Multiobjective Optimization for the Greener Synthesis of Chloromethyl Ethylene Carbonate by CO2 and Epichlorohydrin via Response Surface Methodology
Abstract
1. Introduction
2. Experimental Methods
2.1. Chemicals and Materials
2.2. Catalysts Preparation
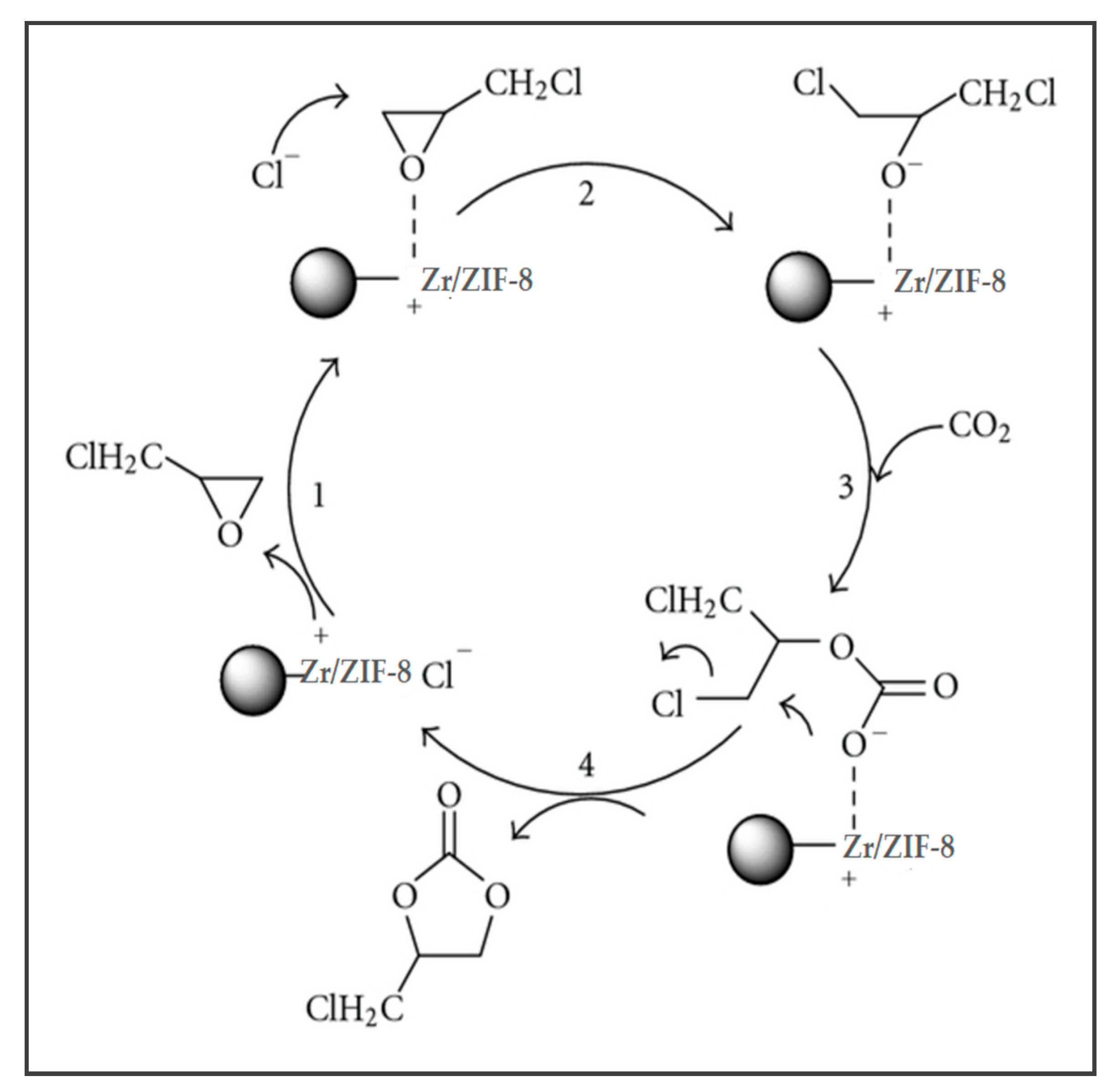
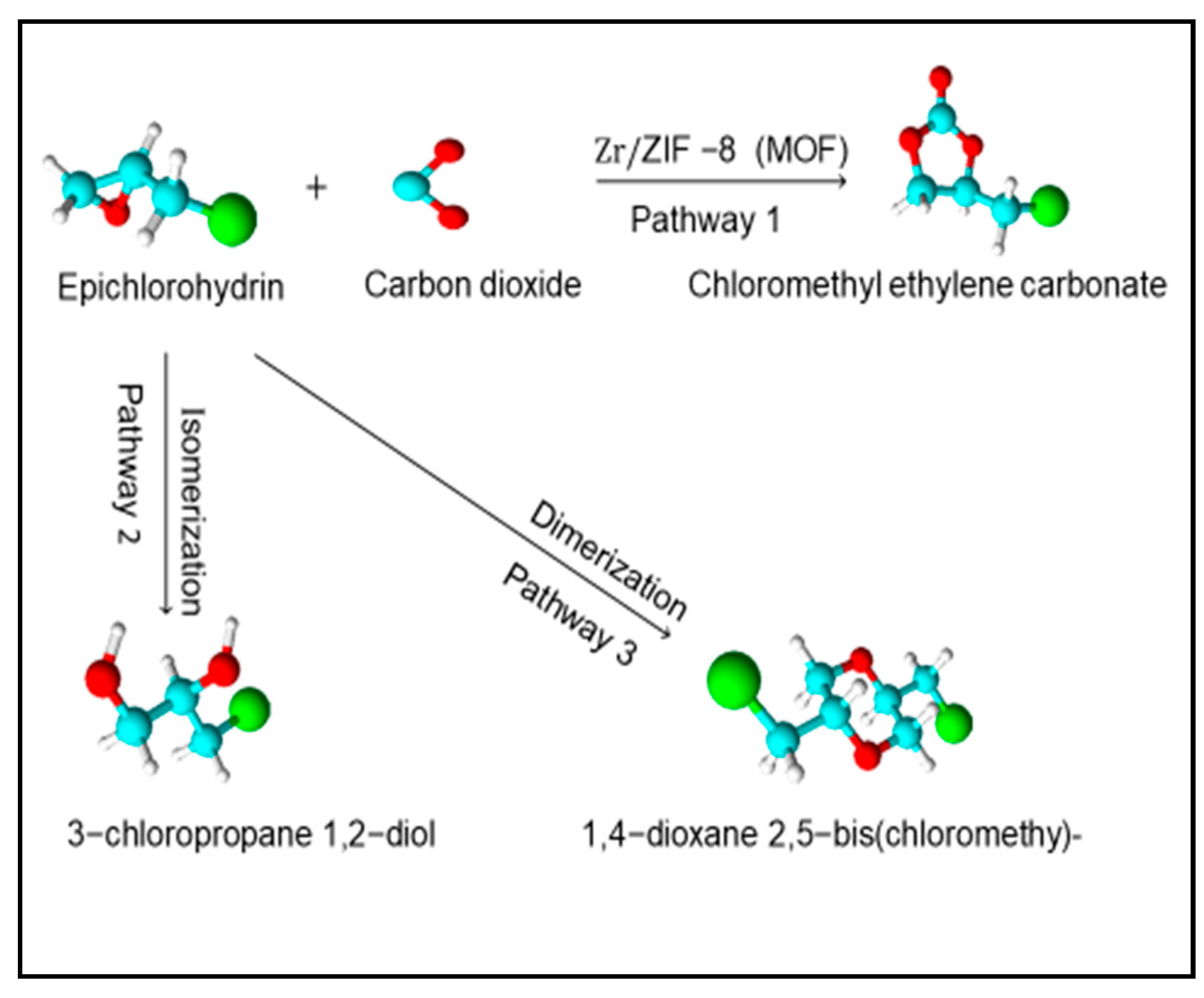
2.3. Proposed Reaction Mechanism and Reaction Pathways
2.4. One-Factor-at-a-Time (OFAT) Analysis
2.5. Experimental Design
2.6. Statistical Analysis
2.7. Experimental Procedures
3. Results and Discussion
3.1. Analysis of Variance (ANOVA)
3.2. Development of Regression Model
3.3. Statistical Analysis of Regression Model
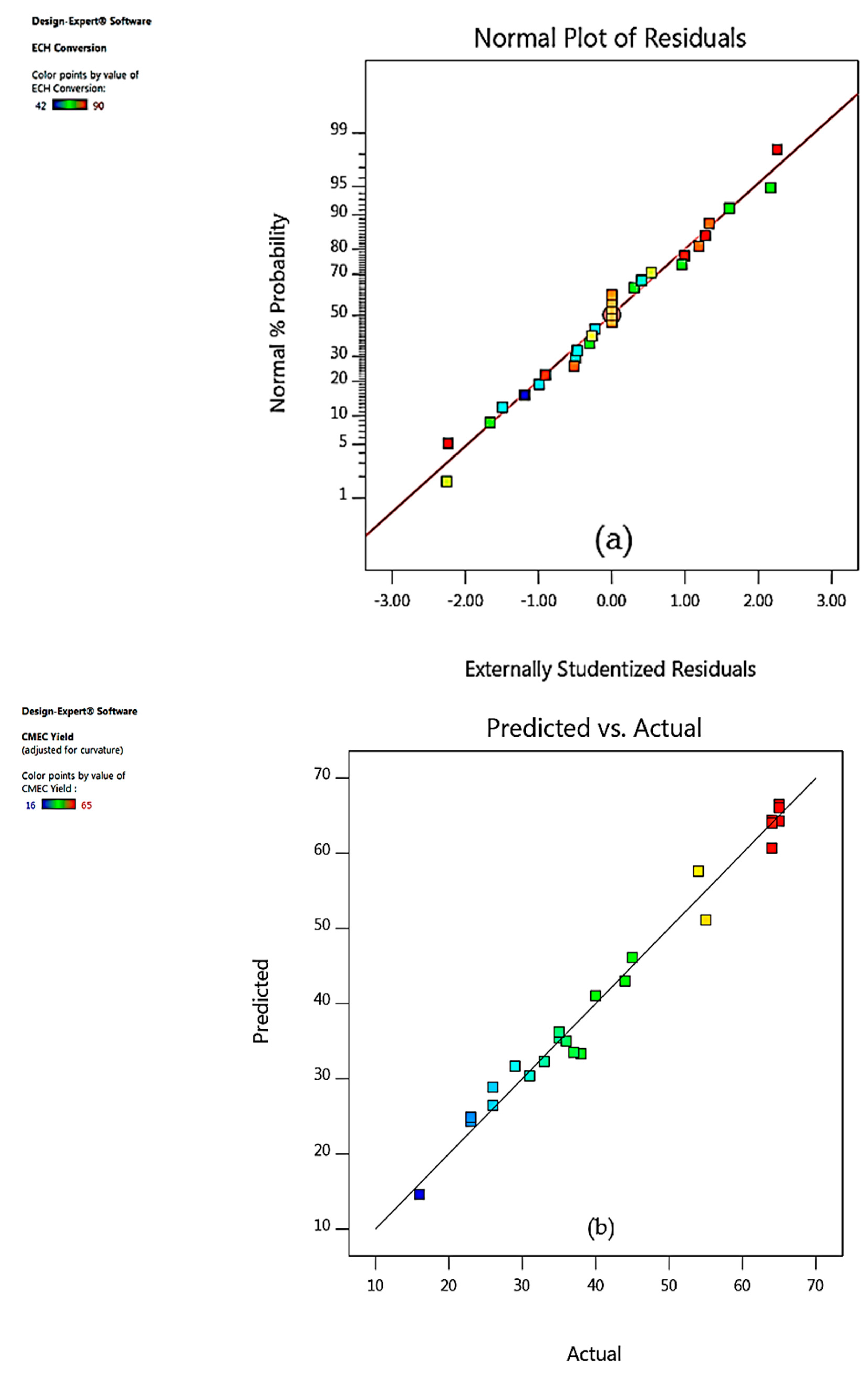
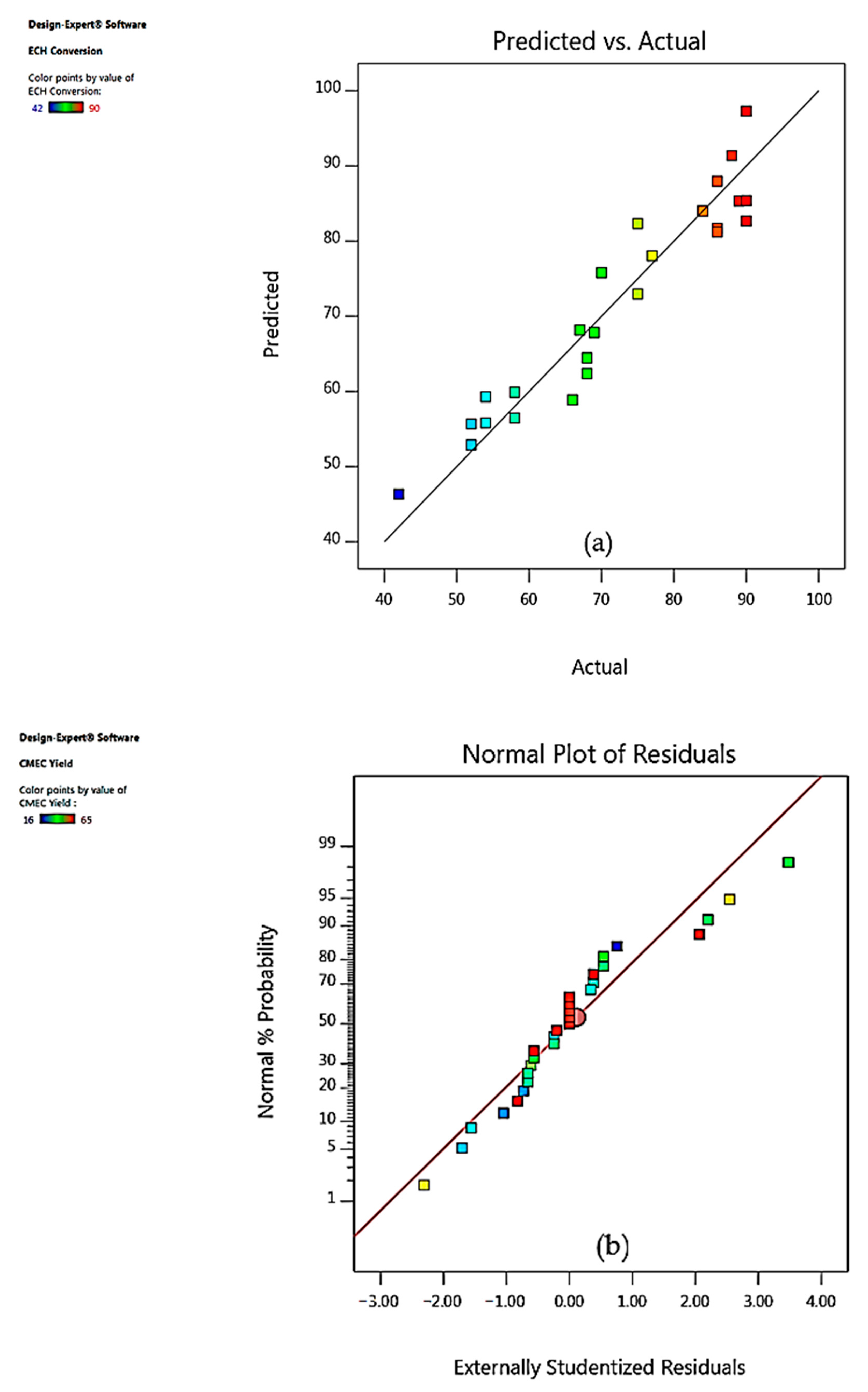
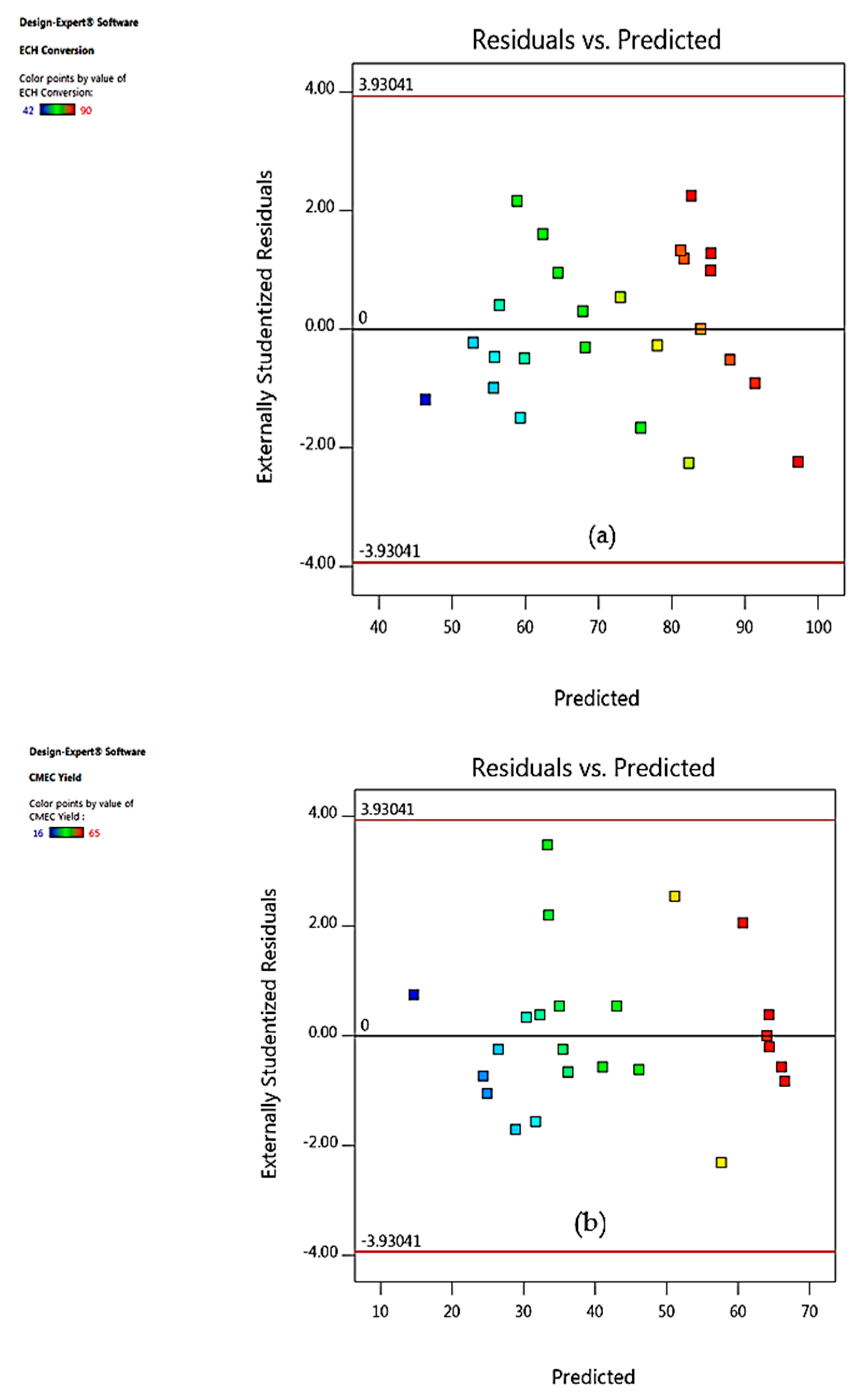
3.4. Model Fitting and Adequacy Checking
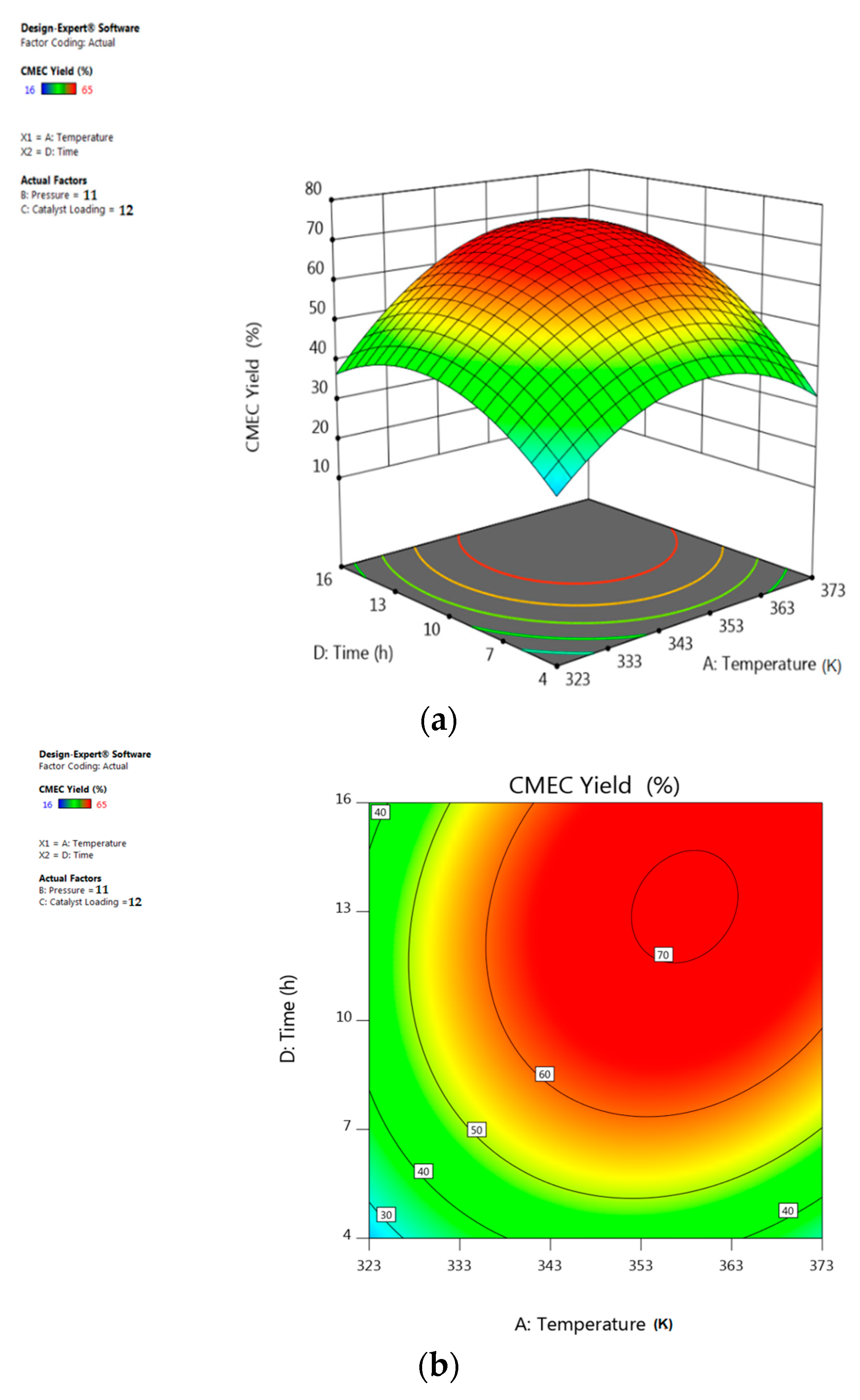
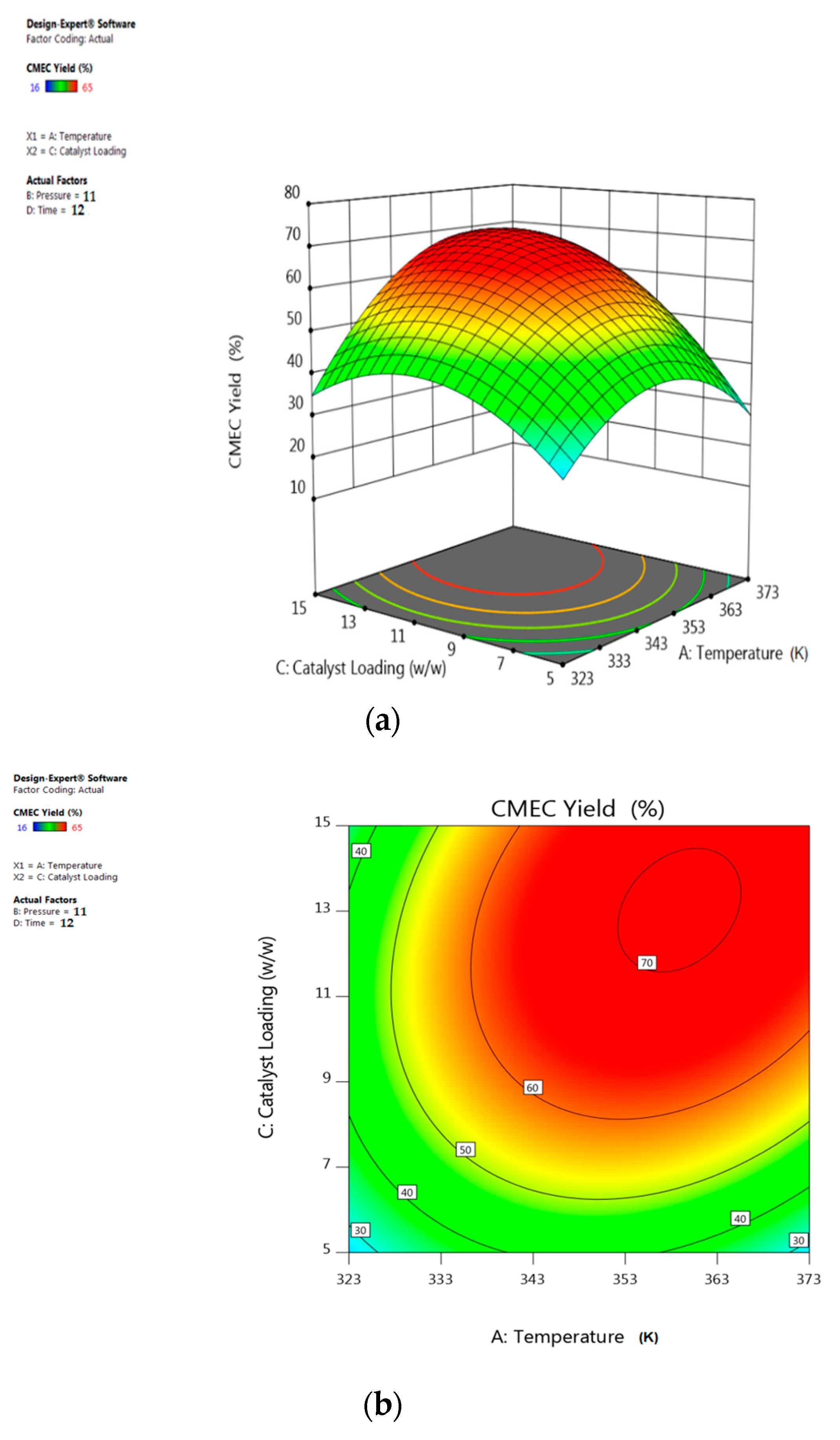
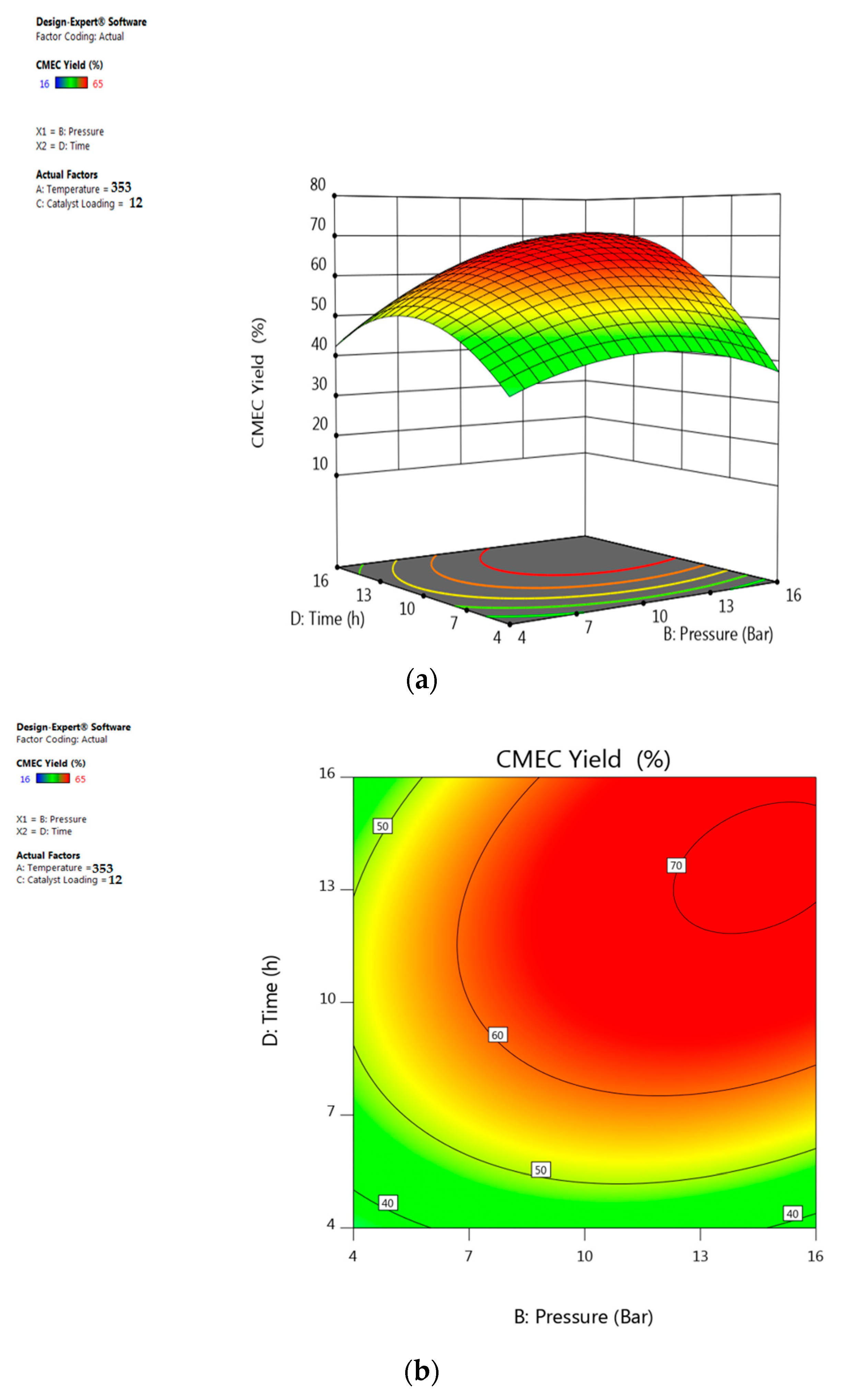
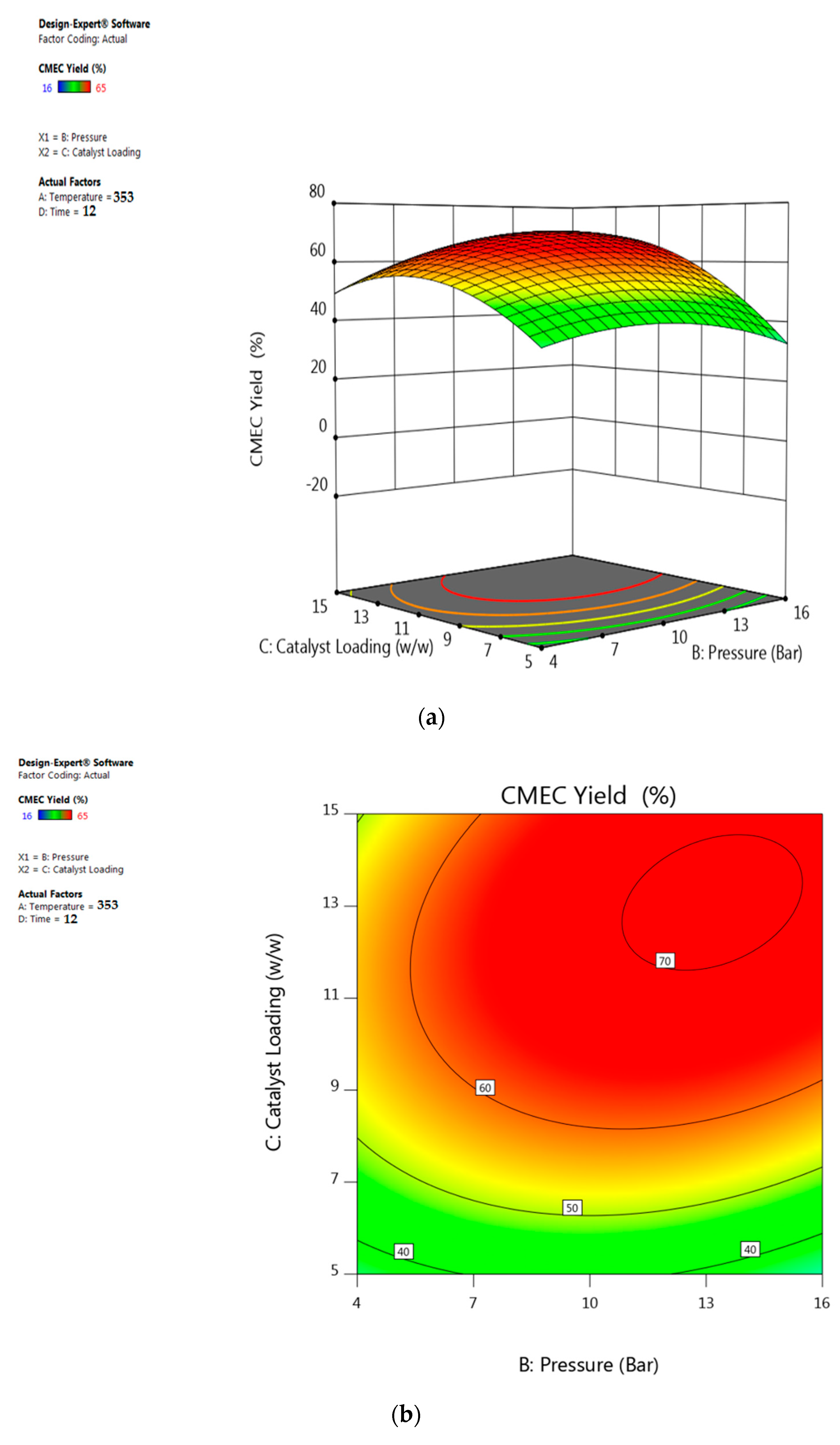
3.5. Response Surface Plots Analysis
4. Effect of One Factor at a Time Experiments on Responses (OFAT)
4.1. Effect of Reaction Temperature
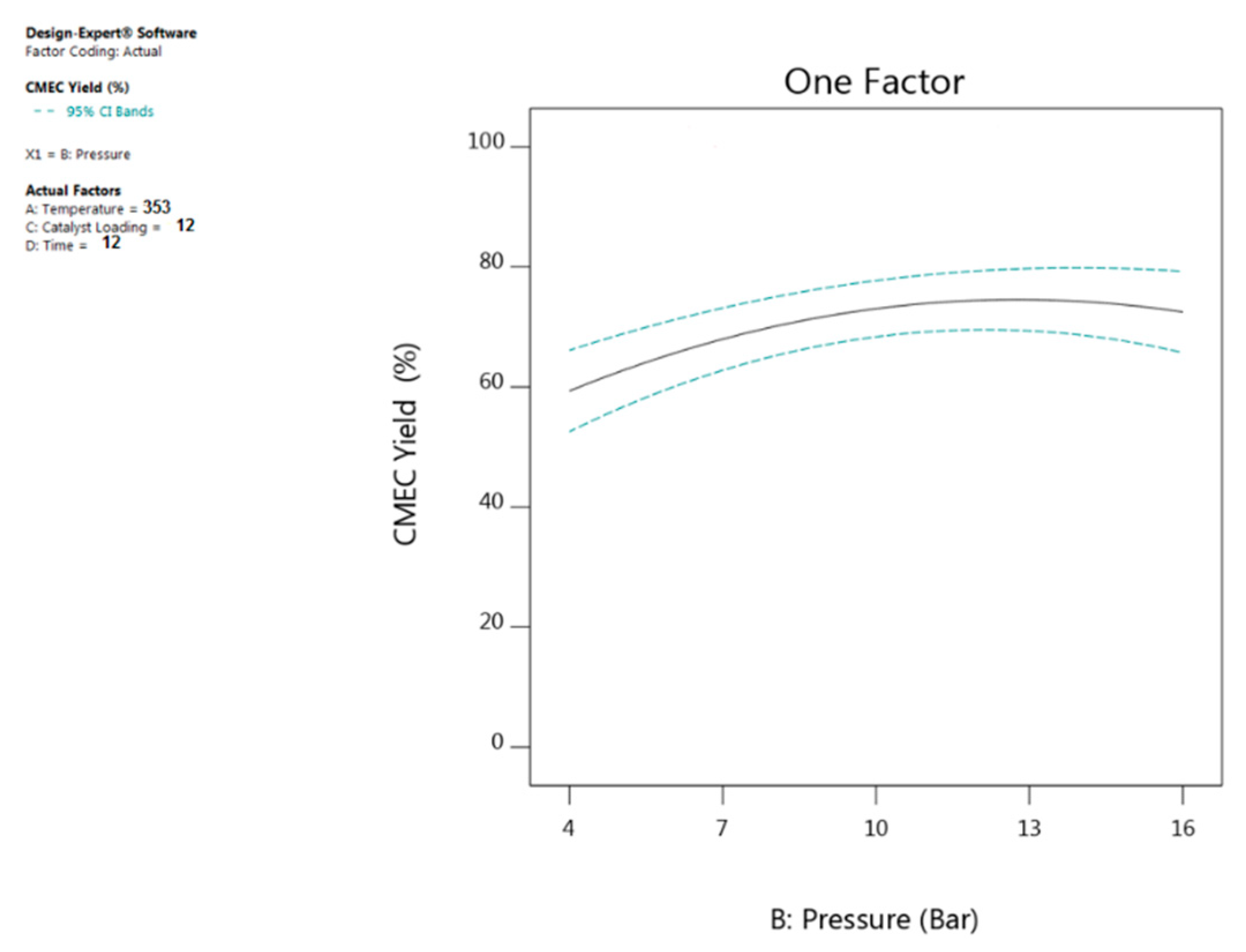
4.2. Effect of Carbon Dioxide (CO2) Pressure
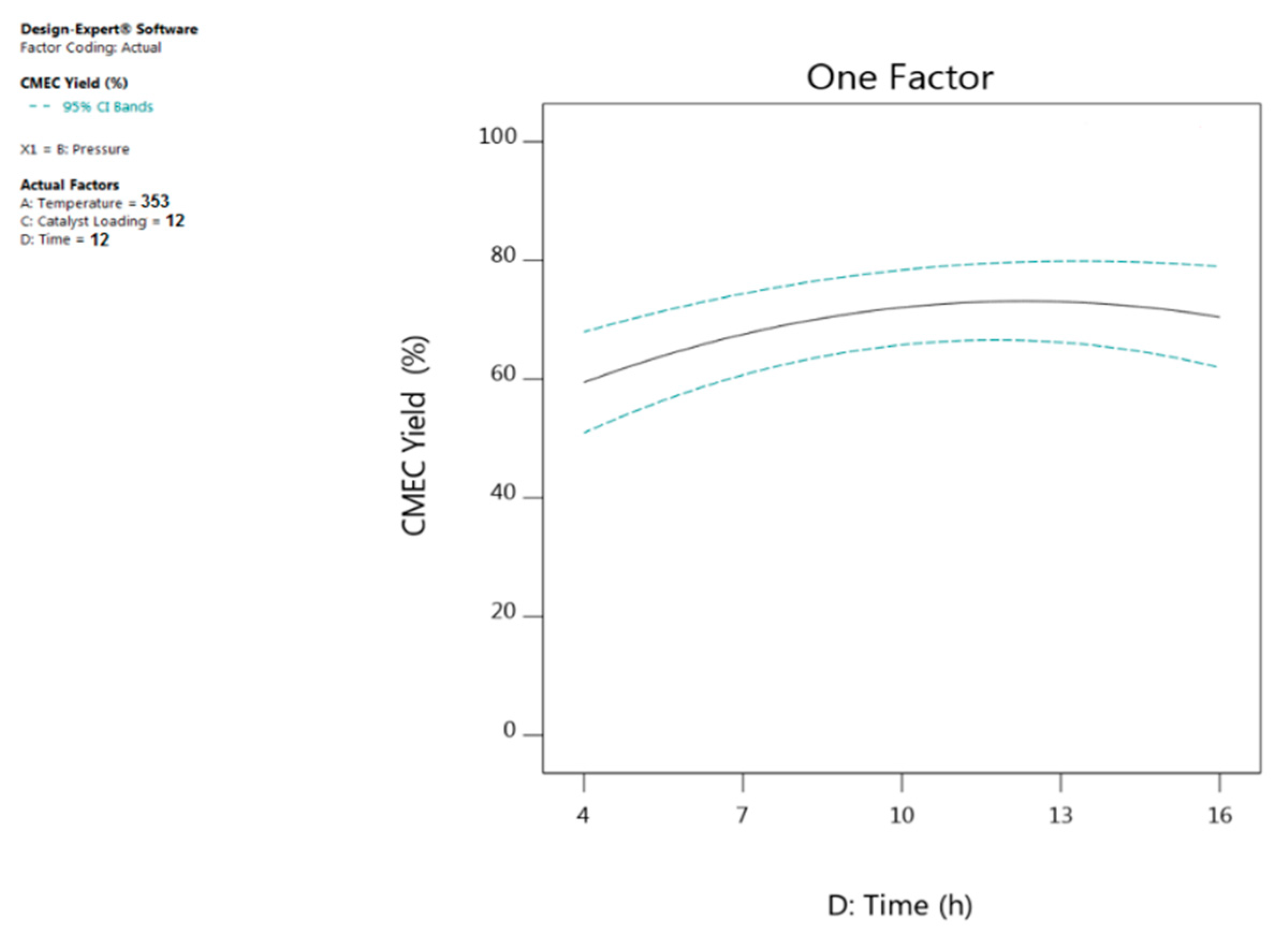
4.3. Effect of Reaction Time
4.4. Effect of Catalyst Loading
5. Interactive Effect of Process Variables on Responses
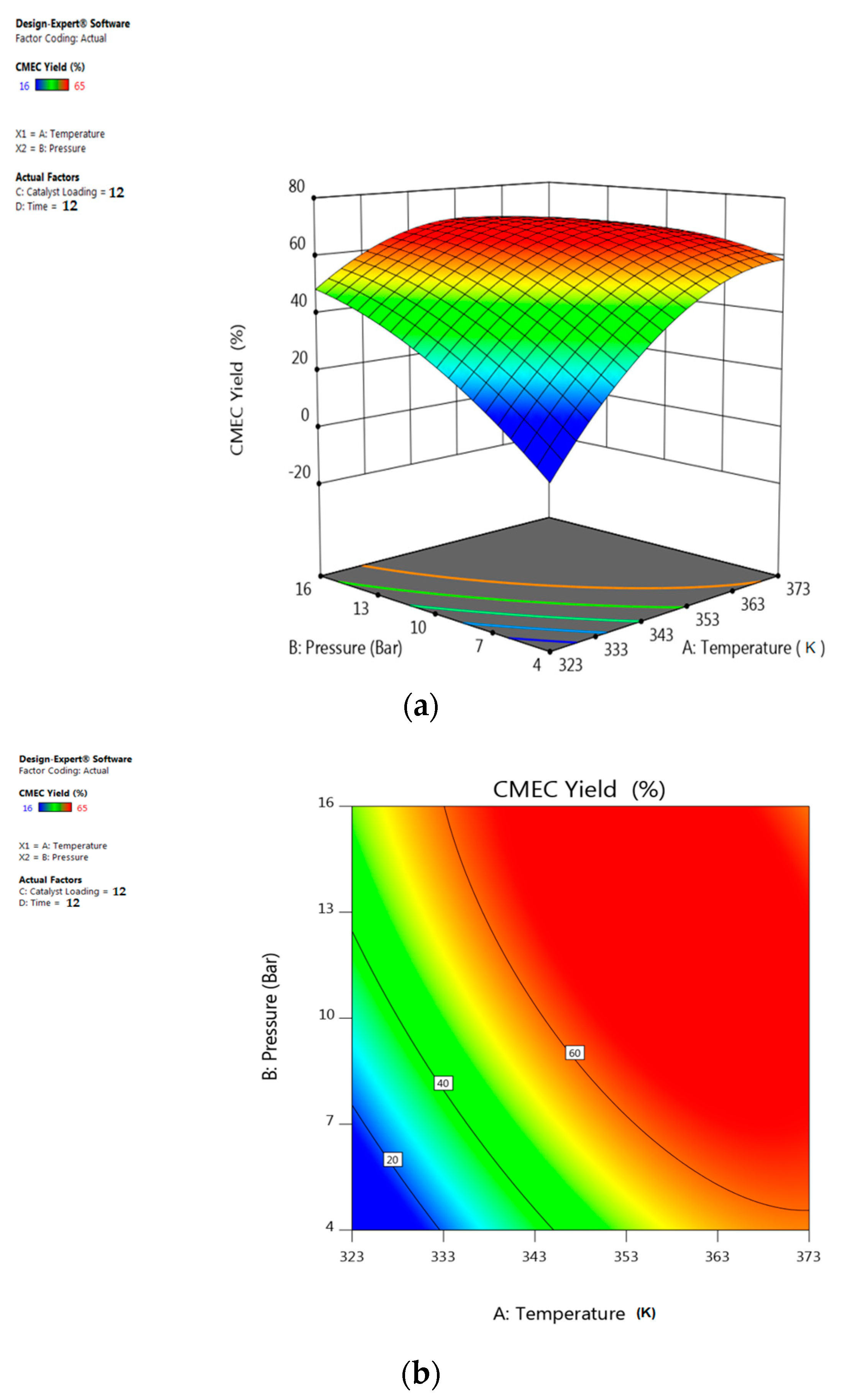
5.1. Interactive Effect of Temperature and Pressure
5.2. Interactive Effect of Temperature and Time
5.3. Interactive Effect of Temperature and Catalyst Loading
5.4. Interactive Effect of Time and Pressure
5.5. Interactive Effect of Time and Catalyst Loading
5.6. Interactive Effect of Catalyst Loading and Pressure
6. Multiobjective Process Optimization
7. Catalyst Reusability Studies
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Miao, C.-X.; Wang, J.-Q.; He, L.-N. Catalytic Processes for Chemical Conversion of Carbon Dioxide into Cyclic Carbonates and Polycarbonates. Open Org. Chem. J. 2008, 2, 68–82. [Google Scholar] [CrossRef][Green Version]
- Liu, B.; Liu, M.; Liang, L.; Sun, J. Guanidine Hydrochloride/ZnI2 as Heterogeneous Catalyst for Conversion of CO2 and Epoxides to Cyclic Carbonates under Mild Conditions. Catalysts 2015, 5, 119–130. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, T.-T.; Liu, X.-H.; Zou, K.; Deng, W.-Q. Capture and conversion of CO2 at ambient conditions by a conjugated microporous polymer. Nat. Commun. 2013, 4, 1960–1967. [Google Scholar] [CrossRef] [PubMed]
- North, M.; Pasquale, R.; Young, C. Synthesis of cyclic carbonates from epoxides and CO2. Green Chem. 2010, 12, 1514–1539. [Google Scholar] [CrossRef]
- Sathe, A.A.; Nambiar, A.M.; Sturgis, N.; Rioux, M. Synthesis of cyclic organic carbonates via catalytic oxidative carboxylation of olefins in flow reactors. Catal. Sci. Technol. 2017, 7, 2–3. [Google Scholar] [CrossRef]
- Liu, A.-H.; Li, Y.-N.; He, L.-N. Organic synthesis using carbon dioxide as phosgene-free carbonyl reagent. Pure Appl. Chem. 2012, 84, 581–602. [Google Scholar] [CrossRef]
- Maeda, C.; Miyazaki, Y.; Ema, T. Recent progress in catalytic conversions of carbon dioxide. Catal. Sci. Technol. 2014, 4, 74–82. [Google Scholar] [CrossRef]
- Adeleye, A.I.; Kellici, S.; Heil, T.; Morgan, D.; Vickers, M.; Saha, B. Greener synthesis of propylene carbonate using graphene-inorganic nanocomposite catalysts. Catal. Today 2015, 256, 347–357. [Google Scholar] [CrossRef]
- Onyenkeadi, V.; Kellici, S.; Saha, B. Greener synthesis of 1,2-butylene carbonate from CO2 using graphene-inorganic nanocomposite catalyst. Energy 2018, 165, 867–876. [Google Scholar] [CrossRef]
- Adeleye, A.I.; Patel, D.; Niyogi, D.; Saha, B. Efficient and greener synthesis of propylene carbonate from carbon dioxide and propylene oxide. Ind. Eng. Chem. Res. 2014, 53, 18647–18657. [Google Scholar] [CrossRef]
- Sakakura, T.; Kohno, K. The synthesis of organic carbonates from carbon dioxide. Chem. Commun. 2009, 10, 1312–1330. [Google Scholar] [CrossRef] [PubMed]
- Shukla, K.; Srivastava, V.C. Synthesis of organic carbonates from alcoholysis of urea: A review. Catal. Rev. Sci. Eng. 2017, 59, 1–43. [Google Scholar] [CrossRef]
- Dalpozzo, R.; Ca, N.D.; Gabriele, B.; Mancuso, R. Recent advances in the chemical fixation of carbon dioxide: A green route to carbonylated heterocycle synthesis. Catalysts 2019, 9, 511. [Google Scholar] [CrossRef]
- Lu, X.B.; Liang, B.; Zhang, Y.J.; Tian, Y.Z.; Wang, Y.M.; Bai, C.X.; Wang, H.; Zhang, R. Asymmetric Catalysis with CO2: Direct Synthesis of Optically Active Propylene Carbonate from Racemic Epoxides. J. Am. Chem. Soc. 2004, 126, 3732–3733. [Google Scholar] [CrossRef]
- Paddock, R.L.; Nguyen, S.T. Chemical CO2 fixation: CR(III) salen complexes as highly efficient catalysts for the coupling of CO2 and epoxides. J. Am. Chem. Soc. 2001, 123, 11498–11499. [Google Scholar] [CrossRef]
- Caló, V.; Nacci, A.; Monopoli, A.; Fanizzi, A. Cyclic carbonate formation from carbon dioxide and oxiranes in tetrabutylammonium halides as solvents and catalysts. Org. Lett. 2002, 4, 2561–2563. [Google Scholar] [CrossRef]
- He, Q.; O’Brien, J.W.; Kitselman, K.A.; Tompkins, L.E.; Curtis, G.C.T.; Kerton, F.M. Synthesis of cyclic carbonates from CO2 and epoxides using ionic liquids and related catalysts including choline chloride-metal halide mixtures. Catal. Sci. Technol. 2014, 4, 1513–1528. [Google Scholar] [CrossRef]
- Olaniyan, B.; Saha, B. Comparison of Catalytic Activity of ZIF-8 and Zr/ZIF-8 for Greener Synthesis of Chloromethyl Ethylene Carbonate by CO2 Utilization. Energies 2020, 13, 521. [Google Scholar] [CrossRef]
- Kihara, N.; Hara, N.; Endo, T. Catalytic Activity of Various Salts in the Reaction of 2,3-Epoxypropyl Phenyl Ether and Carbon Dioxide under Atmospheric Pressure. J. Org. Chem. 1993, 58, 6198–6202. [Google Scholar] [CrossRef]
- Castro-Osma, J.A.; Lamb, K.J.; North, M. Cr(salophen) Complex Catalyzed Cyclic Carbonate Synthesis at Ambient Temperature and Pressure. ACS Catal. 2016, 6, 5012–5025. [Google Scholar] [CrossRef]
- Rulev, Y.A.; Larionov, V.A.; Lokutova, A.V.; Moskalenko, M.A.; Lependina, O.L.; Maleev, V.I.; North, M.; Belokon, Y.N. Chiral Cobalt(III) Complexes as Bifunctional Brønsted Acid-Lewis Base Catalysts for the Preparation of Cyclic Organic Carbonates. ChemSusChem 2016, 9, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Jutz, F.; Grunwaldt, J.D.; Baiker, A. Mn(III)(salen)-catalyzed synthesis of cyclic organic carbonates from propylene and styrene oxide in ‘supercritical’ CO2. J. Mol. Catal. A Chem. 2008, 279, 94–103. [Google Scholar] [CrossRef]
- Kuznetsova, S.A.; Rulev, Y.A.; Larionov, V.A.; Smol’yakov, A.F.; Zubavichus, Y.V.; Maleev, V.I.; Li, H.; North, M.; Saghyan, A.S.; Belokon, Y.N. Self-Assembled Ionic Composites of Negatively Charged Zn(salen) Complexes and Triphenylmethane Derived Polycations as Recyclable Catalysts for the Addition of Carbon Dioxide to Epoxides. ChemCatChem 2019, 11, 511–519. [Google Scholar] [CrossRef]
- Luo, R.; Zhou, X. Metal- and solvent-free synthesis of cyclic carbonates from epoxides and CO2 in the presence of graphite oxide and ionic liquid under mild conditions: A kinetic study. Carbon 2014, 82, 1–11. [Google Scholar] [CrossRef]
- Siewniak, A.; Jasiak, K.; Baj, S. An efficient method for the synthesis of cyclic carbonates from CO2 and epoxides using an effective two-component catalyst system: Polymer-supported quaternary onium salts and aqueous solutions of metal salts. Appl. Catal. A Gen. 2014, 482, 266–274. [Google Scholar] [CrossRef]
- Ma, D.; Li, B.; Liu, K.; Zhang, X.; Zou, W.; Yang, Y.; Li, G.; Shi, Z.; Feng, S. Bifunctional MOF heterogeneous catalysts based on the synergy of dual functional sites for efficient conversion of CO2 under mild and co-catalyst free conditions. J. Mater. Chem. A 2015, 3, 23136–23142. [Google Scholar] [CrossRef]
- Saptal, V.B.; Bhanage, B.M. Current advances in heterogeneous catalysts for the synthesis of cyclic carbonates from carbon dioxide. Curr. Opin. Green Sustain. Chem. 2017, 3, 1–10. [Google Scholar] [CrossRef]
- Adeleye, A.I. (Supervisor-Saha. B) Heterogeneous Catalytic Conversion of Carbon Dioxide to Value Added Chemicals. Ph.D. Thesis, London South Bank University, London, UK, 2015. [Google Scholar]
- Zalomaeva, O.V.; Chibiryaev, A.M.; Kovalenko, K.A.; Kholdeeva, O.A.; Balzhinimaev, B.S.; Fedin, V.P. Cyclic carbonates synthesis from epoxides and CO2 over metal-organic framework Cr-MIL-101. J. Catal. 2013, 298, 179–185. [Google Scholar] [CrossRef]
- Beyzavi, M.H.; Stephenson, C.J.; Liu, Y.; Karagiaridi, O.; Hupp, J.T.; Farha, O.K. Metal Organic Framework-Based Catalysts: Chemical Fixation of CO2 with Epoxides Leading to Cyclic Organic Carbonates. Front. Energy Res. 2015, 2, 1–10. [Google Scholar] [CrossRef]
- Li, Q.; Yang, W.; Li, F.; Cui, A.; Hong, J. Preparation of CoB/ZIF-8 supported catalyst by single step reduction and its activity in hydrogen production. Int. J. Hydrogen Energy 2018, 43, 271–282. [Google Scholar] [CrossRef]
- Jumbri, K.; Al-Haniff Rozy, M.F.; Ashari, S.E.; Mohamad, R.; Basri, M.; Fard Masoumi, H.R. Optimisation and characterisation of lipase catalysed synthesis of a kojic monooleate ester in a solvent-free system by response surface methodology. PLoS ONE 2015, 10, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Koech, J.K.; Mutiso, M.J.; Koskei, J.K. Response Surface Methodology Approach to the Optimization of Potato (Solanum tuberosum) Tuber Yield Using Second-Order Rotatable Design. J. Biom. Biostat. 2017, 8, 22–33. [Google Scholar]
- Manohar, M.; Joseph, J.; Selvaraj, T.; Sivakumar, D. Application of box Behnken design to optimize the parameters for turning inconel 718 using coated carbide tools. Int. J. Sci. Eng. Res. 2013, 4, 620–642. [Google Scholar]
- Jeirani, Z.; Mohamed Jan, B.; Si Ali, B.; Mohd Noor, I.; Chun Hwa, S.; Saphanuchart, W. The optimal mixture design of experiments: Alternative method in optimizing the aqueous phase composition of a microemulsion. Chemom. Intell. Lab. Syst. 2012, 112, 1–7. [Google Scholar] [CrossRef]
- Khuri, A.I.; Mukhopadhyay, S. Response surface methodology. Wiley Interdiscip. Rev. Comput. Stat. 2010, 2, 128–149. [Google Scholar] [CrossRef]
- Saada, R.; AboElazayem, O.; Kellici, S.; Heil, T.; Morgan, D.; Lampronti, G.I.; Saha, B. Greener synthesis of dimethyl carbonate using a novel tin-zirconia/graphene nanocomposite catalyst. Appl. Catal. B Environ. 2018, 226, 451–462. [Google Scholar] [CrossRef]
- Aboelazayem, O.; El-Gendy, N.S.; Abdel-Rehim, A.A.; Ashour, F.; Sadek, M.A. Biodiesel production from castor oil in Egypt: Process optimisation, kinetic study, diesel engine performance and exhaust emissions analysis. Energy 2018, 157, 843–852. [Google Scholar] [CrossRef]
- Onyenkeadi, V.; Aboelazayem, O.; Saha, B. Systematic multivariate optimisation of butylene carbonate synthesis via CO2 utilisation using graphene-inorganic nanocomposite catalysts. Catal. Today 2019, 7, 1–13. [Google Scholar] [CrossRef]
- Kumar, A.; Gupta, M.; Mazumder, A.; Poluri, K.M.; Rao, V.K. Use of Box Behnken Design for Development of High Throughput Quantitative Proton Nuclear Magnetic Resonance Experiments for Industrial Applications. Ind. Eng. Chem. Res. 2017, 56, 2873–2882. [Google Scholar] [CrossRef]
- Sadeghi, N.; Sharifnia, S.; Do, T.O. Optimization and modeling of CO2 photoconversion using a response surface methodology with porphyrin-based metal organic framework. React. Kinet. Mech. Catal. 2018, 125, 411–431. [Google Scholar] [CrossRef]
- Yu, X.L.; He, Y. Application of Box-Behnken designs in parameters optimization of differential pulse anodic stripping voltammetry for lead(II) determination in two electrolytes. Sci. Rep. 2017, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Huang, J.; Wu, G.; Tong, J.; Xie, G.; Duan, J.A.; Qin, M. Multiple responses optimization of ultrasonic-assisted extraction by response surface methodology (RSM) for rapid analysis of bioactive compounds in the flower head of Chrysanthemum morifolium Ramat. Ind. Crops Prod. 2015, 74, 192–199. [Google Scholar] [CrossRef]
- Nwabueze, T.U. Basic steps in adapting response surface methodology as mathematical modelling for bioprocess optimisation in the food systems. Int. J. Food Sci. Technol. 2010, 45, 1768–1776. [Google Scholar] [CrossRef]
- Mostafaei, M.; Ghobadian, B.; Barzegar, M.; Banakar, A. Optimization of ultrasonic assisted continuous production of biodiesel using response surface methodology. Ultrason. Sonochem. 2015, 27, 54–61. [Google Scholar] [CrossRef]
- Schejn, A.; Aboulaich, A.; Balan, L.; Falk, V.; Lalevée, J.; Medjahdi, G.; Aranda, L.; Mozet, K.; Schneider, R. Cu2+ -doped zeolitic imidazolate frameworks (ZIF-8): Efficient and stable catalysts for cycloadditions and condensation reactions. Catal. Sci. Technol. 2015, 5, 1829–1839. [Google Scholar] [CrossRef]
- Thi Thanh, M.; Vinh Thien, T.; Thi Thanh Chau, V.; Dinh Du, P.; Phi Hung, N.; Quang Khieu, D. Synthesis of Iron Doped Zeolite Imidazolate Framework-8 and Its Remazol Deep Black RGB Dye Adsorption Ability. J. Chem. 2017, 8, 635–650. [Google Scholar] [CrossRef]
- Zhou, K.; Mousavi, B.; Luo, Z.; Phatanasri, S.; Chaemchuen, S.; Verpoort, F. Characterization and properties of Zn/Co zeolitic imidazolate frameworks vs. ZIF-8 and ZIF-67. J. Mater. Chem. A 2017, 5, 952–957. [Google Scholar] [CrossRef]
- Saada, R. (Supervisor-Saha. B) Catalytic Conversion of Carbon Dioxide (CO2) into Value Added Chemicals. Ph.D. Thesis, London South Bank University, London, UK, 2015. [Google Scholar]
- Gallardo-Fuentes, S.; Contreras, R.; Isaacs, M.; Honores, J.; Quezada, D.; Landaeta, E.; Ormazábal-Toledo, R. On the mechanism of CO2 electro-cycloaddition to propylene oxides. J. CO2 Util. 2016, 16, 114–120. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, F.; Ni, L.; Meng, M.; Meng, X.; Zhong, G.; Qiu, J. A modeling study by response surface methodology (RSM) on Sr(II) ion dynamic adsorption optimization using a novel magnetic ion imprinted polymer. RSC Adv. 2016, 6, 54679–54692. [Google Scholar] [CrossRef]
- Mäkelä, M. Experimental design and response surface methodology in energy applications: A tutorial review. Energy Convers. Manag. 2017, 151, 630–640. [Google Scholar] [CrossRef]
- Zhu, Z.; Rosendahl, L.; Toor, S.S.; Chen, G. Optimizing the conditions for hydrothermal liquefaction of barley straw for bio-crude oil production using response surface methodology. Sci. Total Environ. 2018, 630, 560–569. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.W.; Marasini, N.; Poudel, B.K.; Kim, J.H.; Cho, H.J.; Moon, B.K.; Choi, H.G.; Yong, C.S.; Kim, J.O. Application of Box-Behnken design in the preparation and optimization of fenofibrate-loaded self-microemulsifying drug delivery system (SMEDDS). J. Microencapsul. 2014, 31, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Rabiee, F.; Mahanpoor, K. Catalytic oxidation of SO2 by novel Mn/copper slag nanocatalyst and optimization by Box-Behnken design. Int. J. Ind. Chem. 2018, 9, 27–38. [Google Scholar] [CrossRef]
- Long, X.; Cai, L.; Li, W. RSM-based assessment of pavement concrete mechanical properties under joint action of corrosion, fatigue, and fiber content. Constr. Build. Mater. 2019, 197, 406–420. [Google Scholar] [CrossRef]
- Mohammadifard, H.; Amiri, M.C. On tailored synthesis of nano CaCO3 particles in a colloidal gas aphron system and evaluating their performance with response surface methodology for heavy metals removal from aqueous solutions. J. Water Environ. Nanotechnol. 2018, 3, 141–149. [Google Scholar]
- Kilic, A.; Durgun, M.; Aytar, E.; Yavuz, R. Synthesis and characterization of novel positively charged organocobaloximes as catalysts for the fi xation of CO2 to cyclic carbonates. J. Organomet. Chem. 2018, 858, 78–88. [Google Scholar] [CrossRef]
- Abhang, R.M.; Wani, K.S.; Patil, V.S. Synthesis and Characterization of ZIF-8 Filler for preparation of Mixed Matrix Membrane. Int. J. Sci. Eng. Res. 2015, 6, 1276–1280. [Google Scholar]
- Zhong, S.; Liang, L.; Liu, B.; Sun, J. ZnBr2/DMF as simple and highly active Lewis acid–base catalysts for the cycloaddition of CO2 to propylene oxide. J. CO2 Util. 2014, 6, 75–79. [Google Scholar] [CrossRef]
- Shi, J.; Song, J.; Ma, J.; Zhang, Z.; Fan, H.; Han, B. Effective synthesis of cyclic carbonates from CO2 and epoxides catalyzed by KI/cucurbit[6]uril. Pure Appl. Chem. 2013, 85, 1633–1641. [Google Scholar] [CrossRef]
- Xuan, K.; Pu, Y.; Li, F.; Li, A.; Luo, J.; Li, L.; Wang, F.; Zhao, N. Direct synthesis of dimethyl carbonate from CO2 and methanol over tri fl uoroacetic acid modulated UiO-66. J. CO2 Util. 2018, 27, 272–282. [Google Scholar] [CrossRef]
- Han, X.; Zhu, G.; Ding, Y.; Miao, Y.; Wang, K.; Zhang, H.; Wang, Y.; Liu, S. Bin Selective catalytic synthesis of glycerol monolaurate over silica gel-based sulfonic acid functionalized ionic liquid catalysts. Chem. Eng. J. 2019, 359, 733–745. [Google Scholar] [CrossRef]
- Bahrami, M.; Amiri, M.J.; Bagheri, F. Optimization of the lead removal from aqueous solution using two starch based adsorbents: Design of experiments using response surface methodology (RSM). J. Environ. Chem. Eng. 2019, 7, 700–793. [Google Scholar] [CrossRef]
- Nandiwale, K.Y.; Bokade, V.V. Process optimization by response surface methodology and kinetic modeling for synthesis of methyl oleate biodiesel over H3PW12O40 anchored montmorillonite K10. Ind. Eng. Chem. Res. 2014, 53, 18690–18698. [Google Scholar] [CrossRef]
- Peng, J.; Wang, S.; Yang, H.; Ban, B.; Wei, Z.; Wang, L.; Lei, B. Highly e ffi cient fi xation of carbon dioxide to cyclic carbonates with new multi-hydroxyl bis- (quaternary ammonium ) ionic liquids as metal-free catalysts under mild conditions. Fuel 2018, 224, 481–488. [Google Scholar] [CrossRef]
- Baş, D.; Boyacı, İ.H. Modeling and optimization I: Usability of response surface methodology. J. Food Eng. 2007, 78, 836–845. [Google Scholar] [CrossRef]
- Bezerra, M.A.; Santelli, R.E.; Oliveira, E.P.; Villar, L.S.; Escaleira, L.A. Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta 2008, 76, 965–977. [Google Scholar] [CrossRef]
- Feilizadeh, M.; Rahimi, M.; Zakeri, S.M.E.; Mahinpey, N.; Vossoughi, M.; Qanbarzadeh, M. Individual and interaction effects of operating parameters on the photocatalytic degradation under visible light illumination: Response surface methodological approach. Can. J. Chem. Eng. 2017, 95, 1228–1235. [Google Scholar] [CrossRef]
- Zhang, Y.; Yin, S.; Luo, S.; Au, C.T. Cycloaddition of CO2 to epoxides catalyzed by carboxyl-functionalized imidazolium-based ionic liquid grafted onto cross-linked polymer. Ind. Eng. Chem. Res. 2012, 51, 3951–3957. [Google Scholar] [CrossRef]
- Bosch, M.; Zhang, M.; Zhou, H.-C. Increasing the Stability of Metal-Organic Frameworks. Adv. Chem. 2014, 2014, 1–8. [Google Scholar] [CrossRef]




| Variables | Code | Range and Levels | ||
|---|---|---|---|---|
| −1 | 0 | +1 | ||
| Temperature (K) | x1 | 313 | 353 | 373 |
| Pressure (bar) | x2 | 4 | 8 | 16 |
| Catalyst loading (w/w) | x3 | 5 | 7 | 15 |
| Time (h) | x4 | 4 | 8 | 16 |
| Run | T x1 (K) | P x2 (bar) | t x3 (h) | Catalyst Loading x4 (w/w) | Actual ECH Conv. (%) | Predicted ECH Conv. (%) | Actual CMEC Yield (%) | Predicted CMEC Yield (%) |
|---|---|---|---|---|---|---|---|---|
| 1 | 313 | 4 | 8 | 7 | 42 | 46.33 | 16 | 14.63 |
| 2 | 353 | 8 | 16 | 5 | 67 | 68.17 | 33 | 32.29 |
| 3 | 353 | 8 | 8 | 7 | 84 | 84.00 | 64 | 64.00 |
| 4 | 313 | 16 | 16 | 7 | 58 | 59.88 | 29 | 31.67 |
| 5 | 353 | 8 | 8 | 7 | 84 | 84.00 | 64 | 64.00 |
| 6 | 353 | 8 | 4 | 5 | 52 | 55.67 | 26 | 26.46 |
| 7 | 353 | 4 | 16 | 7 | 75 | 72.96 | 40 | 41.04 |
| 8 | 313 | 8 | 8 | 5 | 54 | 55.79 | 23 | 24.88 |
| 9 | 353 | 16 | 16 | 7 | 93 | 93.29 | 65 | 66.04 |
| 10 | 313 | 8 | 8 | 15 | 58 | 56.46 | 31 | 30.38 |
| 11 | 353 | 16 | 8 | 5 | 86 | 81.21 | 36 | 35.00 |
| 12 | 373 | 16 | 8 | 7 | 86 | 81.67 | 45 | 46.13 |
| 13 | 373 | 4 | 8 | 15 | 75 | 82.33 | 54 | 57.63 |
| 14 | 353 | 16 | 8 | 15 | 88 | 91.38 | 68 | 68.00 |
| 15 | 373 | 8 | 4 | 7 | 68 | 62.38 | 38 | 33.33 |
| 16 | 353 | 8 | 8 | 7 | 84 | 84.00 | 64 | 64.00 |
| 17 | 373 | 8 | 16 | 15 | 90 | 85.38 | 64 | 60.67 |
| 18 | 373 | 16 | 8 | 5 | 54 | 59.29 | 26 | 28.88 |
| 19 | 313 | 16 | 8 | 7 | 90 | 82.67 | 55 | 51.13 |
| 20 | 373 | 8 | 8 | 15 | 86 | 87.96 | 64 | 64.38 |
| 21 | 353 | 4 | 4 | 7 | 68 | 64.46 | 35 | 36.21 |
| 22 | 353 | 8 | 8 | 15 | 84 | 84.00 | 64 | 64.00 |
| 23 | 353 | 8 | 8 | 7 | 84 | 84.00 | 64 | 64.00 |
| 24 | 353 | 16 | 4 | 15 | 70 | 75.79 | 35 | 36.21 |
| 25 | 313 | 8 | 4 | 15 | 52 | 52.88 | 23 | 24.33 |
| 26 | 353 | 8 | 16 | 15 | 89 | 85.33 | 65 | 64.29 |
| 27 | 353 | 4 | 8 | 5 | 66 | 58.88 | 37 | 33.50 |
| 28 | 353 | 4 | 8 | 15 | 77 | 78.04 | 44 | 43.00 |
| 29 | 373 | 8 | 4 | 15 | 69 | 67.83 | 35 | 35.46 |
| Source | Sum of Square | Diff. | Mean Square | F Value | p-Value | Significance |
|---|---|---|---|---|---|---|
| Model | 5014.09 | 14 | 362.01 | 11.21 | <0.0001 | HS |
| x1-temperature | 827.75 | 1 | 827.75 | 26.02 | 0.0001 | HS |
| x2-pressure | 854.08 | 1 | 854.08 | 27.10 | <0.0001 | HS |
| x3-catalyst loading | 871.33 | 1 | 871.33 | 18.68 | 0.0006 | HS |
| x4-reaction time | 619.00 | 1 | 619.00 | 21.58 | 0.0005 | HS |
| x1x2 | 308.25 | 1 | 308.25 | 9.44 | 0.0060 | HS |
| x1x3 | 177.00 | 1 | 177.00 | 4.98 | 0.0283 | S |
| x1x4 | 58.00 | 1 | 58.00 | 1.95 | 0.1842 | NS |
| x2x3 | 18.25 | 1 | 18.25 | 0.62 | 0.4451 | NS |
| x2x4 | 38.25 | 1 | 38.25 | 1.29 | 0.2754 | NS |
| x3x4 | 5.15 | 1 | 5.15 | 0.19 | 0.6691 | NS |
| x12 | 789.39 | 1 | 789.39 | 25.73 | 0.0001 | HS |
| x22 | 4.16 | 1 | 4.16 | 0.15 | 0.7030 | NS |
| x32 | 353.82 | 1 | 353.82 | 11.13 | 0.0049 | S |
| x42 | 336.95 | 1 | 336.95 | 9.40 | 0.0061 | S |
| Residual | 448.08 | 14 | 34.86 | |||
| Lack of Fit | 448.08 | 10 | 44.81 | 0.44 | 0.56 | NS |
| Pure Error | 0.000 | 4 | 0.000 | |||
| Cor Total | 5553.17 | 28 |
| Sum of Square | Difference | Mean Square | F Value | p-Value | Significance | |
|---|---|---|---|---|---|---|
| Model | 7335.55 | 14 | 431.90 | 68.68 | <0.0001 | HS |
| x1-temperature | 1023.00 | 1 | 1023.00 | 139.85 | <0.0001 | HS |
| x2-pressure | 468.75 | 1 | 468.75 | 60.53 | <0.0001 | HS |
| x3-catalyst loading | 1260.75 | 1 | 1260.75 | 162.80 | <0.0001 | HS |
| x4-time | 901.33 | 1 | 901.33 | 116.39 | <0.0001 | HS |
| x1x2 | 576.00 | 1 | 576.00 | 74.38 | <0.0001 | HS |
| x1x3 | 225.00 | 1 | 225.00 | 29.05 | <0.0001 | HS |
| x1x4 | 100.00 | 1 | 100.00 | 12.91 | 0.0029 | HS |
| x2x3 | 119.00 | 1 | 119.00 | 15.62 | 0.0014 | HS |
| x2x4 | 146.25 | 1 | 146.25 | 20.18 | 0.0005 | HS |
| x3x4 | 128.25 | 1 | 128.25 | 17.08 | 0.0010 | HS |
| x12 | 1258.78 | 1 | 1258.78 | 176.11 | <0.0001 | HS |
| x22 | 347.29 | 1 | 347.29 | 42.52 | <0.0001 | HS |
| x32 | 897.34 | 1 | 897.34 | 128.27 | <0.0001 | HS |
| x42 | 897.05 | 1 | 897.05 | 120.62 | <0.0001 | HS |
| Residual | 104.24 | 14 | 7.87 | |||
| Lack of Fit | 104.24 | 10 | 10.43 | 1.35 | 0.325 | NS |
| Pure Error | 0.000 | 4 | 0.000 | |||
| Cor Total | 7444.79 | 28 |
| Factor | Code | Goal | Limits | |
|---|---|---|---|---|
| Lower | Upper | |||
| Temperature (K) | x1 | Minimize | 313 | 373 |
| Pressure (bar) | x2 | In range | 2 | 16 |
| Catalyst loading (%) | x3 | In range | 5 | 15 |
| Time (h) | x4 | Minimize | 2 | 16 |
| ECH conversion | Y1 | Maximize | 60 | 93 |
| CMEC yield | Y2 | Maximize | 30 | 68 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olaniyan, B.; Saha, B. Multiobjective Optimization for the Greener Synthesis of Chloromethyl Ethylene Carbonate by CO2 and Epichlorohydrin via Response Surface Methodology. Energies 2020, 13, 741. https://doi.org/10.3390/en13030741
Olaniyan B, Saha B. Multiobjective Optimization for the Greener Synthesis of Chloromethyl Ethylene Carbonate by CO2 and Epichlorohydrin via Response Surface Methodology. Energies. 2020; 13(3):741. https://doi.org/10.3390/en13030741
Chicago/Turabian StyleOlaniyan, Bisi, and Basudeb Saha. 2020. "Multiobjective Optimization for the Greener Synthesis of Chloromethyl Ethylene Carbonate by CO2 and Epichlorohydrin via Response Surface Methodology" Energies 13, no. 3: 741. https://doi.org/10.3390/en13030741
APA StyleOlaniyan, B., & Saha, B. (2020). Multiobjective Optimization for the Greener Synthesis of Chloromethyl Ethylene Carbonate by CO2 and Epichlorohydrin via Response Surface Methodology. Energies, 13(3), 741. https://doi.org/10.3390/en13030741






