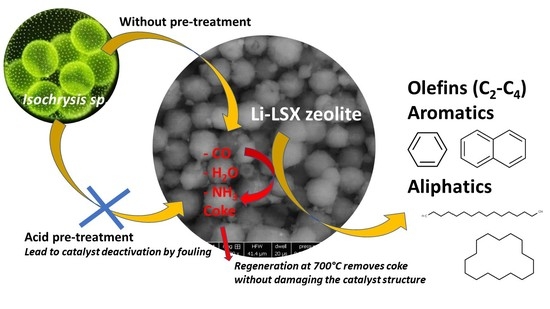
Stability of Li-LSX Zeolite in the Catalytic Pyrolysis of Non-Treated and Acid Pre-Treated Isochrysis sp. Microalgae
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterisation Techniques
2.3. Pyrolysis Apparatus and Procedure
2.4. Catalysts Regeneration Procedure
3. Results
3.1. Microalgae Pre-Treatment
3.2. Characterisation of Pyrolysis Products
3.2.1. Elemental Analyses (EA) of Bio-Chars and Bio-Oils
3.2.2. 1H-NMR of Bio-Oils
3.2.3. GC-MS Analyses
3.2.4. Gas Analyses
3.2.5. Pyrolysis Mechanism
3.3. Catalyst Characterisation
3.3.1. Surface Analyses
3.3.2. SEM-EDS Analysis of Spent Catalysts
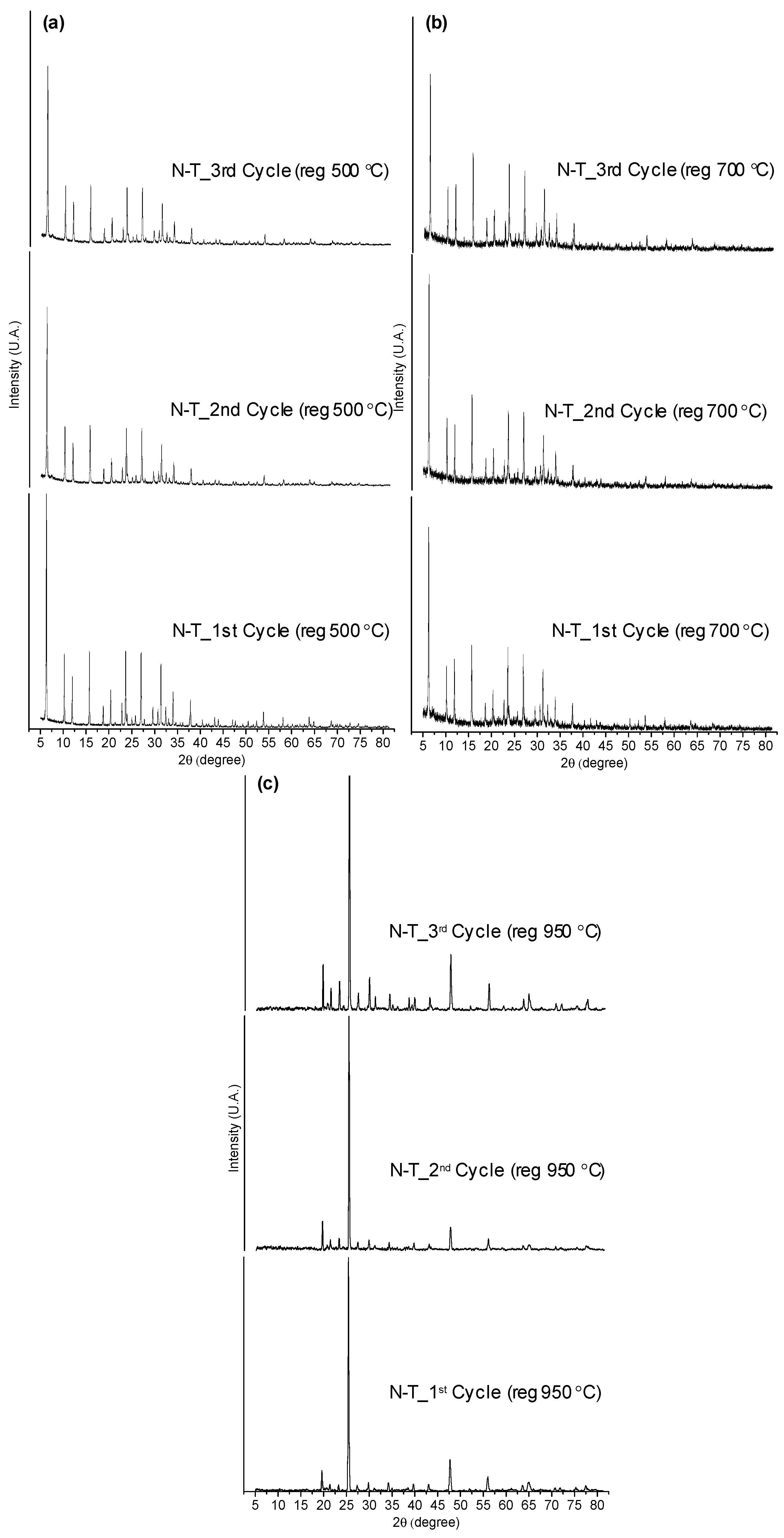
3.3.3. Regeneration Temperature Study
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sanna, A.; Abd Rahman, N.A. Conversion of Microalgae Bio-oil into Bio-diesel. Algal Biorefin. Prod. Refin. Des. 2015, 2, 493–510. [Google Scholar]
- Bridgwater, A.V. Review of fast pyrolysis of biomass and product upgrading. Biomass Bioenergy 2012, 38, 68–94. [Google Scholar] [CrossRef]
- Chisti, Y. Biodiesel from microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhao, B.; Tang, X.; Yang, X. Comparison of direct and indirect pyrolysis of micro-algae Isochrysis. Bioresour. Technol. 2015, 179, 58–62. [Google Scholar] [CrossRef]
- Aysu, T.; Abd Rahman, N.A.; Sanna, A. Catalytic pyrolysis of Tetraselmis and Isochrysis microalgae by nickel ceria based catalysts for hydrocarbon production. Energy 2016, 103, 205–214. [Google Scholar] [CrossRef]
- Rahman, N.A.A.; Fermoso, J.; Sanna, A. Effect of Li-LSX-zeolite on the in-situ catalytic deoxygenation and denitrogenation of Isochrysis sp. microalgae pyrolysis vapours. Fuel Process. Technol. 2018, 173, 253–261. [Google Scholar] [CrossRef]
- Thangalazhy-Gopakumar, S.; Adhikari, S.; Chattanathan, S.A.; Gupta, R.B. Catalytic pyrolysis of green algae for hydrocarbon production using H +ZSM-5 catalyst. Bioresour. Technol. 2012, 118, 150–157. [Google Scholar] [CrossRef]
- Pan, P.; Hu, C.; Yang, W.; Li, Y.; Dong, L.; Zhu, L.; Tong, D.; Qing, R.; Fan, Y. The direct pyrolysis and catalytic pyrolysis of Nannochloropsis sp. residue for renewable bio-oils. Bioresour. Technol. 2010, 101, 4593–4599. [Google Scholar] [CrossRef]
- Zainan, N.H.; Srivatsa, S.C.; Bhattacharya, S. Catalytic pyrolysis of microalgae Tetraselmis suecica and characterization study using in situ Synchrotron-based Infrared Microscopy. Fuel 2015, 161, 345–354. [Google Scholar] [CrossRef]
- Fahmi, R.; Bridgwater, A.V.; Donnison, I.; Yates, N.; Jones, J.M. The effect of lignin and inorganic species in biomass on pyrolysis oil yields, quality and stability. Fuel 2008, 87, 1230–1240. [Google Scholar] [CrossRef]
- Ross, A.B.; Jones, J.M.; Kubacki, M.L.; Bridgeman, T. Classification of macroalgae as fuel and its thermochemical behaviour. Bioresour. Technol. 2008, 99, 6494–6504. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.J.; Ryu, C.; Jeon, J.K.; Park, J.; Suh, D.J.; Suh, Y.W.; Chang, D.; Park, Y.K. The characteristics of bio-oil produced from the pyrolysis of three marine macroalgae. Bioresour. Technol. 2011, 102, 3512–3520. [Google Scholar] [CrossRef] [PubMed]
- Ross, A.B.; Anastasakis, K.; Kubacki, M.; Jones, J.M. Investigation of the pyrolysis behaviour of brown algae before and after pre-treatment using PY-GC/MS and TGA. J. Anal. Appl. Pyrolysis 2009, 85, 3–10. [Google Scholar] [CrossRef]
- Choi, J.; Choi, J.W.; Suh, D.J.; Ha, J.M.; Hwang, J.W.; Jung, H.W.; Lee, K.Y.; Woo, H.C. Production of brown algae pyrolysis oils for liquid biofuels depending on the chemical pretreatment methods. Energy Convers. Manag. 2014, 86, 371–378. [Google Scholar] [CrossRef]
- French, R.; Czernik, S. Catalytic pyrolysis of biomass for biofuels production. Fuel Process. Technol. 2010, 91, 25–32. [Google Scholar] [CrossRef]
- Cheng, S.; Wei, L.; Zhao, X.; Julson, J. Application, deactivation, and regeneration of heterogeneous catalysts in bio-oil upgrading. Catalysts 2016, 6, 195. [Google Scholar] [CrossRef]
- Zhang, B.; Zhong, Z.-P.; Wang, X.-B.; Ding, K.; Song, Z.-W. Catalytic upgrading of fast pyrolysis biomass vapors over fresh, spent and regenerated ZSM-5 zeolites. Fuel Process. Technol. 2015, 138, 430–434. [Google Scholar] [CrossRef]
- Paasikallio, V.; Kalogiannis, K.; Lappas, A.; Lehto, J.; Lehtonen, J. Catalytic Fast Pyrolysis: Influencing Bio-Oil Quality with the Catalyst-to-Biomass Ratio. Energy Technol. 2017, 5, 94–103. [Google Scholar] [CrossRef]
- Shao, S.; Zhang, H.; Xiao, R.; Li, X.; Cai, Y. Controlled regeneration of ZSM-5 catalysts in the combined oxygen and steam atmosphere used for catalytic pyrolysis of biomass-derivates. Energy Convers. Manag. 2018, 155, 175–181. [Google Scholar] [CrossRef]
- Channiwala, S.A.; Parikh, P.P. A unified correlation for estimating HHV of solid, liquid and gaseous fuels. Fuel 2002, 81, 1051–1063. [Google Scholar] [CrossRef]
- López, A.; de Marco, I.; Caballero, B.M.; Adrados, A.; Laresgoiti, M.F. Deactivation and regeneration of ZSM-5 zeolite in catalytic pyrolysis of plastic wastes. Waste Manag. 2011, 31, 1852–1858. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.T.; Horne, P.A. The influence of catalyst regeneration on the composition of zeolite-upgraded biomass pyrolysis oils. Fuel 1995, 74, 1839–1851. [Google Scholar] [CrossRef]
- Liu, C.; Van Santen, R.A.; Poursaeidesfahani, A.; Vlugt, T.J.H.; Pidko, E.A.; Hensen, E.J.M. Hydride Transfer versus Deprotonation Kinetics in the Isobutane-Propene Alkylation Reaction: A Computational Study. ACS Catal. 2017, 7, 8613–8627. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.; Shobana, S.; Chen, W.-H.; Bach, Q.-V.; Kim, S.-H.; Atabani, A.E.; Chang, J.-S. A review of thermochemical conversion of microalgal biomass for biofuels: Chemistry and processes. Green Chem. 2017, 19, 44–67. [Google Scholar] [CrossRef]
- Li, H.; Liu, Z.; Zhang, Y.; Li, B.; Lu, H.; Duan, N.; Liu, M.; Zhu, Z.; Si, B. Conversion efficiency and oil quality of low-lipid high-protein and high-lipid low-protein microalgae via hydrothermal liquefaction. Bioresour. Technol. 2014, 154, 322–329. [Google Scholar] [CrossRef]
- Kodasma, R.; Fermoso, J.; Sanna, A. Li-LSX-zeolite evaluation for post-combustion CO2 capture. Chem. Eng. J. 2019, 358, 1351–1362. [Google Scholar] [CrossRef]






| Sample | Concentration (wt %) | |||||||
|---|---|---|---|---|---|---|---|---|
| Na | Si | P | S | K | Ca | Fe | Zn | |
| Raw microalgae | 8.944 | 0.026 | 1.055 | 0.485 | 0.672 | 0.384 | 0.045 | 0.005 |
| Acid-washed microalgae (1 wt % H2SO4) | 3.702 | 0.036 | 0.323 | 1.239 | 0.411 | 0.576 | 0.029 | 0.138 |
| Without pre-Treatment | With pre-Treatment | |||||
|---|---|---|---|---|---|---|
| Elemental Analysis (wt %) | 1st Cycle | 2nd Cycle | 3rd Cycle | 1st Cycle | 2nd Cycle | 3rd Cycle |
| Bio-char | ||||||
| C | 53.7 | 62.3 | 58.6 | 69.8 | 71.3 | 72.5 |
| H | 10.0 | 1.7 | 1.6 | 5.3 | 4.7 | 4.2 |
| N | 2.8 | 5.0 | 4.7 | 7.9 | 8.9 | 8.0 |
| O | 33.5 | 31.0 | 35.1 | 16.0 | 15.1 | 15.3 |
| H/C molar ratio | 2.24 | 0.3 | 0.32 | 0.90 | 0.78 | 0.69 |
| O/C molar ratio | 0.47 | 0.37 | 0.45 | 0.19 | 0.16 | 0.16 |
| HHV (MJ/kg) | 27.3 | 19.2 | 17.3 | 27.8 | 27.6 | 27.5 |
| Bio-oil | ||||||
| C | 74.2 | 75.6 | 76.0 | 64.5 | 68.6 | 69.2 |
| H | 10.1 | 10.4 | 10.3 | 8.9 | 9.6 | 9.7 |
| N | 2.9 | 3.1 | 3.9 | 2.6 | 3.1 | 3.1 |
| O | 12.8 | 10.9 | 9.8 | 24.0 | 18.7 | 18.0 |
| H/C molar ratio | 1.63 | 1.65 | 1.62 | 1.65 | 1.68 | 1.69 |
| O/C molar ratio | 0.13 | 0.11 | 0.10 | 0.28 | 0.20 | 0.20 |
| HHV (MJ/kg) | 36.83 | 37.95 | 37.93 | 30.58 | 33.52 | 34.00 |
| Proton % | Without pre-Treatment | With pre-Treatment | |||||
|---|---|---|---|---|---|---|---|
| Chemical Shift Region (ppm) | Type of Protons | 1st Cycle | 2nd Cycle | 3rd Cycle | 1st Cycle | 2nd Cycle | 3rd Cycle |
| 0.0−1.6 | CH3. –CH2- | 69.23 | 61.11 | 57.02 | 70.68 | 69.08 | 69.04 |
| 1.6−2.2 | –CH2-, aliphatic OH | 7.57 | 15.06 | 15.69 | 12.37 | 13.15 | 11.99 |
| 2.2−3.0 | –CH3OC, –CH3–Ar, –CH2Ar | 3.17 | 6.21 | 7.33 | 6.68 | 6.98 | 6.73 |
| 3.0−4.2 | CH3O–, –CH2O-, =CHO | 1.95 | 1.60 | 3.91 | 0.13 | 0.48 | 1.37 |
| 4.2−6.4 | =CHO, ArOH, HC=C (nonconjugated) | 4.48 | 4.56 | 4.88 | 2.31 | 3.42 | 4.40 |
| 6.4−6.8 | HC=C (nonconjugated) | 0.00 | 0.14 | 0.24 | 0.36 | 0.57 | 0.47 |
| 6.8−8.0 | ArH, HC=C (conjugated) | 13.41 | 13.20 | 10.57 | 7.39 | 6.14 | 5.66 |
| 8.0−10.0 | –CHO, –COOH, downfiled ArH | 0.23 | 0.12 | 0.36 | 0.08 | 0.18 | 0.34 |
| Non-Treated | Pre-Treated | |||||
|---|---|---|---|---|---|---|
| Gas Product Distribution (wt %) | 1st Cycle | 2nd Cycle | 3rd Cycle | 1st Cycle | 2nd Cycle | 3rd Cycle |
| H2 | 0.96 | 1.43 | 1.88 | 0.96 | 1.29 | 1.11 |
| CO | 19.68 | 18.96 | 20.87 | 18.75 | 15.60 | 17.42 |
| CO2 | 1.55 | 1.63 | 1.29 | 2.57 | 1.87 | 1.08 |
| CH4 | 4.57 | 3.13 | 2.83 | 4.58 | 9.10 | 7.82 |
| H2O | 11.78 | 16.31 | 17.07 | 11.80 | 11.97 | 17.03 |
| HCN, NH3 | 1.87 | 1.37 | 2.38 | 1.86 | 0.58 | 0.88 |
| Olefins (C2-C4) | 43.34 | 44.77 | 42.99 | 43.26 | 24.27 | 25.85 |
| Alkanes (C2-C5) | 13.61 | 10.02 | 8.52 | 13.55 | 33.67 | 26.68 |
| Sample | Cycle Number | BET (m2/g) | Micropore Vol. (cm3/g) | Micropore Area (m2/g) | Ext. Surface Area (m2/g) |
|---|---|---|---|---|---|
| Raw catalyst | - | 662 | 0.31 | 620 | 42 |
| Catalyst without pre-treatment | 1st cycle | 353 | 0.16 | 302 | 51 |
| 2nd cycle | 299 | 0.14 | 265 | 34 | |
| 3rd cycle | 229 | 0.10 | 197 | 32 | |
| Catalyst with pre-treatment | 1st cycle | 409 | 0.18 | 339 | 70 |
| 2nd cycle | 176 | 0.07 | 123 | 53 | |
| 3rd cycle | 121 | 0.05 | 98 | 23 |
| Elemental Analysis (wt %) | |||
|---|---|---|---|
| C | H | N | |
| After calcinaton at 500 °C | |||
| 1 | 6.41 | 2.06 | 0.60 |
| 2 | 7.05 | 1.80 | 0.75 |
| 3 | 7.30 | 1.39 | 0.86 |
| After calcination 700 °C | |||
| 1 | 0.16 | 1.64 | 0.00 |
| 2 | 0.16 | 1.00 | 0.00 |
| 3 | 0.15 | 0.40 | 0.00 |
| After calcination 950 °C | |||
| 1 | 0.00 | 0.00 | 0.00 |
| 2 | 0.00 | 0.00 | 0.00 |
| 3 | 0.00 | 0.00 | 0.00 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abd Rahman, N.A.; Fermoso, J.; Sanna, A. Stability of Li-LSX Zeolite in the Catalytic Pyrolysis of Non-Treated and Acid Pre-Treated Isochrysis sp. Microalgae. Energies 2020, 13, 959. https://doi.org/10.3390/en13040959
Abd Rahman NA, Fermoso J, Sanna A. Stability of Li-LSX Zeolite in the Catalytic Pyrolysis of Non-Treated and Acid Pre-Treated Isochrysis sp. Microalgae. Energies. 2020; 13(4):959. https://doi.org/10.3390/en13040959
Chicago/Turabian StyleAbd Rahman, Nur Adilah, Javier Fermoso, and Aimaro Sanna. 2020. "Stability of Li-LSX Zeolite in the Catalytic Pyrolysis of Non-Treated and Acid Pre-Treated Isochrysis sp. Microalgae" Energies 13, no. 4: 959. https://doi.org/10.3390/en13040959
APA StyleAbd Rahman, N. A., Fermoso, J., & Sanna, A. (2020). Stability of Li-LSX Zeolite in the Catalytic Pyrolysis of Non-Treated and Acid Pre-Treated Isochrysis sp. Microalgae. Energies, 13(4), 959. https://doi.org/10.3390/en13040959