Selective Hydrogenation of Phenol to Cyclohexanol over Ni/CNT in the Absence of External Hydrogen
Abstract
:1. Introduction
2. Experimental
2.1. Catalyst Characterization
2.2. Activity of Various Catalysts for the Hydrogenation of Phenol
2.3. Influence of Hydrogen-Donor Solvents
2.4. Influence of Reaction Temperature and Reaction Time
2.5. Scope of the Substrates
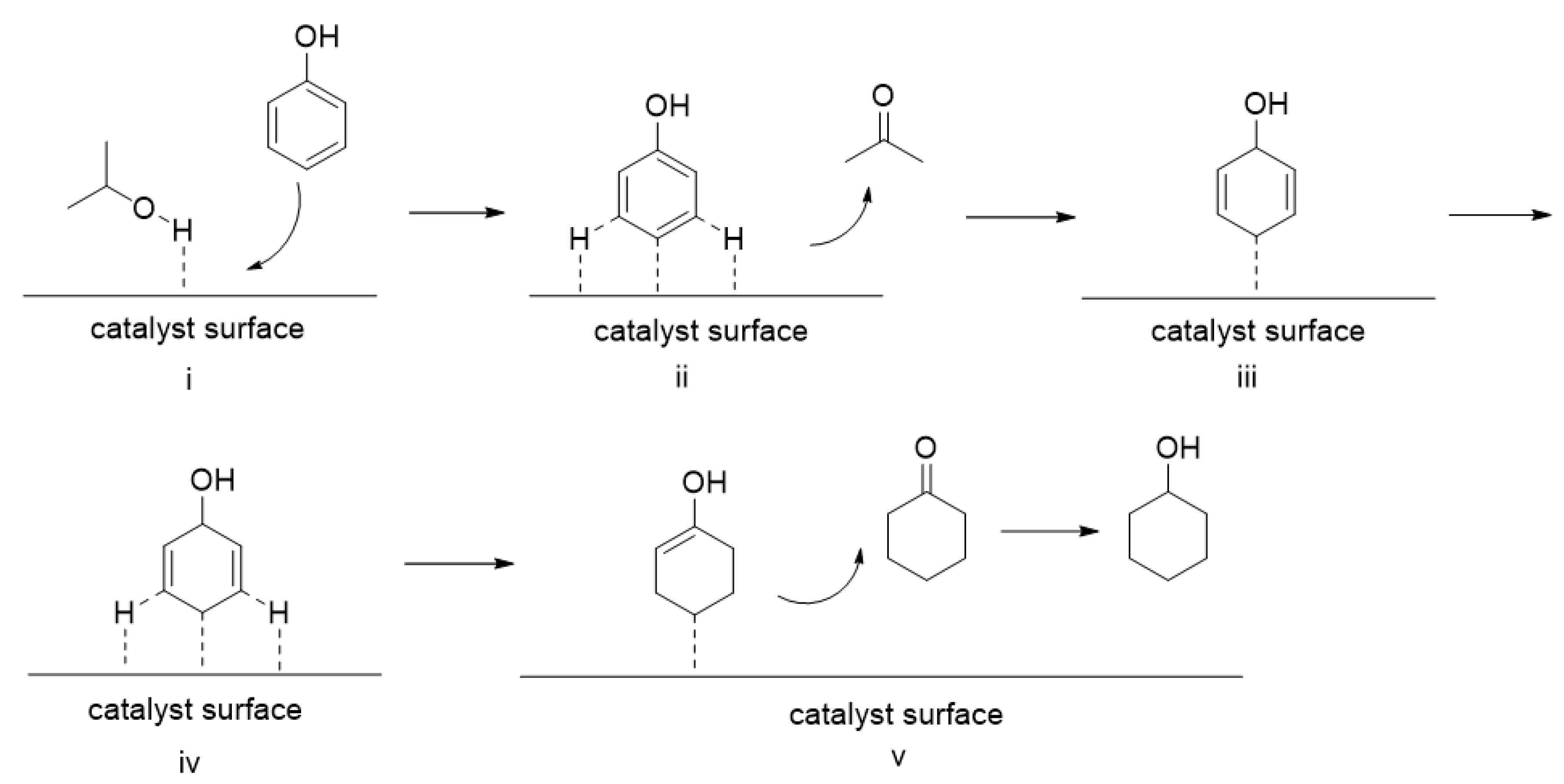
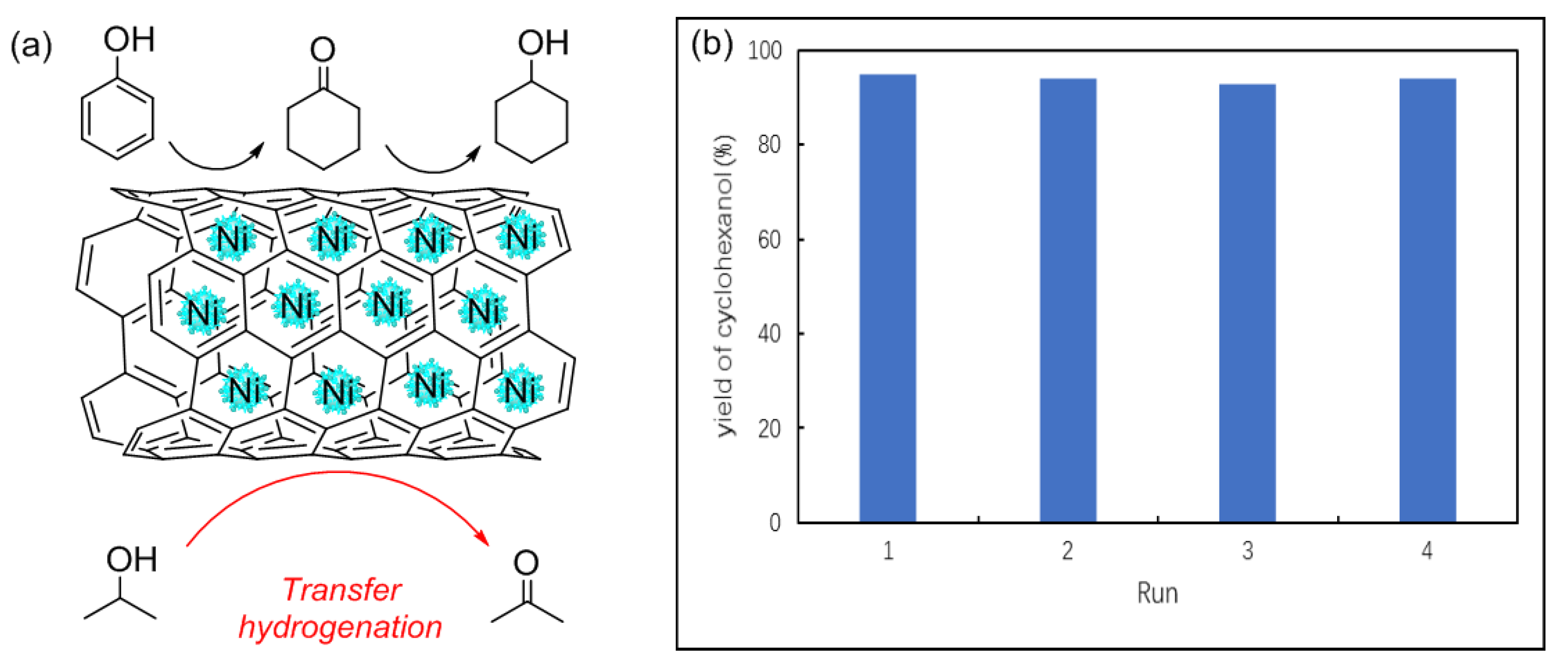
2.6. Mechanism Studies and the Recyclability of Ni/CNT
3. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CNT | Carbon nanotube |
| ICP | Inductive Coupled Plasma Emission Spectrometer |
| XRD | Powder X-ray diffraction |
| XPS | X-Ray photoelectron spectroscopy |
| TEM | Transmission electron microscopy |
| BET | Brunauer- Emmett- Teller |
| GC/MS | Gas Chromatograph/Mass Spectrometer |
References
- Xiang, Y.; Ma, L.; Lu, C.; Zhang, Q.; Li, X. Aqueous system for the improved hydrogenation of phenol and its derivatives. Green Chem. 2008, 10, 939–943. [Google Scholar] [CrossRef]
- Xiang, Y.; Kong, L.; Xie, P.; Xu, T.; Wang, J.; Li, X. Carbon nanotubes and activated carbons supported catalysts for phenol in situ hydrogenation: Hydrophobic/hydrophilic effect. Ind. Eng. Chem. Res. 2014, 53, 2197–2203. [Google Scholar] [CrossRef]
- Porwal, G.; Gupta, S.; Sreedhala, S.; Elizabeth, J.; Khan, T.S.; Haider, M.A.; Vinod, C.P. Mechanistic insights into the pathways of phenol hydrogenation on Pd nanostructures. ACS Sustain. Chem. Eng. 2019, 7, 17126–17136. [Google Scholar] [CrossRef]
- Song, Z.; Ren, D.; Wang, T.; Jin, F.; Jiang, Q.; Huo, Z. Highly selective hydrothermal production of cyclohexanol from biomass-derived cyclohexanone over Cu powder. Catal. Today 2016, 274, 94–98. [Google Scholar] [CrossRef]
- Sikhwivhilu, L.M.; Coville, N.J.; Naresh, D.; Chary, K.V.; Vishwanathan, V. Nanotubular titanate supported palladium catalysts: The influence of structure and morphology on phenol hydrogenation activity. Appl. Catal. A Gen. 2007, 324, 52–61. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Y.; Du, R.; Yuan, H.; Wang, Y.; Yao, J.; Li, H. Selective one-step aerobic oxidation of cyclohexane to ϵ-caprolactone mediated by N-hydroxyphthalimide (NHPI). ChemCatChem 2019, 11, 2260–2264. [Google Scholar] [CrossRef]
- Alazman, A.; Belic, D.; Alotaibi, A.; Kozhevnikova, E.F.; Kozhevnikov, I.V. Isomerization of cyclohexane over bifunctional Pt-, Au-, and PtAu-heteropoly acid Catalysts. ACS Catal. 2019, 9, 5063–5073. [Google Scholar] [CrossRef]
- Kim, A.R.; Ahn, S.; Yoon, T.U.; Notestein, J.M.; Farha, O.K.; Bae, Y.S. Fast cyclohexane oxidation under mild reaction conditions through a controlled creation of Redox-active Fe (II/III) Sites in a Metal-organic Framework. ChemCatChem 2019, 11, 5650–5656. [Google Scholar] [CrossRef]
- Chary, K.V.; Naresh, D.; Vishwanathan, V.; Sadakane, M.; Ueda, W. Vapour phase hydrogenation of phenol over Pd/C catalysts: A relationship between dispersion, metal area and hydrogenation activity. Catal. Commun. 2007, 8, 471–477. [Google Scholar] [CrossRef]
- Nelson, N.C.; Manzano, J.S.; Sadow, A.D.; Overbury, S.H.; Slowing, I.I. Selective hydrogenation of phenol catalyzed by palladium on high-surface-area ceria at room temperature and ambient pressure. ACS Catal. 2015, 5, 2051–2061. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Du, Y.; Jiang, H.; Liu, Y.; Chen, R. Insights into the stability of Pd/CN catalyst in liquid phase hydrogenation of phenol to cyclohexanone: Role of solvent. Catal. Lett. 2019, 149, 1–10. [Google Scholar] [CrossRef]
- Liu, T.; Zhou, H.; Han, B.; Gu, Y.; Li, S.; Zheng, J.; Zhong, X.; Zhuang, G.L.; Wang, J.G. Enhanced selectivity of phenol hydrogenation in low-pressure CO2 over supported Pd catalysts. ACS Sustain. Chem. Eng. 2017, 5, 11628–11636. [Google Scholar] [CrossRef]
- Sanyal, U.; Song, Y.; Singh, N.; Fulton, J.L.; Herranz, J.; Jentys, A.; Gutiérrez, O.Y.; Lercher, J.A. Structure sensitivity in hydrogenation reactions on Pt/C in aqueous-phase. ChemCatChem 2019, 11, 575–582. [Google Scholar] [CrossRef]
- Singh, N.; Lee, M.S.; Akhade, S.A.; Cheng, G.; Camaioni, D.M.; Gutiérrez, O.Y.; Glezakou, V.A.; Rousseau, R.; Lercher, J.A.; Campbell, C.T. Impact of pH on aqueous-phase phenol hydrogenation catalyzed by carbon-supported Pt and Rh. ACS Catal. 2018, 9, 1120–1128. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, J.; Yi, X.; Zheng, A.; Deng, F.; Chen, C.; Ji, Y.; Liu, F.; Meng, X.; Xiao, F.S. Mesoporous ZSM-5 zeolite-supported Ru nanoparticles as highly efficient catalysts for upgrading phenolic biomolecules. ACS Catal. 2015, 5, 2727–2734. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Ishikawa, M.; Tamura, M.; Tomishige, K. Selective production of cyclohexanol and methanol from guaiacol over Ru catalyst combined with MgO. Green Chem. 2014, 16, 2197–2203. [Google Scholar] [CrossRef]
- Martinez-Espinar, F.; Blondeau, P.; Nolis, P.; Chaudret, B.; Claver, C.; Castillón, S.; Godard, C. NHC-stabilised Rh nanoparticles: Surface study and application in the catalytic hydrogenation of aromatic substrates. J. Catal. 2017, 354, 113–127. [Google Scholar] [CrossRef]
- Kinoshita, A.; Nakanishi, K.; Yagi, R.; Tanaka, A.; Hashimoto, K.; Kominami, H. Hydrogen-free ring hydrogenation of phenol to cyclohexanol over a rhodium-loaded titanium (IV) oxide photocatalyst. Appl. Catal. A Gen. 2019, 578, 83–88. [Google Scholar] [CrossRef]
- Wang, Y.; Yao, J.; Li, H.; Su, D.; Antonietti, M. Highly selective hydrogenation of phenol and derivatives over a Pd@carbon nitride catalyst in aqueous media. J. Am. Chem. Soc. 2011, 133, 2362–2365. [Google Scholar] [CrossRef]
- Zhao, C.; Song, W.; Lercher, J.A. Aqueous phase hydroalkylation and hydrodeoxygenation of phenol by dual functional catalysts comprised of Pd/C and H/La-BEA. ACS Catal. 2012, 2, 2714–2723. [Google Scholar] [CrossRef]
- Li, Z.; Liu, J.; Xia, C.; Li, F. Nitrogen-functionalized ordered mesoporous carbons as multifunctional supports of ultrasmall Pd nanoparticles for hydrogenation of phenol. ACS Catal. 2013, 3, 2440–2448. [Google Scholar] [CrossRef]
- Galkin, M.V.; Sawadjoon, S.; Rohde, V.; Dawange, M.; Samec, J.S. Mild Heterogeneous Palladium-Catalyzed Cleavage of β-O-4′-Ether Linkages of Lignin Model Compounds and Native Lignin in Air. ChemCatChem 2014, 6, 179–184. [Google Scholar] [CrossRef]
- Paone, E.; Espro, C.; Pietropaolo, R.; Mauriello, F. Selective arene production from transfer hydrogenolysis of benzyl phenyl ether promoted by a co-precipitated Pd/Fe3O4 catalyst. Catal. Sci. Technol. 2016, 6, 7937–7941. [Google Scholar] [CrossRef]
- Wu, H.; Song, J.; Xie, C.; Wu, C.; Chen, C.; Han, B. Efficient and mild transfer hydrogenolytic cleavage of aromatic ether bonds in lignin-derived compounds over Ru/C. ACS Sustain. Chem. Eng. 2018, 6, 2872–2877. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, H.; Gao, P.; Wang, L. PVP-NiB amorphous catalyst for selective hydrogenation of phenol and its derivatives. Chin. J. Catal. 2014, 35, 1793–1799. [Google Scholar] [CrossRef]
- Gilkey, M.J.; Xu, B. Heterogeneous transfer hydrogenation as an effective pathway in biomass upgrading. ACS Catal. 2016, 6, 1420–1436. [Google Scholar] [CrossRef]
- Croston, M.; Langston, J.; Sangoi, R.; Santhanam, K.S.V. Catalytic oxidation of p-toluidine at multiwalled functionalized carbon nanotubes. Int. J. Nanosci. 2002, 1, 277–283. [Google Scholar] [CrossRef]
- Croston, M.; Langston, J.; Takacs, G.; Morrill, T.C.; Miri, M.; Santhanam, K.S.V.; Ajayan, P. Conversion of aniline to azobenzene at functionalized carbon nanotubes: A possible case of a nanodimensional reaction. Int. J. Nanosci. 2002, 1, 285–293. [Google Scholar] [CrossRef]
- Gordon, M.; Santhanam, K.S.V. High product yield in a narrow column configuration of carbon nanotubes: A Pathway for nanosynthetic machine. Proc. Electrochem. Soc. 2003, 13, 285–288. [Google Scholar]
- Zhou, M.; Tian, L.; Niu, L.; Li, C.; Xiao, G.; Xiao, R. Upgrading of liquid fuel from fast pyrolysis of biomass over modified Ni/CNT catalysts. Fuel Process. Technol. 2014, 126, 12–18. [Google Scholar] [CrossRef]
- Zhou, M.; Zhu, H.; Niu, L.; Xiao, G.; Xiao, R. Catalytic hydroprocessing of furfural to cyclopentanol over Ni/CNTs catalysts: Model reaction for upgrading of bio-oil. Catal. Lett. 2014, 144, 235–241. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, C.; Yan, W.; Duan, F.; Zhang, B.; Gao, Z.; Qin, Y. Ni nanoparticles supported on CNTs with excellent activity produced by atomic layer deposition for hydrogen generation from the hydrolysis of ammonia borane. Catal. Sci. Technol. 2016, 6, 2112–2119. [Google Scholar] [CrossRef]
- Li, G.; Han, J.; Wang, H.; Zhu, X.; Ge, Q. Role of dissociation of phenol in its selective hydrogenation on Pt (111) and Pd (111). ACS Catal. 2015, 5, 2009–2016. [Google Scholar] [CrossRef]
- Song, Y.; Chia, S.H.; Sanyal, U.; Gutiérrez, O.Y.; Lercher, J.A. Integrated catalytic and electrocatalytic conversion of substituted phenols and diaryl ethers. J. Catal. 2016, 344, 263–272. [Google Scholar] [CrossRef] [Green Version]
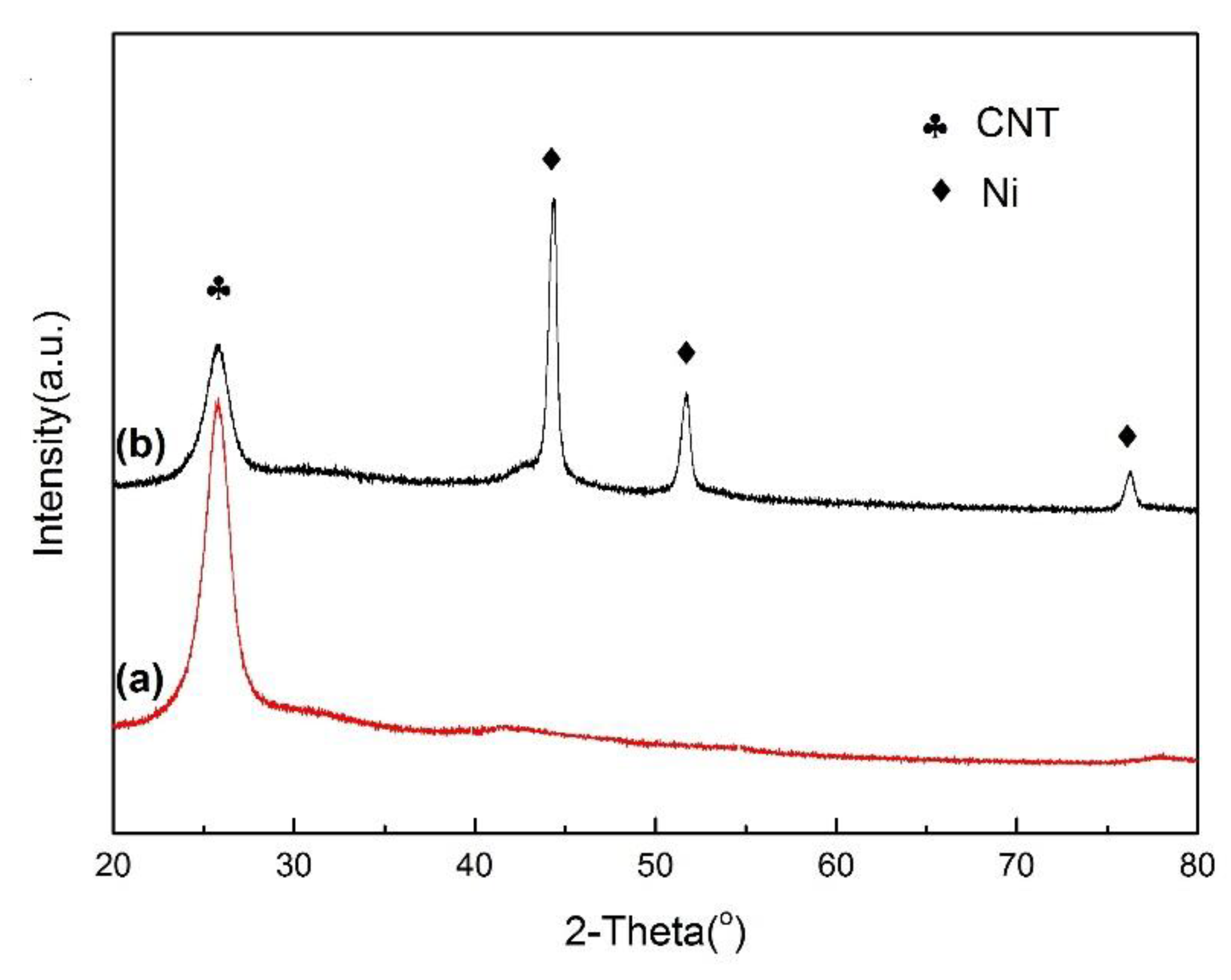
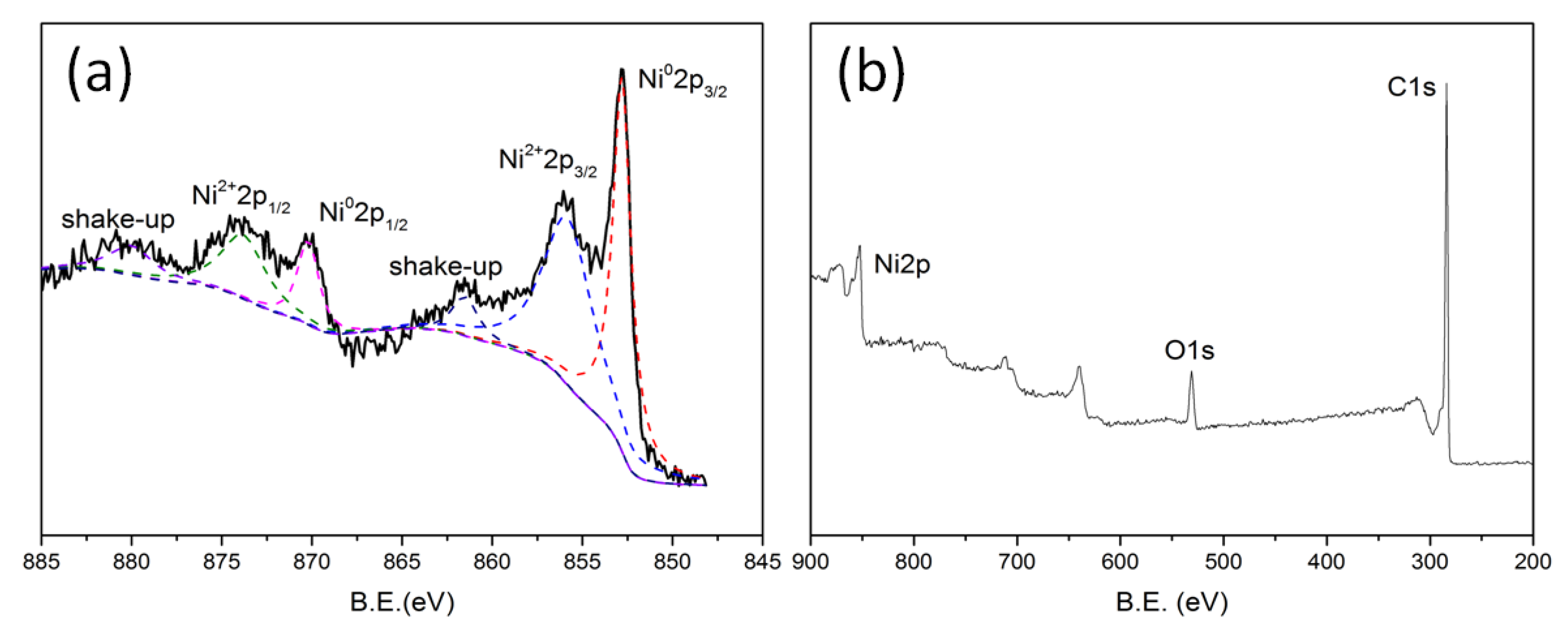
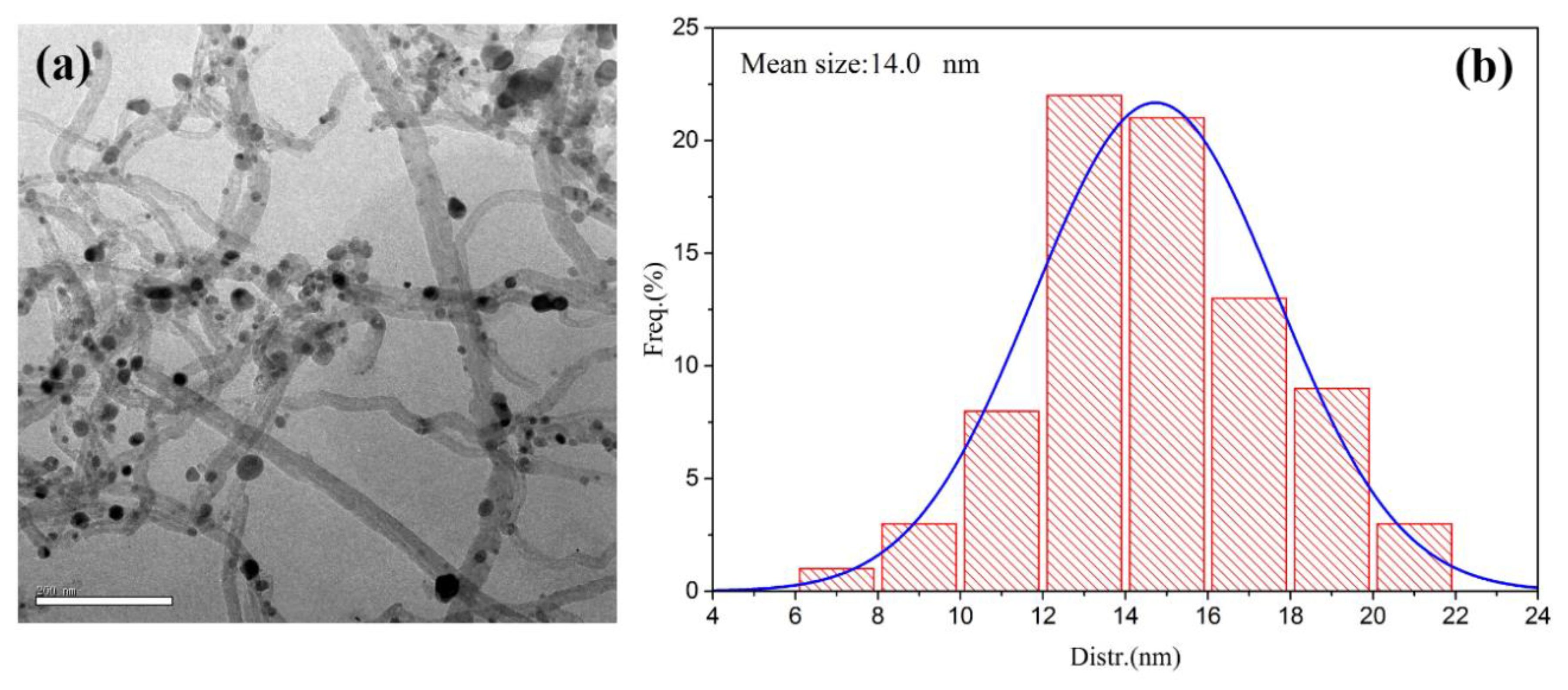

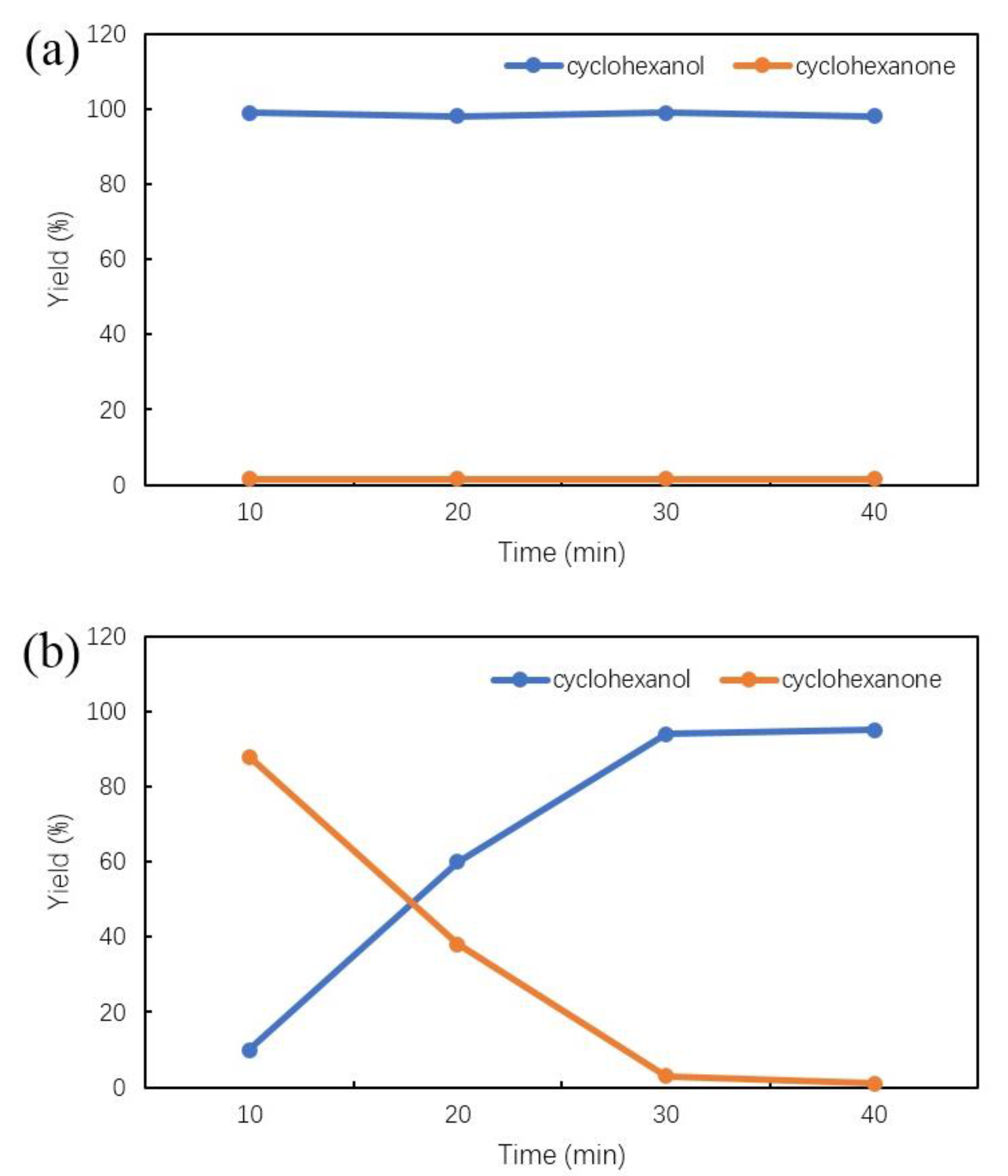
| Catalysts | Composition (wt.%) a | SBET b (m2/g) | Average Metal Size c (nm) | Average Metal Size d (nm) |
|---|---|---|---|---|
| Ni | ||||
| CNT | / | 180.6 | / | / |
| 5% Ni/CNT | 4.32 | 149.3 | 12.1 | 12.8 |
| 10% Ni/CNT | 10.55 | 145.6 | 12.6 | 13.1 |
| 15% Ni/CNT | 14.66 | 135.8 | 12.8 | 13.5 |
| 20% Ni/CNT | 19.68 | 132.4 | 13.1 | 14.0 |
| 25% Ni/CNT | 24.09 | 120.5 | 15.6 | 16.5 |
| Entry | Catalyst | T.(°C)/ t.(h) | Con. (%) | Yield (%) b | |
|---|---|---|---|---|---|
| 2a | 3a | ||||
| 1 | none | 180/4 | 0 | 0 | 0 |
| 2 | CNT | 180/4 | 0 | 0 | |
| 3 | 10% Fe/CNT | 180/4 | 8 | 2 | 0 |
| 4 | 10% Co/CNT | 180/4 | 48 | 5 | 5 |
| 5 | 10% Mo/CNT | 180/4 | 27 | 4 | 22 |
| 6 | 5% Ni/CNT | 180/4 | 73 | 3 | 66 |
| 7 | 10% Ni/CNT | 180/4 | 81 | 4 | 68 |
| 8 | 15% Ni/CNT | 180/4 | 95 | 3 | 88 |
| 9 | 20% Ni/CNT | 180/4 | 100 | 2 | 95 |
| 10 | 25% Ni/CNT | 180/4 | 100 | 2 | 94 |
| Entry | Solvent | T.(°C)/ t.(h) | Con. (%) | Yield (%) b | |
|---|---|---|---|---|---|
| 2a | 3a | ||||
| 1 | isopropanol | 180/4 | 100 | 2 | 95 |
| 2 | methanol | 180/4 | 15 | 3 | 6 |
| 3 | ethanol | 180/4 | 13 | 2 | 5 |
| 4 | H2O | 180/4 | 5 | 0 | 0 |
| Entry | Substrate | T (°C)/t (min) | Conversion (%) | Yield of Product (%) |
|---|---|---|---|---|
| 1 | 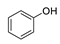 | 220/60 | 100 |  95 95 |
| 2 |  | 240/60 | 89 |  80 80 |
| 3 | 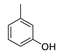 | 240/60 | 90 |  82 82 |
| 4 |  | 240/60 | 85 | 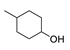 78 78 |
| 5 |  | 240/60 | 88 |  79 79 |
| Catalyst | Temperature/Time | Conversion | 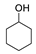 |  | 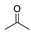 |  |
|---|---|---|---|---|---|---|
| Ni/CNT | 220 °C/60 min | 100% | 95% | 2% | 5.8 equiv | 33.6 equiv |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.; Liu, P.; Zhou, M.; Sharma, B.K.; Jiang, J. Selective Hydrogenation of Phenol to Cyclohexanol over Ni/CNT in the Absence of External Hydrogen. Energies 2020, 13, 846. https://doi.org/10.3390/en13040846
Chen C, Liu P, Zhou M, Sharma BK, Jiang J. Selective Hydrogenation of Phenol to Cyclohexanol over Ni/CNT in the Absence of External Hydrogen. Energies. 2020; 13(4):846. https://doi.org/10.3390/en13040846
Chicago/Turabian StyleChen, Changzhou, Peng Liu, Minghao Zhou, Brajendra K. Sharma, and Jianchun Jiang. 2020. "Selective Hydrogenation of Phenol to Cyclohexanol over Ni/CNT in the Absence of External Hydrogen" Energies 13, no. 4: 846. https://doi.org/10.3390/en13040846
APA StyleChen, C., Liu, P., Zhou, M., Sharma, B. K., & Jiang, J. (2020). Selective Hydrogenation of Phenol to Cyclohexanol over Ni/CNT in the Absence of External Hydrogen. Energies, 13(4), 846. https://doi.org/10.3390/en13040846





