Improving the Efficiency of PEM Electrolyzers through Membrane-Specific Pressure Optimization
Abstract
:1. Introduction
2. Model
2.1. Electrolysis Cell and System
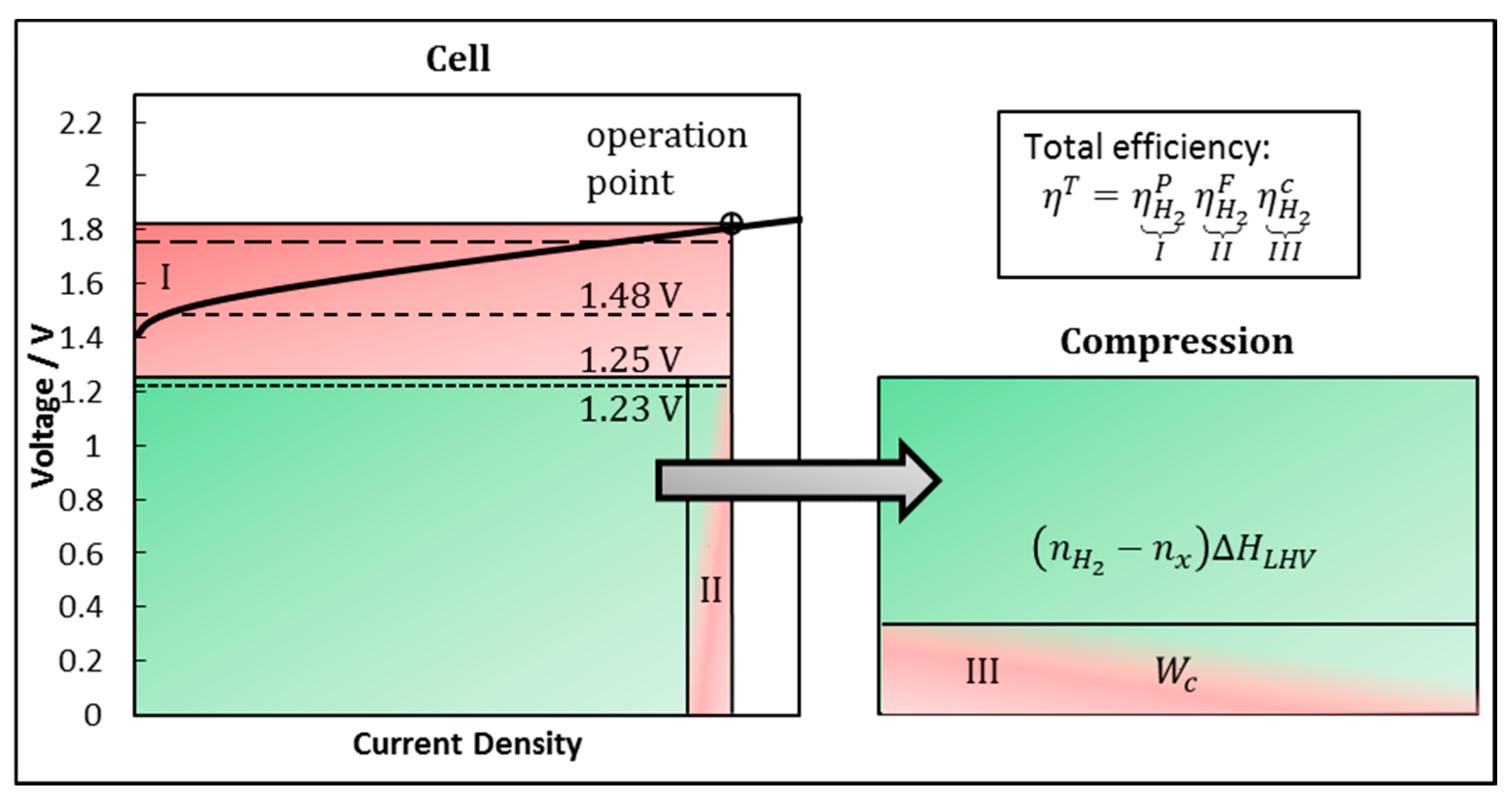
2.2. Fundamentals of System Efficiency
2.3. Hydrogen Production Efficiency
2.4. Faraday Efficiency
2.5. Compression Effort
2.6. Parameter Values
2.6.1. Parameter Values from Literature
2.6.2. Parameter Values Evaluated
3. Results and Discussion
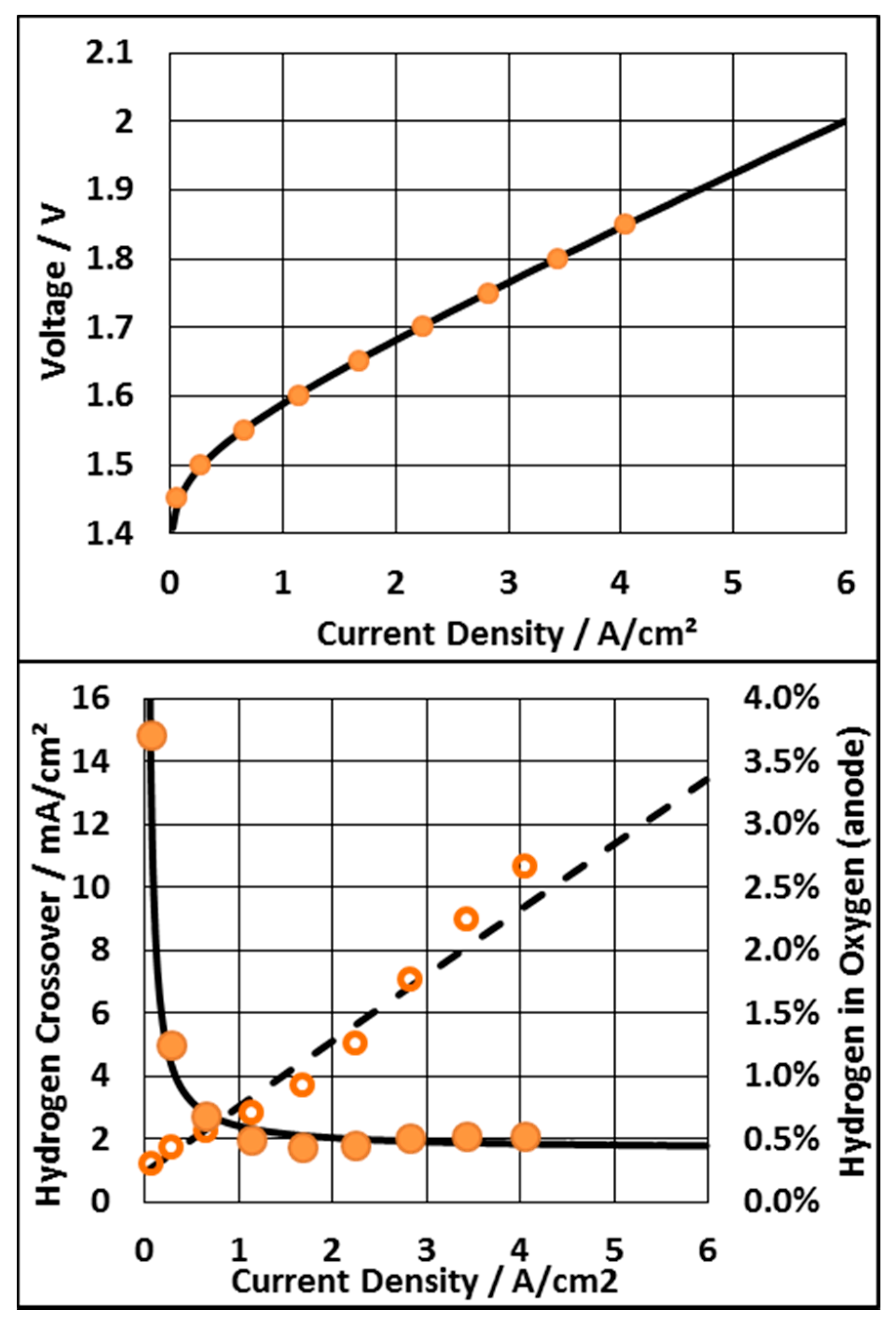
3.1. The Ideal PEM Electrolyzer
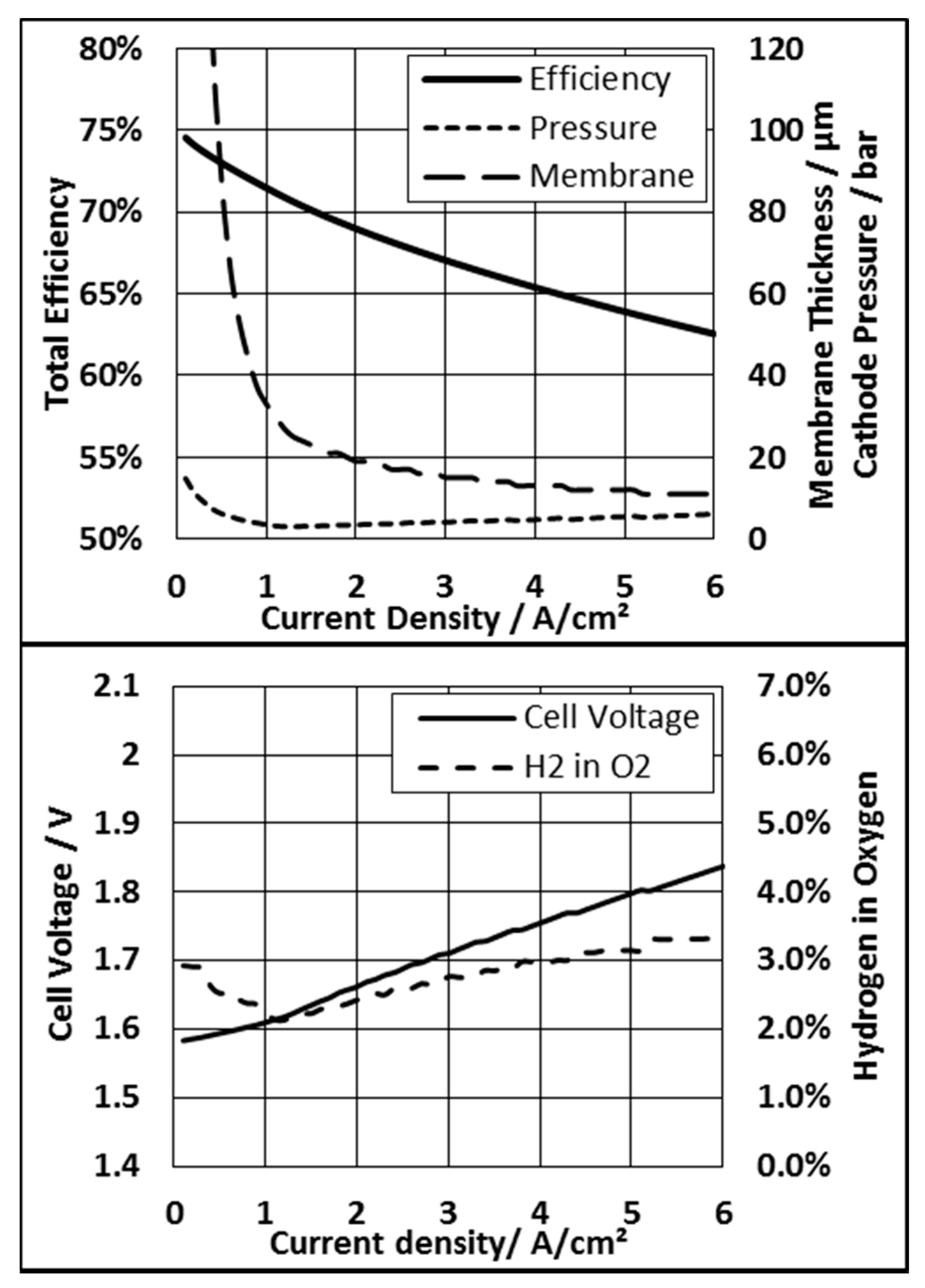
3.2. Voltage Limitation and Constant Membrane Thickness
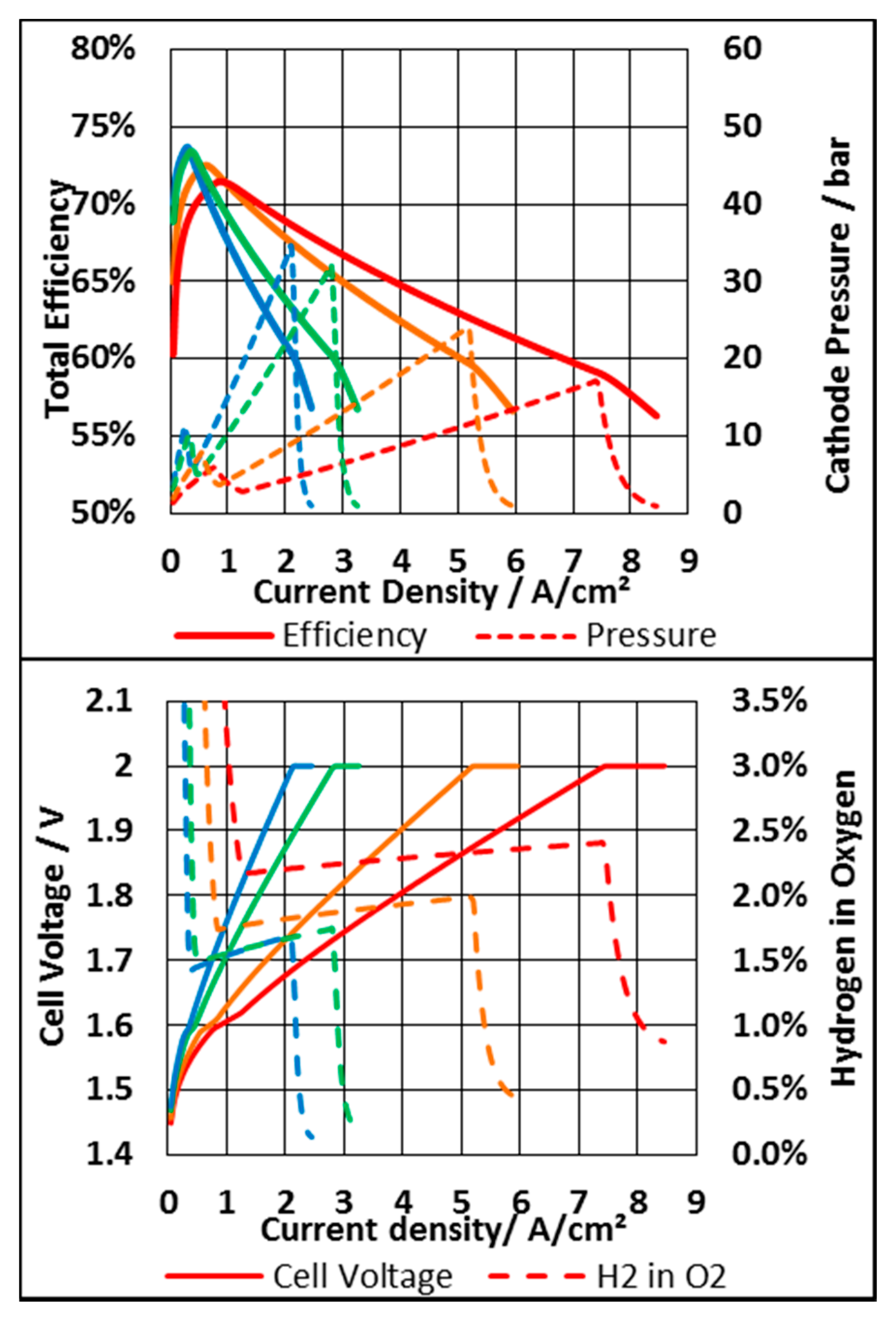
3.3. Membrane Thickness and Pressure Mode
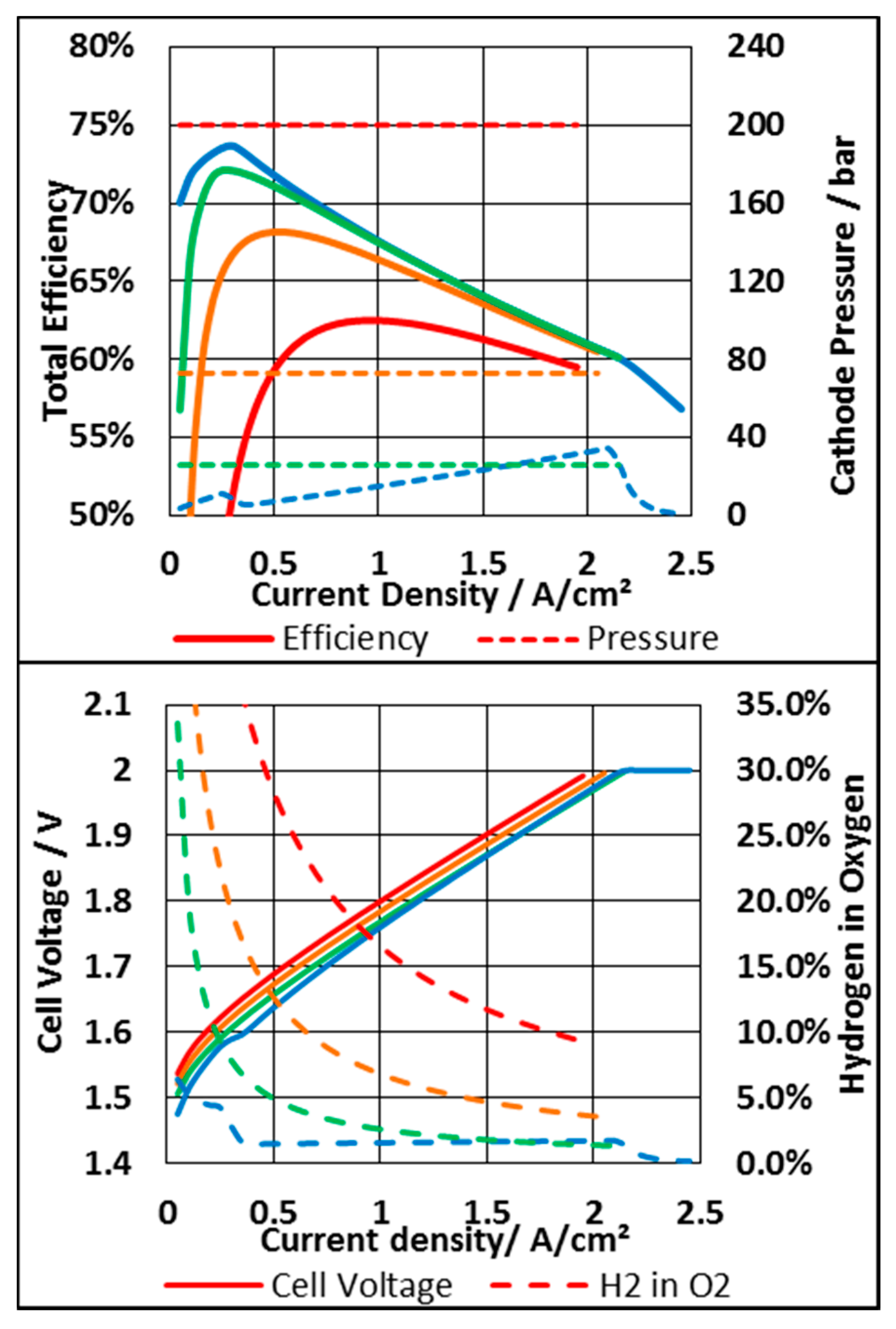
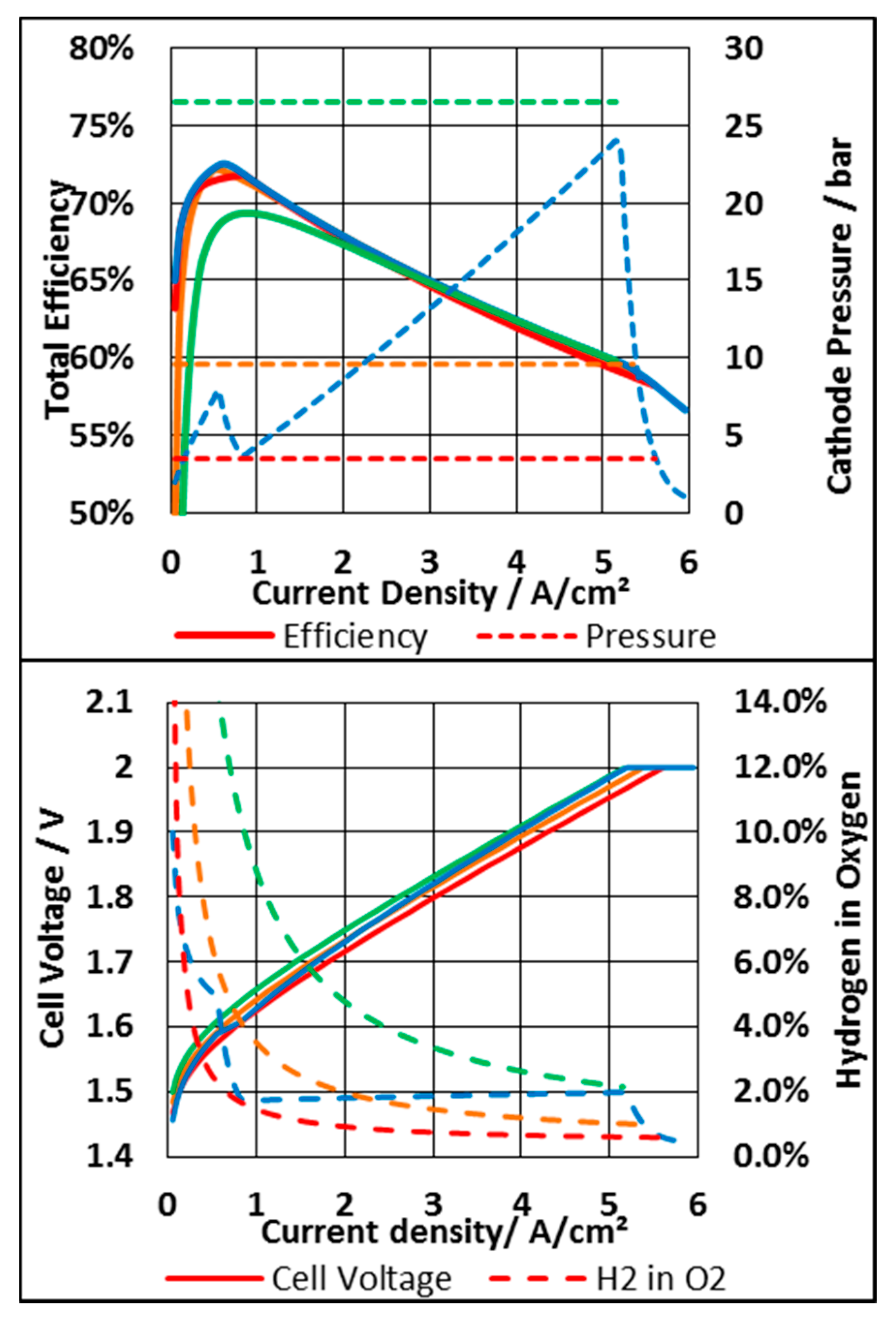
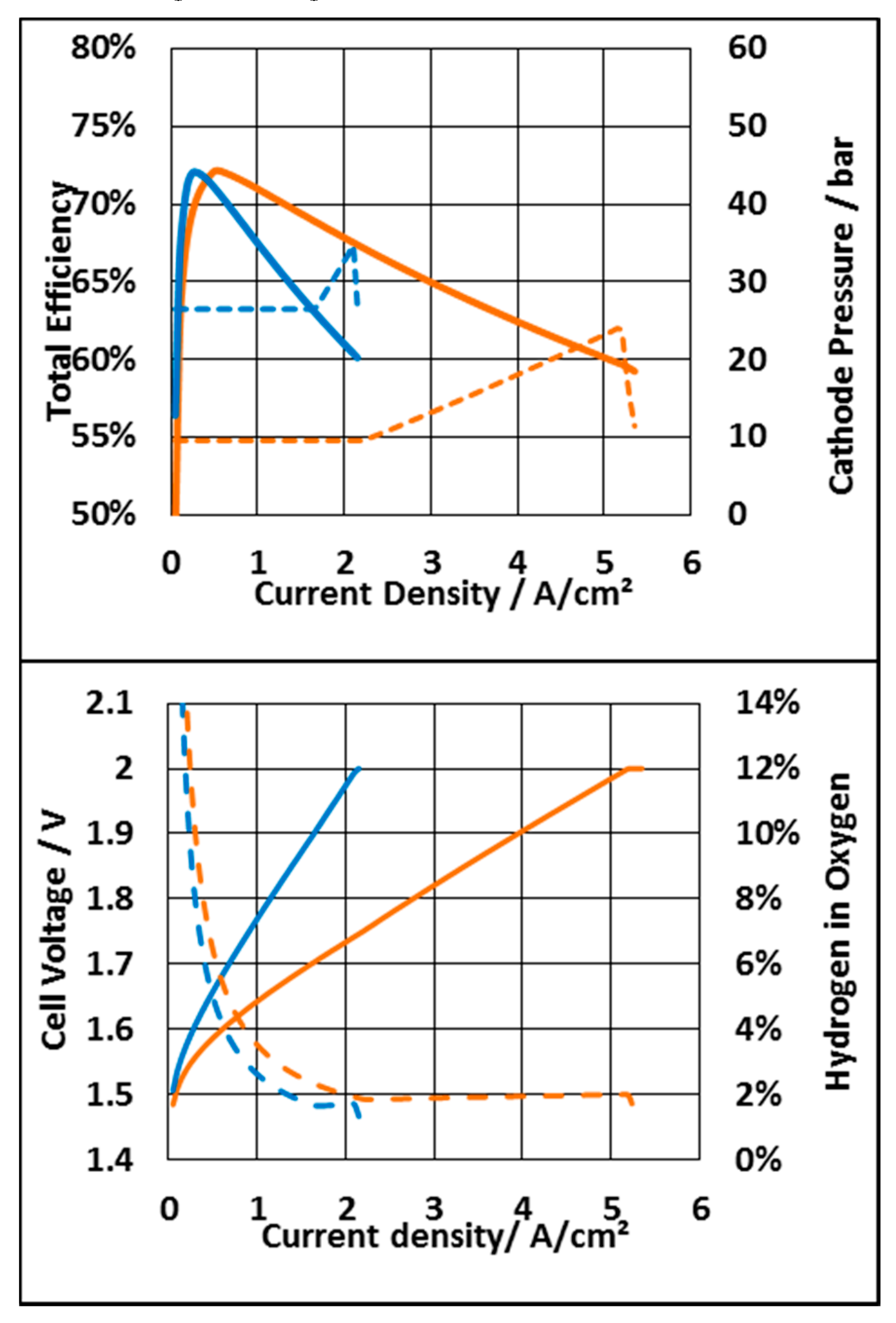
3.3.1. System Based on an N117 Membrane
3.3.2. System Based on N212 Membrane
3.3.3. Economic Consideration
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Nomenclature | |
| A | ampere |
| a | current density effect on permeation |
| compression factor | |
| d | membrane thickness |
| E | energy |
| F | Faraday constant |
| G | Gibbs free energy |
| H | enthalpy |
| I | current |
| j | current density |
| K | Kelvin |
| molar flow | |
| P | power |
| permeability coefficient | |
| p | pressure |
| heat flow | |
| R | gas constant |
| electric resistance | |
| S | entropy |
| s | compressor stages |
| T | temperature |
| U | voltage |
| V | volt |
| W | pressure–volume work |
| Z | compressibility factor |
| z | number of transferred electrons |
| Greek Symbols | |
| charge transfer coefficient | |
| difference | |
| swelling factor | |
| efficiency | |
| average heat capacity ratio | |
| conductivity | |
| volume fraction | |
| Subscripts | |
| 0 | standard |
| 1 | one step |
| act | activation |
| an | anode |
| c | compression |
| cat | cathode |
| el | electric |
| F | Faraday |
| i | index number |
| load | vapor loading |
| N | Nernst |
| p | production |
| res | resistance |
| rev | reversible |
| s | storage |
| T | total |
| vap | vaporization |
| x | crossover |
References
- Gür, T.M. Review of electrical energy storage technologies, materials and systems: Challenges and prospects for large-scale grid storage. Energy Environ. Sci. 2018, 11, 2696–2767. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, J.; Yu, B.; Zhang, W.; Chen, J.; Qiao, J.; Zhang, J. A review of high temperature co-electrolysis of H2O and CO2 to produce sustainable fuels using solid oxide electrolysis cells (SOECs): Advanced materials and technology. Chem. Soc. Rev. 2017, 46, 1427–1463. [Google Scholar] [CrossRef] [PubMed]
- Phillips, R.; Dunnill, C.W. Zero gap alkaline electrolysis cell design for renewable energy storage as hydrogen gas. RSC Adv. 2016, 6, 100643–100651. [Google Scholar] [CrossRef] [Green Version]
- Carmo, M.; Fritz, D.L.; Mergel, J.; Stolten, D. A comprehensive review on PEM water electrolysis. Int. J. Hydrogen Energy 2013, 38, 4901–4934. [Google Scholar] [CrossRef]
- Hank, C.; Gelpke, S.; Schnabl, A.; White, R.J.; Full, J.; Wiebe, N.; Smolinka, T.; Schaadt, A.; Henning, H.M.; Hebling, C. Economics & carbon dioxide avoidance cost of methanol production based on renewable hydrogen and recycled carbon dioxide—Power-to-methanol. Sustain. Energy Fuels 2018, 2, 1244–1261. [Google Scholar]
- Shaner, M.R.; Atwater, H.A.; Lewis, N.S.; McFarland, E.W. A comparative technoeconomic analysis of renewable hydrogen production using solar energy. Energy Environ. Sci. 2016, 9, 2354–2371. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Higgins, D.; Yu, A.; Zhang, L.; Zhang, J. A review on non-precious metal electrocatalysts for PEM fuel cells. Energy Environ. Sci. 2011, 4, 3167–3192. [Google Scholar] [CrossRef]
- Xing, L.; Shi, W.; Su, H.; Xu, Q.; Das, P.K.; Mao, B.; Scott, K. Membrane electrode assemblies for PEM fuel cells: A review of functional graded design and optimization. Energy 2019, 177, 445–464. [Google Scholar] [CrossRef]
- Yigit, T.; Selamet, O.F. Mathematical modeling and dynamic Simulink simulation of high-pressure PEM electrolyzer system. Int. J. Hydrogen Energy 2016, 41, 13901–13914. [Google Scholar] [CrossRef]
- Niermann, M.; Drünert, S.; Kaltschmitt, M.; Bonhoff, K. Liquid organic hydrogen carriers (LOHCs)—Techno-economic analysis of LOHCs in a defined process chain. Energy Environ. Sci. 2019, 12, 290–307. [Google Scholar] [CrossRef]
- Jorschick, H.; Preuster, P.; Dürr, S.; Seidel, A.; Müller, K.; Bösmann, A.; Wasserscheid, P. Hydrogen storage using a hot pressure swing reactor. Energy Environ. Sci. 2017, 10, 1652–1659. [Google Scholar] [CrossRef] [Green Version]
- Sartory, M.; Wallnöfer-Ogris, E.; Salman, P.; Fellinger, T.; Justl, M.; Trattner, A.; Klell, M. Theoretical and experimental analysis of an asymmetric high pressure PEM water electrolyser up to 155 bar. Int. J. Hydrogen Energy 2017, 42, 30493–30508. [Google Scholar] [CrossRef]
- Trinke, P.; Haug, P.; Brauns, J.; Bensmann, B.; Hanke-Rauschenbach, R.; Turek, T. Hydrogen crossover in PEM and alkaline water electrolysis: Mechanisms, direct comparison and mitigation strategies. J. Electrochem. Soc. 2018, 165, F502–F513. [Google Scholar] [CrossRef]
- Grigoriev, S.A.; Porembskiy, V.I.; Korobtsev, S.V.; Fateev, V.N.; Auprêtre, F.; Millet, P. High-pressure PEM water electrolysis and corresponding safety issues. Int. J. Hydrogen Energy 2011, 36, 2721–2728. [Google Scholar] [CrossRef]
- Gubler, L. Polymer Design Strategies for Radiation-Grafted Fuel Cell Membranes. Adv. Energy Mater. 2014, 4, 1300827. [Google Scholar] [CrossRef]
- Klose, C. Membrane Interlayer with Pt Recombination Particles for Reduction of the Anodic Hydrogen Content in PEM Water Electrolysis. J. Electrochem. Soc. 2018, 165, F1271–F1276. [Google Scholar] [CrossRef]
- Schalenbach, M.; Carmo, M.; Fritz, D.L.; Mergel, J.; Stolten, D. Pressurized PEM water electrolysis: Efficiency and gas crossover. Int. J. Hydrogen Energy 2013, 38, 14921–14933. [Google Scholar] [CrossRef]
- Schalenbach, M. Corrigendum to “Pressurized PEM water electrolysis: Efficiency and gas crossover”. Int. J. Hydrogen Energy 2016, 41, 729–732. [Google Scholar] [CrossRef]
- Tjarks, G.; Gibelhaus, A.; Lanzerath, F.; Müller, M.; Bardow, A.; Stolten, D. Energetically-optimal PEM electrolyzer pressure in power-to-gas plants. Appl. Energy 2018, 218, 192–198. [Google Scholar] [CrossRef]
- Bensmann, B.; Hanke-Rauschenbach, R.; Peña Arias, I.K.; Sundmacher, K. Energetic evaluation of high pressure PEM electrolyzer systems for intermediate storage of renewable energies. Electrochim. Acta 2013, 110, 570–580. [Google Scholar] [CrossRef]
- Suermann, M.; Kiupel, T.; Schmidt, T.J.; Buchi, F.N. Electrochemical Hydrogen Compression: Efficient Pressurization Concept Derived from an Energetic Evaluation. J. Electrochem. Soc. 2017, 164, F1187–F1195. [Google Scholar] [CrossRef]
- Marangio, F.; Santarelli, M.; Calì, M. Theoretical model and experimental analysis of a high pressure PEM water electrolyser for hydrogen production. Int. J. Hydrogen Energy 2009, 34, 1143–1158. [Google Scholar] [CrossRef]
- García-Valverde, R.; Espinosa, N.; Urbina, A. Simple PEM water electrolyser model and experimental validation. Int. J. Hydrogen Energy 2012, 37, 1927–1938. [Google Scholar] [CrossRef]
- Selamet, Ö.F.; Acar, M.C.; Mat, M.D.; Kaplan, Y. Effects of operating parameters on the performance of a high-pressure proton exchange membrane electrolyzer. Int. J. Energy Res. 2013, 37, 457–467. [Google Scholar] [CrossRef]
- Awasthi, A.; Scott, K.; Basu, S. Dynamic modeling and simulation of a proton exchange membrane electrolyzer for hydrogen production. Int. J. Hydrogen Energy 2011, 36, 14779–14786. [Google Scholar] [CrossRef]
- Santarelli, M.; Medina, P.; Calì, M. Fitting regression model and experimental validation for a high-pressure PEM electrolyzer. Int. J. Hydrogen Energy 2009, 34, 2519–2530. [Google Scholar] [CrossRef]
- Kurzweil, P. Brennstoffzellentechnik: Grundlagen, Komponenten, Systeme, Anwendungen, 2nd ed.; Springer Vieweg: Wiesbaden, Germany, 2013. [Google Scholar]
- Tjarks, G.; Stolten, D.; Weßling, M. PEM-Elektrolyse-Systeme zur Anwendung in Power-to-Gas Anlagen; Forschungszentrum, Zentralbibliothek: Jülich, Germany, 2017. [Google Scholar]
- Stähler, M.; Stähler, A.; Scheepers, F.; Carmo, M.; Stolten, D. A completely slot die coated membrane electrode assembly. Int. J. Hydrogen Energy 2019, 44, 7053–7058. [Google Scholar] [CrossRef]
- Harrison, K.W.; Hernández-Pacheco, E.; Mann, M.; Salehfar, H. Semiempirical Model for Determining PEM Electrolyzer Stack Characteristics. J. Electrochem. Energy Convers. Storage 2005, 3, 220–223. [Google Scholar] [CrossRef]
- Barbir, F. Chapter Three—Fuel Cell Electrochemistry. In PEM Fuel Cells, 2nd ed.; Barbir, F., Ed.; Academic Press: Boston, MA, USA, 2013; pp. 33–72. [Google Scholar]
- Grigoriev, S.A.; Millet, P.; Korobtsev, S.V.; Porembskiy, V.I.; Pepic, M.; Etievant, C.; Puyenchet, C.; Fateev, V.N. Hydrogen safety aspects related to high-pressure polymer electrolyte membrane water electrolysis. Int. J. Hydrogen Energy 2009, 34, 5986–5991. [Google Scholar] [CrossRef]
- Bernt, M.; Siebel, A.; Gasteiger, H.A. Analysis of Voltage Losses in PEM Water Electrolyzers with Low Platinum Group Metal Loadings. J. Electrochem. Soc. 2018, 165, F305–F314. [Google Scholar] [CrossRef]
- Yadav, R. Analysis of EIS Technique and Nafion 117 Conductivity as a Function of Temperature and Relative Humidity. J. Electrochem. Soc. 2012, 159, B340–B346. [Google Scholar] [CrossRef]
- Trinke, P.; Bensmann, B.; Reichstein, S.; Hanke-Rauschenbach, R.; Sundmacher, K. Hydrogen Permeation in PEM Electrolyzer Cells Operated at Asymmetric Pressure Conditions. J. Electrochem. Soc. 2016, 163, F3164–F3170. [Google Scholar] [CrossRef]
- Sakai, T.; Takenaka, H.; Wakabayashi, N.; Kawami, Y.; Torikai, E. Gas Permeation Properties of Solid Polymer Electrolyte (SPE) Membranes. J. Electrochem. Soc. 1985, 132, 1328–1332. [Google Scholar] [CrossRef]
- Matsushima, H.; Kiuchi, D.; Fukunaka, Y. Measurement of dissolved hydrogen supersaturation during water electrolysis in a magnetic field. Electrochim. Acta 2009, 54, 5858–5862. [Google Scholar] [CrossRef]
- Stähler, M.; Stähler, A.; Scheepers, F.; Lehnert, W.; Stolten, D. Impact of porous transport layer compression on hydrogen permeation in PEM Water electrolysis. Int. J. Hydrogen Energy 2019, 45, 4008–4014. [Google Scholar] [CrossRef]
- Albert, A.; Barnett, A.O.; Thomassen, M.S.; Schmidt, T.J.; Gubler, L. Radiation-Grafted Polymer Electrolyte Membranes for Water Electrolysis Cells: Evaluation of Key Membrane Properties. ACS Appl. Mater. Interfaces 2015, 7, 22203–22212. [Google Scholar] [CrossRef] [PubMed]
- Trinke, P.; Bensmann, B.; Hanke-Rauschenbach, R. Current density effect on hydrogen permeation in PEM water electrolyzers. Int. J. Hydrogen Energy 2017, 42, 14355–14366. [Google Scholar] [CrossRef]
- Sethuraman, V.A. Hydrogen peroxide formation rates in a PEMFC anode and cathode: Effect of humidity and temperature. J. Electrochem. Soc. 2008, 155, B50–B57. [Google Scholar] [CrossRef] [Green Version]
- Tzimas, E.; Filiou, C.; Peteves, S.; Veyret, J. Hydrogen Storage: State-of-the-Art and Future Perspective. In EU Commission, JRC Petten, EUR 20995EN; European Commission (Directorate General Joint Research Ceneter (DG JRC)): Petten, The Netherlands, 2003. [Google Scholar]
- Lemmon, E.W.; Huber, M.L.; Leachman, J.W. Revised Standardized Equation for Hydrogen Gas Densities for Fuel Consumption Applications. J. Res. Natl. Inst. Stand. Technol. 2008, 113, 341–350. [Google Scholar] [CrossRef]
- Alduchov, O.A.; Eskridge, R.E. Improved Magnus Form Approximation of Saturation Vapor Pressure. J. Appl. Meteorol. 1996, 35, 601–609. [Google Scholar] [CrossRef]
- Shi, S.; Weber, A.Z.; Kusoglu, A. Structure/property relationship of Nafion XL composite membranes. J. Membr. Sci. 2016, 516, 123–134. [Google Scholar] [CrossRef] [Green Version]
- Deffenbaugh, D.M.; Brun, K.; Harris, R.E.; Harrell, J.P.; Mckee, R.J.; Moore, J.J.; Siebenaler, S.P.; Svedeman, S.J. Advanced Reciprocating Compression Technology (ARCT); Southwest Research Institute: San Antonio, TX, USA, 2005. [Google Scholar]
- Dehnen, M. Hofer Diaphragm Compressors; Hofer Hochdrucktechnik GmbH: Mülheim an der Ruhr, Germany, 2007. [Google Scholar]
- Witkowski, A.; Rusin, A.; Majkut, M.; Stolecka, K. Comprehensive analysis of hydrogen compression and pipeline transportation from thermodynamics and safety aspects. Energy 2017, 141, 2508–2518. [Google Scholar] [CrossRef]
- Ayers, K.; Danilovic, N.; Ouimet, R.; Carmo, M.; Pivovar, B.; Bornstein, M. Perspectives on Low-Temperature Electrolysis and Potential for Renewable Hydrogen at Scale. Annu. Rev. Chem. Biomol. Eng. 2019, 10, 219–239. [Google Scholar] [CrossRef] [PubMed]
- Esposito, D.V. Membraneless electrolyzers for low-cost hydrogen production in a renewable energy future. Joule 2017, 1, 651–658. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, O.; Gambhir, A.; Staffell, I.; Hawkes, A.; Nelson, J.; Few, S. Future cost and performance of water electrolysis: An expert elicitation study. Int. J. Hydrogen Energy 2017, 42, 30470–30492. [Google Scholar] [CrossRef]
- Smolinka, T.; Wiebe, N.; Sterchele, P.; Palzer, A. Study IndWEDe—Brief Overview; Fraunhofer Institute for Manufacturing Engineering and Automation IPA: Berlin, Germany, 2018. [Google Scholar]
- Bertuccioli, L.; Chan, A.; Hart, D.; Lehner, F.; Madden, B.; Standen, E. Study on Development of Water Electrolysis in the EU; e4tech: Lausanne, Switzerland, 2014. [Google Scholar]
- Mayyas, A.T.; Ruth, M.F.; Pivovar, B.S.; Bender, G.; Wipke, K.B. Manufacturing Cost Analysis for Proton Exchange Membrane Water Electrolyzers; National Renewable Energy Lab. (NREL): Golden, CO, USA, 2019.
- Feng, Q.; Yuan, X.Z.; Liu, G.; Wei, B.; Zhang, Z.; Li, H.; Wang, H. A review of proton exchange membrane water electrolysis on degradation mechanisms and mitigation strategies. J. Power Sources 2017, 366, 33–55. [Google Scholar] [CrossRef]


| Parameter | Value | Unit |
|---|---|---|
| 47,948 | Pa | |
| 41,572 | J mol−1 | |
| mol (cm s Pa)−1 | ||
| 0.137 | S cm−1 | |
| 1.15 | - | |
| 2.75 | - | |
| Tin | 300 | K |
| 1.4 | - | |
| ηc | 0.825 | - |
| 200 | bar |
| Parameter | Value | Unit |
|---|---|---|
| α | 0.43 | - |
| j0 | A cm−2 | |
| ax | cm | |
| R0 | 27 | mΩ cm2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scheepers, F.; Stähler, M.; Stähler, A.; Rauls, E.; Müller, M.; Carmo, M.; Lehnert, W. Improving the Efficiency of PEM Electrolyzers through Membrane-Specific Pressure Optimization. Energies 2020, 13, 612. https://doi.org/10.3390/en13030612
Scheepers F, Stähler M, Stähler A, Rauls E, Müller M, Carmo M, Lehnert W. Improving the Efficiency of PEM Electrolyzers through Membrane-Specific Pressure Optimization. Energies. 2020; 13(3):612. https://doi.org/10.3390/en13030612
Chicago/Turabian StyleScheepers, Fabian, Markus Stähler, Andrea Stähler, Edward Rauls, Martin Müller, Marcelo Carmo, and Werner Lehnert. 2020. "Improving the Efficiency of PEM Electrolyzers through Membrane-Specific Pressure Optimization" Energies 13, no. 3: 612. https://doi.org/10.3390/en13030612
APA StyleScheepers, F., Stähler, M., Stähler, A., Rauls, E., Müller, M., Carmo, M., & Lehnert, W. (2020). Improving the Efficiency of PEM Electrolyzers through Membrane-Specific Pressure Optimization. Energies, 13(3), 612. https://doi.org/10.3390/en13030612





