Possibility of Calcium Oxide from Natural Limestone Including Impurities for Chemical Heat Pump
Abstract
:1. Introduction
2. Operating Principle of CaO/H2O/Ca(OH)2 Chemical Heat Pump
3. Sample Materials
4. Experimental Analysis
4.1. Repeated Hydration Reaction Cycle (Muffle Furnace and TGA-51)
4.2. Repeated Hydration Reaction Cycle (TGA-51)
5. Results and Discussion
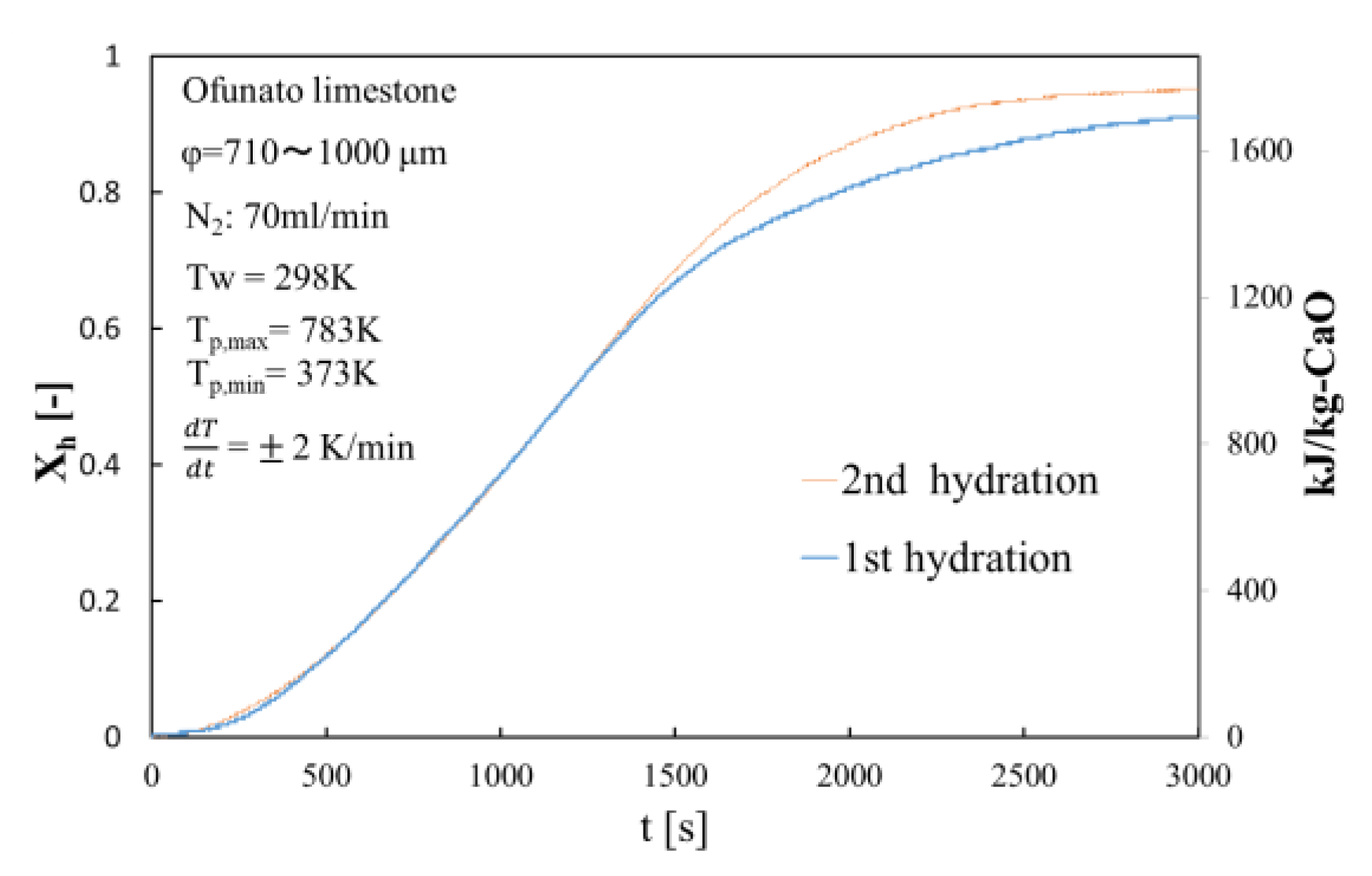
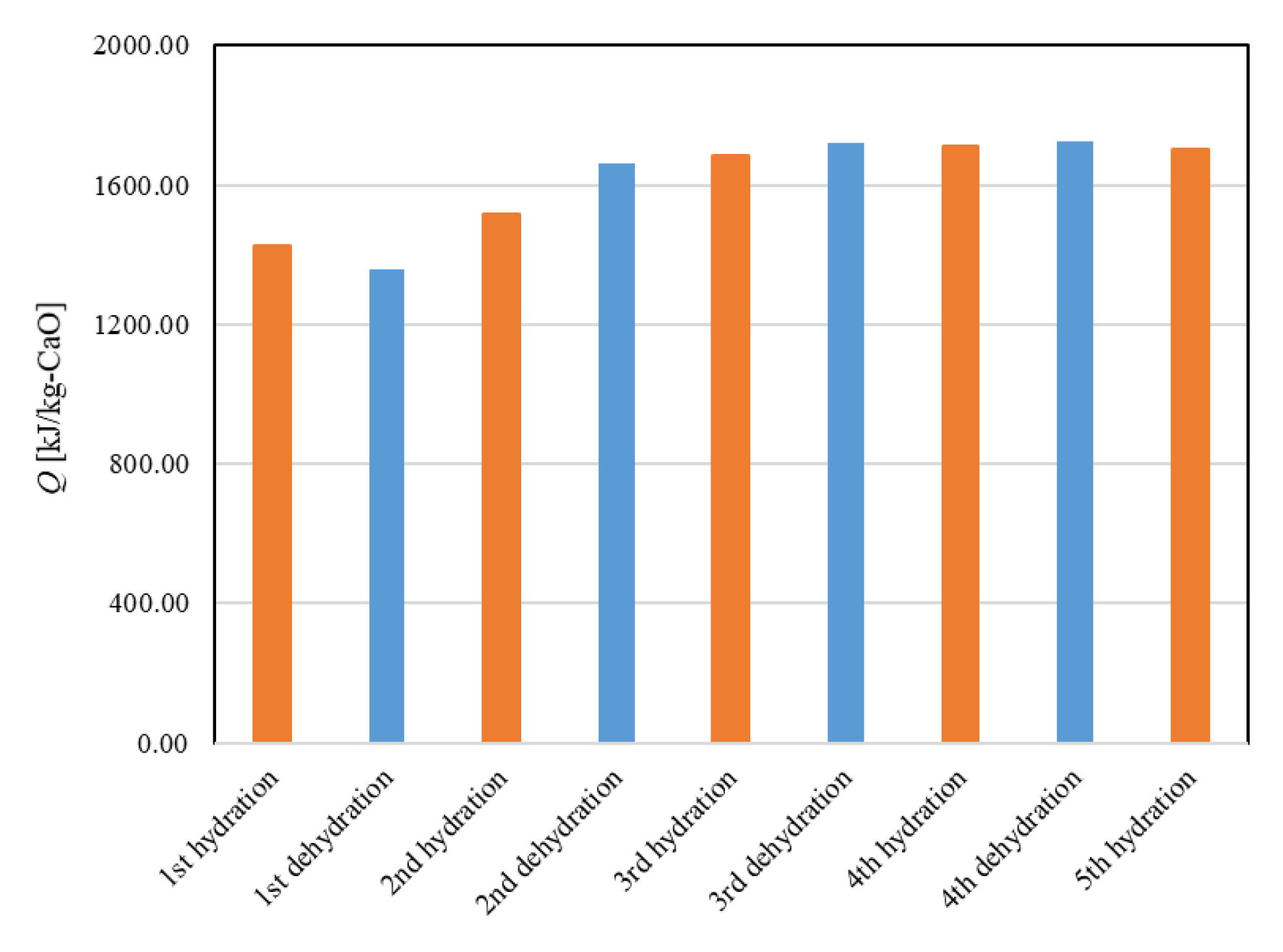
5.1. Repeated Hydration Experiments of 3 Different Kinds of CaO
5.2. Effects of Different Decarbonization Temperature
5.3. Effects of Decarbonization Conditions
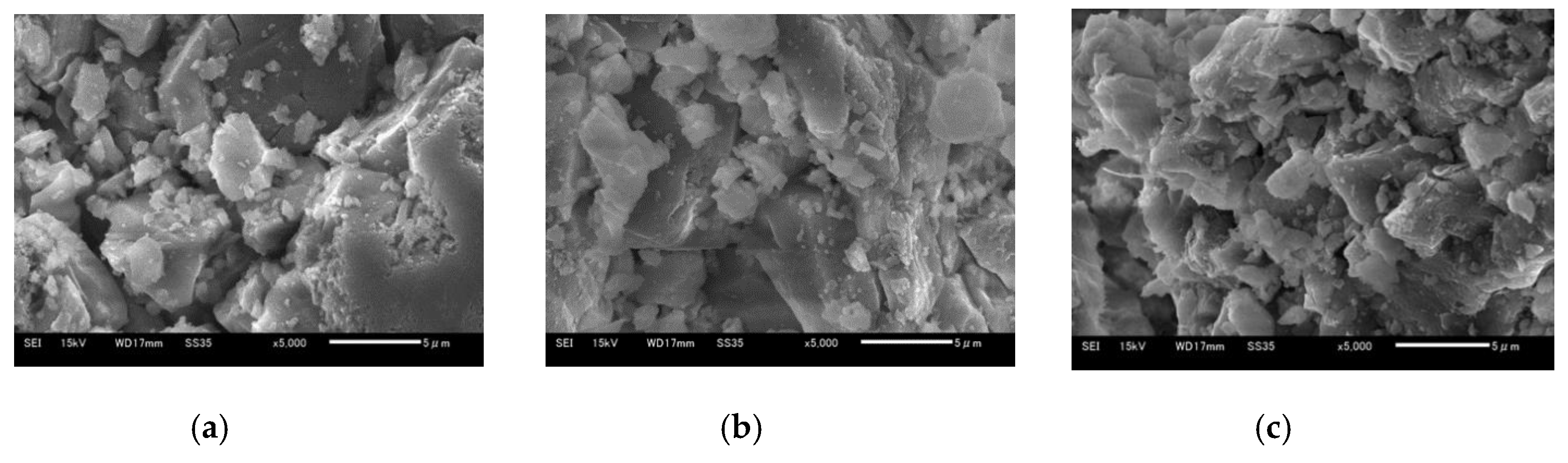
5.4. Crystal Grain Structure
5.5. Effects of Impurities in Ofunato CaO
6. Conclusions
- The high temperature sintering of impurities in Ofunato limestone occurs easier than that in Kawara limestone with lower impurities.
- In the first hydration reaction, the reaction rate of Ofunato CaO is relatively low. However, the reactivity of Ofunato CaO could be improved by repeated hydration reaction experiments.
- For practical development of a chemical heat pump, it is viable to use cheaper Ofunato limestone instead of high quality “kansuiseki” (Kawara limestone), which is more expensive.
- The impurities adhered to the surface of the CaO particles make the specific surface area of CaO particles smaller, which could inhibit the hydration reactions of the CaO particles.
- The adverse effects of impurities on the hydration characteristics can be minimalized by controlling the decarbonization temperature and holding time.
Author Contributions
Funding
Conflicts of Interest
References
- Ogura, H.; Yamamoto, T.; Kage, H. Efficiencies of CaO/H2O/Ca(OH)2 chemical heat pump for heat storing and heating/cooling. Energy 2003, 28, 1479–1493. [Google Scholar] [CrossRef]
- Ogura, H.; Hamaguchi, N.; Kage, H.; Mujumdar, A.S. Energy and cost estimation for application of chemical heat pump dryer to industrial ceramics drying. Dry. Technol. 2004, 22, 307–323. [Google Scholar] [CrossRef]
- Ogura, H.; Saigusa, A.; Shimadu, T. Fundamental experiments for independent heating/cooling system driven by solar energy using CaSO4/ CaSO4⋅1/2H2O chemical heat pump. In Proceedings of the Japan Solar Energy Society 2013, Naha, Japan, 28–29 November 2013; p. 122. [Google Scholar]
- Ogura, H.; Haba, Y.; Kobayashi, E.; Ren, Y.; Aoki, R.; Hirose, Y. Improvement of Solar Chemical Heat Pump Performance for Refrigeration/Cooling/Heating. In Proceedings of the Grand Renewable Energy 2018, Yokohama, Japan, 17–22 June 2018; p. a90458. [Google Scholar]
- Ogura, H.; Fujita, H. Possibility of waste heat recycle utilization for automobiles by chemical heat pump. Trans. JSAE 2016, 47, 579–585. [Google Scholar] [CrossRef]
- Kuwata, K.; Esaki, T.; Yasuda, M.; Matsuda, T.; Kobayashi1, N.; Shiren, Y.; Aman, Y. Durability of thermochemical heat storage demonstrated through long-term repetitive CaCl2/H2O reversible reactions. J. Renew. Sustain. Energy 2017, 9, 024102. [Google Scholar] [CrossRef]
- Li, J.; Zeng, T.; Kobayashi, N.; Xu, H.; Bai, Y.; Deng, L.; He, Z.; Huang, H. Lithium Hydroxide Reaction for Low Temperature Chemical Heat Storage: Hydration and Dehydration Reaction. Energies 2019, 12, 3741. [Google Scholar] [CrossRef] [Green Version]
- Ryo, K.; Junichi, R. Effect of LiOH Addition on Dehydration Reaction of Mg(OH)2. J. Chem. Eng. Jpn. 2019, 52, 152–158. [Google Scholar] [CrossRef] [Green Version]
- Ogura, H.; Haguro, M.; Shibata, Y.; Otsubo, Y. Reaction characteristics of CaSO4/CaSO4⋅1/2H2O reversible reaction for chemical heat pump. J. Chem. Eng. Jpn. 2007, 40, 1252–1256. [Google Scholar] [CrossRef] [Green Version]
- Esaki, T.; Kobayashi, N. Reaction rate characteristics of SrBr2 hydration system for chemical heat pump cooling mode. J. Mater. Sci. Chem. Eng. 2016, 4, 106–115. [Google Scholar] [CrossRef] [Green Version]
- Esaki, T.; Kuwata, K.; Ichinose, A.; Kobayashi, N. Reaction rate analysis with unreacted-core shell model for chemical heat pump cooling mode with SrBr2 hydration. Jpn. Soc. Mech. Eng. 2017, 83, 16-00439. [Google Scholar] [CrossRef] [Green Version]
- Kato, Y.; Yamashita, N.; Kobayashi, K.; Yoshizawa, Y. Kinetic study of the hydration of magnesium oxide for a chemical heat pump. Appl. Therm. Eng. 1996, 16, 853–862. [Google Scholar] [CrossRef]
- Tomari, K.; Kishimoto, A.; Toshima, M.; Kansha, Y.; Ishizuka, M.; Tsutsumi, A. Energy saving process for steel heating by using chemical heat pump. J. Jpn. Inst. Energy 2016, 25, 166–167. [Google Scholar] [CrossRef]
- Anzai, T.; Watanabe, T. Present status in lime industry and the future prospects. J. Soc. Inorg. Mater. Jpn. 2016, 23, 49–53. [Google Scholar]
- Zhang, H.; Ogura, H. Studies on hydration reaction rates of various size CaO particles for chemical heat storage/pump. J. Chem. Eng. Jpn. 2014, 47, 587–594. [Google Scholar] [CrossRef]
- Ogura, H.; Zhang, H.; Umezu, M.; Imai, T.; Ishii, M.; Komatsu, K. Reactivity of Garou Limestone for CaO Chemical Heat Pump/Storage. In Proceedings of the 135th Academic Conference of the Society of Inorganic Materials, Kumamoto, Japan, 16–17 November 2017; p. 45. [Google Scholar]
- Kikuchi, Y.; Ishiyama, Y.; Fijita, H.; Hirata, K.; Nakajyo, T.; Ogura, H. Performance enhancement of chemical heat pump unit driven by industrial waste gas heat. J. Jpn. Soc. Energy Resour. 2019, 40, 111–118. [Google Scholar]
- Lai, L.; Kikuchi, Y.; Ogura, H.; Imai, T.; Umezu, M.; Ishii, M. Reactivity of Calcium Oxide from Natural Limestones for Chemical Heat Pump/Storage. In Proceedings of the 137th Academic Conference of the Society of Inorganic Materials, Toyobashi, Japan, 15–16 November 2018; p. 37. [Google Scholar]
- Yoshimura, T. Characteristic and application of white crystalline limestone (kansuiseki). Limest. Assoc. Jpn. 1997, 56, 69–74. [Google Scholar]
- Tagawa, H.; Sugawara, H.; Fujimori, K. Effect of impurities on the shrinkage of limestone during burning. J. Soc. Chem. Ind. Jpn. 1959, 62, 1809–1915. [Google Scholar] [CrossRef]
- Limestone Mining Association. Japanese Limestone; Yasuki Printing Office Co., Ltd.: Tokyo, Japan, 1983; pp. 261–262. [Google Scholar]
- Zhang, H.; Ogura, H.; Umezu, M.; Imai, T.; Ishii, M. Hydration Reaction characteristics of CaO from various local limestones as chemical heat pump/storage materials. J. Mater. Sci. 2017, 52, 11360–11369. [Google Scholar] [CrossRef]
- The Society of Inorganic Materials. Cement·Gypsum·Lime Handbook, 4th ed.; Gihodo Publishing Co., Ltd.: Tokyo, Japan, 1996; pp. 337–339. ISBN 4-7655-0026-8. [Google Scholar]
- Wu, X.; Zhou, T.; Chen, Y.; Zhang, Z.; Piao, G.; Kobayashi, N.; Mori, S.; Itaya, Y. Mineral melting behavior of chinese blended coal ash under gasification condition. Asia Pac. J. Chem. Eng. 2011, 6, 220–230. [Google Scholar] [CrossRef]
- Sohn, H.Y.; Szekely, J. The effect of reaction order in non-catalytic gas-solid reactions. Chem. Eng. Sci. 1972, 27, 763–778. [Google Scholar] [CrossRef]
- Sohn, H.Y. The effects of reactant starvation and mass transfer in the rate measurement of fluid–solid reactions with small equilibrium constants. Chem. Eng. Sci. 2004, 59, 4361–4368. [Google Scholar] [CrossRef]
- Watanabe, T.; Hayashi, M.; Matsuda, H.; Hasatani, M. Enhancement of reactivity of CaO produced from limestone by means of hydration-dehydration treatment. J. Chem. Eng. Jpn. 1993, 19, 91–98. [Google Scholar] [CrossRef] [Green Version]
- Ogura, H.; Abliz, S.; Kage, H. Studies on applicability of scallop material to calcium oxide/calcium chemical heat pump. Fuel Process. Technol. 2004, 85, 1259–1269. [Google Scholar] [CrossRef]














| Sample | Ig.Ioss | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | SO3 | K2O | TiO2 | Others | Purity (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Kawara limestone | 43.22 | 0.00 | 0.03 | 0.02 | 56.08 | 0.28 | 0.00 | 0.00 | 0.00 | 0.02 | 99.66 |
| Ofunado limestone | 42.13 | 1.26 | 0.84 | 0.38 | 54.83 | 0.18 | 0.02 | 0.11 | 0.05 | 0.14 | 96.96 |
| Garou limestone | 42.94 | 2.19 | 0.13 | 0.09 | 51.50 | 2.38 | 0.05 | 0.00 | 0.00 | 0.15 | 95.01 |
| Samples | Pre-Heating | Decarbonization | Hydration | Dehydration | ||
|---|---|---|---|---|---|---|
| Temperature | ||||||
| − | 673–683 K | 1183 K | 1223 K | 783 K | 373 K | 783 K |
| Three different kinds of CaO | 7 K/min | 3 K/min | − | 3 K/min | 2 K/min | 2 K/min |
| Ofunato CaO | 10 K/min | − | 10 K/min | 2 K/min | 2 K/min | 2 K/min |
| Sample | Ofunato CaO | |
|---|---|---|
| Heat treatment temperature (K) | 1273 | 1573 |
| Specific surface area (m2/g) | 3.31 | 1.11 |
| Mean pore diameter (4V/A) (nm) | 28.41 | 16.95 |
| Sample | Ofunato CaO | Ofunato Ca(OH)2 |
|---|---|---|
| Heat treatment temperature (K) | 1223 K | 1223 K |
| Specific surface area (m2/g) | 6.69 | 12.20 |
| Fitting Coefficient: 0.69 | |||||||
|---|---|---|---|---|---|---|---|
| Element | (keV) | Mass% | Error% | Mol% | Compound | Compound Mass% | K |
| Mg K | 1.25 | 0.14 | 3.20 | 0.33 | MgO | 0.24 | 0.11 |
| Fe K | 6.40 | 0.96 | 15.96 | 1.04 | Fe3O4 | 1.33 | 1.13 |
| Al K | 1.49 | 0.16 | 3.50 | 0.16 | Al2O3 | 0.30 | 0.15 |
| Si K | 1.74 | 0.67 | 4.16 | 1.35 | SiO2 | 1.44 | 0.74 |
| Ca K | 3.69 | 69.10 | 5.34 | 97.11 | CaO | 96.69 | 97.88 |
| O | − | 28.96 | − | − | − | − | − |
| Total | − | 100.00 | − | 100.00 | − | 100.00 | − |
| Fitting Coefficient: 0.69 | |||||||
|---|---|---|---|---|---|---|---|
| Element | (keV) | Mass% | Error% | Mol% | Compound | Compound Mass% | K |
| Mg K | 1.25 | 2.40 | 2.50 | 6.06 | MgO | 3.98 | 2.56 |
| Fe K | 6.40 | 6.26 | 12.82 | 7.40 | Fe2O3 | 8.66 | 9.71 |
| Al K | 1.49 | 8.09 | 2.82 | 9.21 | Al2O3 | 15.29 | 9.84 |
| Si K | 1.74 | 10.22 | 3.62 | 22.36 | SiO2 | 21.87 | 13.64 |
| Ca K | 3.69 | 35.88 | 4.49 | 54.97 | CaO | 50.20 | 64.24 |
| O | − | 37.14 | − | − | − | − | − |
| Total | − | 100.00 | − | 100.00 | − | 100.00 | − |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lai, L.; Imai, T.; Umezu, M.; Ishii, M.; Ogura, H. Possibility of Calcium Oxide from Natural Limestone Including Impurities for Chemical Heat Pump. Energies 2020, 13, 803. https://doi.org/10.3390/en13040803
Lai L, Imai T, Umezu M, Ishii M, Ogura H. Possibility of Calcium Oxide from Natural Limestone Including Impurities for Chemical Heat Pump. Energies. 2020; 13(4):803. https://doi.org/10.3390/en13040803
Chicago/Turabian StyleLai, LanXin, Toshio Imai, Motohiro Umezu, Mamoru Ishii, and Hironao Ogura. 2020. "Possibility of Calcium Oxide from Natural Limestone Including Impurities for Chemical Heat Pump" Energies 13, no. 4: 803. https://doi.org/10.3390/en13040803
APA StyleLai, L., Imai, T., Umezu, M., Ishii, M., & Ogura, H. (2020). Possibility of Calcium Oxide from Natural Limestone Including Impurities for Chemical Heat Pump. Energies, 13(4), 803. https://doi.org/10.3390/en13040803






