperature GT exhausts that are directly fed to the cathode side of the fuel cell at a temperature of 608 °C.
This technical choice minimizes the energy penalty associated with the CO2 capture section, because the cathode reactants (GT exhausts) do not need to be pre-heated up to the MCFC’s working temperature, and the waste heat released by the fuel cell is recovered for steam production in the HRSG. The GT model and operating conditions are exactly the same as the base case, while the steam cycle and the HRSG are newly-designed to manage the larger amount of thermal power to be recovered downstream of the MCFC compared to the NGCC baseline.
Therefore, this MCFC–NGCC integrated scheme should exploit synergies between the MCFC and NGCC integration, and it is expected to feature improved thermodynamic performance relative to either of the stand-alone MCFC and NGCC power plants with post-combustion capture. In this CCS arrangement, the anode exhaust, a CO
2-rich flow containing the unconverted H
2/CO stream, first generates steam in the HRSG, and afterwards is processed in the Gas Processing Unit (GPU) section, where CO
2 is separated from the fuel stream via the cryogenic, auto-refrigerated process described by Spinelli et al. [
21]. The unconverted fuel, separated as a vapor phase by the GPU, contains nearly all of the H
2 and CO released at the anode, some CH
4, and also a residual amount of CO
2, and is subsequently recycled back to the fuel cell anode. In this way, all the fuel introduced in the fuel cell is eventually converted into electricity. As a result, the overall fuel utilization factor approaches 100%, even though the single-pass utilization factor across the MCFC anode is 80%. The anode-side heat recovery section has been designed with the aim of preventing metal dusting, which may damage carbon steel surfaces, affecting the durability of the heat recovery section [
46,
47]. To avoid this metal dusting, the mixture of CO
2 and unconverted fuel leaving the MCFC anode is cooled down to a temperature of 125 °C by raising IP steam at a temperature of 254 °C (@35 bar). The high heat transfer coefficient of this quench boiler ensures a wall temperature lower than 350 °C, which is generally recognized as a safety limit to prevent metal dusting.
To reduce the MCFC active area and the related investment cost, the fuel cell is here assumed to operate with a current density of 1500 A/m
2. The cell is simulated with the GS software, and its voltage and performance are calculated by means of an ad hoc correlation developed on the basis of experimental results at FCE laboratories, where a fuel cell test rig (single cell with an active layer of 250 cm
2) has been tested over a wide range of current densities (from 800 up to 1600 A/m
2) using NGCC flue gas and other combustion effluents (CO
2 concentration ranging from 4% to 20% on a molar basis) as cathode reactants [
48].
This correlation computes the Nernst voltage as a function of the average partial pressures of the anode/cathode reactants and of their average temperature. In addition, voltage losses are also evaluated as a function of the composition of the reactants, temperature and current density, which strongly influences the ohmic resistance of the fuel cell. The anode is fed with a mixture of natural gas and recovered syngas, to which is added sufficient steam to achieve the
S/
C ratio equal to 2. As stated above, the amount of fuel converted in a single pass of the MCFC (U
F) is set equal to 80%, while the CO
2 utilization factor is limited in order to leave a CO
2 molar concentration equal to 1% at the cathode outlet to limit concentration overpotential losses inside the MCFC. Long-term, experimental tests have proven the MCFC capability of reaching such a low CO
2 concentration at the cathode outlet, as reported in the work by Ghezel-Ayagh et al. [
49] in 2017. During these trials, the cathode of a short MCFC stack has been fed with coal combustion effluents (13.5%
mol CO
2 at the cathode inlet), and the CO
2 utilization has been kept constantly higher than 90% (hence corresponding to a CO
2 concentration at the cathode outlet close to 1% on a molar basis). Furthermore, the flux of CO
2 separated has been varied significantly from 100 up to 180 Ncm
3/m
2/s, where the upper value corresponds to current densities close to 1600 A/m
2. According to [
49], during the long-term experiment, no additional voltage losses (i.e., the polarization curve remains fairly constant over time) or significant material degradation were observed; however, during this test campaign, the short stack was operated with a low current density (~1000 A/m
2) for most of the runtime (~8000 h of the total 16,000 h). Hence, further long-term, experimental activities should be dedicated to demonstrating the durability of MCFC materials, as well as the stable operation without significant performance degradation, when both high current densities and high CO
2 utilizations are selected as design parameters. A sensitivity on the impact of the CO
2 utilization factor on performance and costs has been carried out by Spinelli et al. [
21].
In the present work, given that the MCFC cathode processes the CO2 from gas turbine flue gases (@3.98% CO2, 12.39% O2), the obtained CO2 utilization is 75.9%. The oxygen utilization (UO2 = 12.1%) is a direct consequence of the assumption on CO2 utilization, because each mole of CO2 transferred from the fuel cell cathode to the anode involves the permeation of 0.5 mol of oxygen, due to the formation of the CO32− ion. In this operating condition, the resulting MCFC voltage is 0.7 V, and this determines the waste heat released by the electrochemical process, which is partially exploited to drive the internal natural gas reforming. Because of this energy balance, the anode and cathode streams leave the fuel cell at a temperature of 652 °C, and are both used for steam production in the HRSC.
6. Supersonic Flow Driven CO2 Anti-Sublimation and Inertial Separation (SSD)
The block flow diagram of the Supersonic flow-driven CO
2 anti-sublimation process evaluated in this work is depicted in
Figure 6. The scheme resembles those proposed in several studies [
16,
51,
52,
53,
54]. The power island is the reference NGCC, which discharges the flue gas directly into the capture island. Here, flue gas is dehydrated through (i) bulk water condensation and removal in a cooler (operating at 28 °C) connected to a knock-out drum, followed by, (ii) deep water removal in a molecular sieve drier to achieve the target moisture content of 10 ppmv. Then, a train of inter-cooled compression stages bring the flue gas to the operational pressure required at the inlet of the supersonic extraction system, i.e., between 2 and 5 bar according to Balepin et al. [
16]. A chiller may be optionally included to the purpose of precooling the gases while achieving CO
2 separation with a lower compression ratio compared to the case without cooling. Finally, the “Inertial CO
2 Extraction System” carries out the CO
2 separation and makes available CO
2 ready for compression, transport and storage, as well as decarbonized flue gas at conditions suitable for the stack.
The performances of this plant (NGCC + SSD) have been estimated as follows. The NGCC section is identical to the reference case, and therefore produces 1330.6 kg/s of flue gas at 1.01 bar, 87 °C, containing 3.96% of CO
2 on a molar basis (corresponding to 81.63 kg/s of CO
2). The conventional equipment units of the capture section (namely, gas cooler, water knock-out drum and intercooled compressor) have been modeled in detail with Aspen Plus
®, while the power consumption of the unconventional units (the molecular sieve drier and the auxiliary system for flue gas refrigeration) have been estimated through the assumptions reported in
Table 4.
It is important to note that accurate modeling of the supersonic CO
2 separator is extremely challenging for two main reasons: (i) the technology is still under development at the bench scale (TRL-4 according to the developers) [
9], and its geometry, set up and balance of plant are likely to be modified in the future; (ii) within the device, a large number of complex physical phenomena occur, namely phase-transition in a multicomponent mixture with particles nucleation and growth, thermodynamic non-equilibrium effects, shock-waves, marked three-dimensionality of velocity and temperature profiles.
Several approaches have been proposed in the literature for the performance assessment of supersonic separators, and they can be classified as follows:
- (a)
Detailed modeling and simulation of the system with a CFD and/or multi-physics approach [
54,
55,
56];
- (b)
High-level analysis of the system, assuming experimentally-measured performance as an input of the overall technology evaluation (see [
51,
57]).
Given the existing uncertainty on the configuration of the system, as well as the lack of relevant experimental data required for CFD model calibration, in this work a high-level analysis approach has been adopted. We have assumed the correlation between achievable CO
2 capture level and inlet conditions (pressure and temperature) of the nozzle found by Hammer et al. [
51]. However, the capture limits and operating conditions adopted in this paper were estimated by Hammer et al. (2014) [
51] from process simulations based on a simplified model (assuming homogeneous phase-equilibrium), and should be taken as theoretical calculations, since they have not been confirmed with experimental investigation, yet. For instance, a report from Balepin et al. [
58] discloses the most interesting experimental results, derived from a 3-years R&D project, involving several bench-scale test campaigns and process modeling activities aimed at investigating the potential of a “supersonic post-combustion Inertial CO
2 Extraction System”. They tested flue gas with CO
2 concentrations ranging from 2.1% to 9.9% on a mass basis, but without identifying a clear correlation between CO
2 concentrations, inlet pressures, temperatures at the system inlet and achievable CO
2 removal levels, the latter being quite variable during tests (half of the tests featured CCL > 50%, and only one third of the tests reported CCL > 80%).
Should relevant experimental data become available in the future, the accuracy of the performance analysis for this technology may be substantially improved.
Six different operating conditions have been considered by varying the inlet nozzle pressure and temperature within the theoretical ranges identified by Hammer et al. [
51] and Sipocz et al. [
57]. The matrix of the cases is reported in
Table 8, together with the main performance assumptions. However, according to Castrogiovanni [
9], the supersonic separator should include internal cold recovery (i.e., the solid CO
2 is pressurized and then liquefied by supplying the thermal power removed from flue gas to be refrigerated) and a so-called “posimetric compressor”.
Due to the lack of data, no cold recovery has been considered, and it is assumed that the CO
2 solid particles are adiabatically compressed from 0.1 to 10 bar as solids, then liquefied and adiabatically pumped to 110 bar in the liquid phase. This results in an overall electricity consumption for CO
2 pumping of 13.2 kJ/kg of CO
2 captured. The overall parasitic load for CO
2 capture includes the following contributions: flue gas compressor power; electric consumption for heat removal from pre-, inter- and after-coolers (equal to 2% of the thermal load); electric consumption of the chiller (where required); electric consumption of the aforementioned solid and liquid pumps; electric consumption of drier auxiliaries, assumed equal to 4250 kW
el/(t
water removed/h) [
1].