Effects of Increasing Nitrogen Content on Process Stability and Reactor Performance in Anaerobic Digestion
Abstract
:1. Introduction
2. Materials and Methods
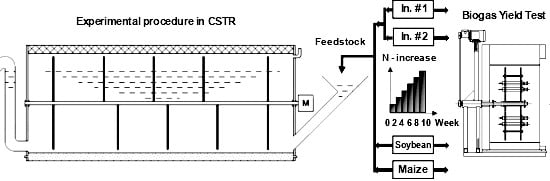
2.1. Reactor Design
2.2. Inocula and N-Increase in Feeding Regimes
2.3. Trace Elements Supplementation
2.4. Analytical Methods
2.5. Calculation of FAN and TAN
2.6. Statistical Analysis
2.6.1. Inhibition in SMY
2.6.2. Analysis of the effect of TAN and FAN on inhibition
3. Results and Discussion
3.1. The Starting Phase
3.2. The Experimental Period
3.3. Results of Statistical Analysis
3.3.1. Results of analysis on inhibition in SMY
3.3.2. Results of analysis of the effect of TAN and FAN on inhibition
3.4. Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yirong, C.; Zhang, W.; Heaven, S.; Banks, C.J. Influence of ammonia in the anaerobic digestion of food waste. J. Environ. Chem. Eng. 2017, 5, 5131–5142. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Wang, F.; Pei, M.; Qiu, L.; Qiang, H.; Yao, Y. Performance of Anaerobic Digestion of Chicken Manure Under Gradually Elevated Organic Loading Rates. Int. J. Environ. Res. Public Health 2019, 16, 2239. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Hu, J.; Lee, D.-J. Biogas from anaerobic digestion processes: Research updates. Renew. Energy 2016, 98, 108–119. [Google Scholar] [CrossRef]
- Tian, H.; Fotidis, I.A.; Mancini, E.; Treu, L.; Mahdy, A.; Ballesteros, M.; González-Fernández, C.; Angelidaki, I. Acclimation to extremely high ammonia levels in continuous biomethanation process and the associated microbial community dynamics. Bioresour. Technol. 2018, 247, 616–623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korres, N. Bioenergy Production by Anaerobic Digestion; Routledge: Abingdon, UK, 2013; ISBN 9780203137697. [Google Scholar]
- Carlos, A.; De Lemos, C. Anaerobic Reactors. Biological Wastewater Treatment Series; IWA Publishing: London, UK, 2007; Volume 4, ISBN 9781843391647. [Google Scholar]
- Anthonisen, A.C.; Loehr, R.C.; Prakasam, T.B.; Srinath, E.G. Inhibition of Nitrification by Ammonia and Nitrous Acid. J. Water Pollut. Control Fed. 1976, 48, 835–852. [Google Scholar]
- Poirier, S.; Madigou, C.; Bouchez, T.; Chapleur, O. Improving anaerobic digestion with support media: Mitigation of ammonia inhibition and effect on microbial communities. Bioresour. Technol. 2017, 235, 229–239. [Google Scholar] [CrossRef]
- Kayhanian, M. Ammonia Inhibition in High-Solids Biogasification: An Overview and Practical Solutions. Environ. Technol. 1999, 20, 355–365. [Google Scholar] [CrossRef]
- Krakat, N.; Demirel, B.; Anjum, R.; Dietz, D. Methods of ammonia removal in anaerobic digestion: A review. Water Sci. Technol. 2017, 76, 1925–1938. [Google Scholar] [CrossRef]
- Fotidis, I.A.; Karakashev, D.; Kotsopoulos, T.A.; Martzopoulos, G.G.; Angelidaki, I. Effect of ammonium and acetate on methanogenic pathway and methanogenic community composition. Fems Microbiol. Ecol. 2013, 83, 38–48. [Google Scholar] [CrossRef] [Green Version]
- Rajagopal, R.; Massé, D.I.; Singh, G. A critical review on inhibition of anaerobic digestion process by excess ammonia. Bioresour. Technol. 2013, 143, 632–641. [Google Scholar] [CrossRef]
- Chen, Y.; Cheng, J.J.; Creamer, K.S. Inhibition of anaerobic digestion process: A review. Bioresour. Technol. 2008, 99, 4044–4064. [Google Scholar] [CrossRef] [PubMed]
- Perry, L.; McCarty, P.L.; McKenney, R.E. Salt Toxicity in Anaerobic Digestion. J. Artic. 1961, 33, 399–415. [Google Scholar]
- Chen, J.L.; Ortiz, R.; Steele, T.W.J.; Stuckey, D.C. Toxicants inhibiting anaerobic digestion: A review. Biotechnol. Adv. 2014, 32, 1523–1534. [Google Scholar] [CrossRef]
- Theuerl, S.; Klang, J.; Prochnow, A. Process Disturbances in Agricultural Biogas Production—Causes, Mechanisms and Effects on the Biogas Microbiome: A Review. Energies 2019, 12, 365. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Wang, W.; Xue, L.; Chen, C.; Liu, G.; Zhang, R. Effects of Ammonia on Anaerobic Digestion of Food Waste: Process Performance and Microbial Community. Energy Fuels 2016, 30, 5749–5757. [Google Scholar] [CrossRef]
- Siegrist, H.; Vogt, D.; Garcia-Heras, J.L.; Gujer, W. Mathematical Model for Meso- and Thermophilic Anaerobic Sewage Sludge Digestion. Environ. Sci. Technol. 2002, 36, 1113–1123. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Zhang, Y.; Sui, Q.; Zhang, J.; Wang, R.; Yu, D.; Wang, Y.; Wei, Y. Biochemical Conversion and Microbial Community in Response to Ternary pH Buffer System during Anaerobic Digestion of Swine Manure. Energies 2018, 11, 2991. [Google Scholar] [CrossRef] [Green Version]
- Yenigün, O.; Demirel, B. Ammonia inhibition in anaerobic digestion: A review. Process Biochem. 2013, 48, 901–911. [Google Scholar] [CrossRef]
- Hashimoto, A.G. Ammonia inhibition of methanogenesis from cattle wastes. Agric. Wastes 1986, 17, 241–261. [Google Scholar] [CrossRef]
- Tian, H.; Fotidis, I.A.; Mancini, E.; Angelidaki, I. Different cultivation methods to acclimatise ammonia-tolerant methanogenic consortia. Bioresour. Technol. 2017, 232, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Angenent, L.T.; Sung, S.; Raskin, L. Methanogenic population dynamics during startup of a full-scale anaerobic sequencing batch reactor treating swine waste. Water Res. 2002, 36, 4648–4654. [Google Scholar] [CrossRef]
- Koster, I.W.; Lettinga, G. Anaerobic digestion at extreme ammonia concentrations. Biol. Wastes 1988, 25, 51–59. [Google Scholar] [CrossRef]
- Kayhanian, M. Performance of a high-solids anaerobic digestion process under various ammonia concentrations. J. Chem. Technol. Biotechnol. 1994, 59, 349–352. [Google Scholar] [CrossRef]
- Shanmugam, P.; Horan, N.J. Optimising the biogas production from leather fleshing waste by co-digestion with MSW. Bioresour. Technol. 2009, 100, 4117–4120. [Google Scholar] [CrossRef] [PubMed]
- Molaey, R.; Bayrakdar, A.; Sürmeli, R.Ö.; Çalli, B. Anaerobic digestion of chicken manure: Mitigating process inhibition at high ammonia concentrations by selenium supplementation. Biomass Bioenergy 2018, 108, 439–446. [Google Scholar] [CrossRef]
- Moestedt, J.; Müller, B.; Westerholm, M.; Schnürer, A. Ammonia threshold for inhibition of anaerobic digestion of thin stillage and the importance of organic loading rate. Microb. Biotechnol. 2016, 9, 180–194. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Sun, Y.; Wu, S.; Kong, X.; Yuan, Z.; Dong, R. The performance efficiency of bioaugmentation to prevent anaerobic digestion failure from ammonia and propionate inhibition. Bioresour. Technol. 2017, 231, 94–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lauterböck, B.; Ortner, M.; Haider, R.; Fuchs, W. Counteracting ammonia inhibition in anaerobic digestion by removal with a hollow fiber membrane contactor. Water Res. 2012, 46, 4861–4869. [Google Scholar] [CrossRef] [PubMed]
- Grimsby, L.K.; Fjørtoft, K.; Aune, J.B. Nitrogen mineralization and energy from anaerobic digestion of jatropha press cake. Energy Sustain. Dev. 2013, 17, 35–39. [Google Scholar] [CrossRef]
- Fotidis, I.A.; Wang, H.; Fiedel, N.R.; Luo, G.; Karakashev, D.B.; Angelidaki, I. Bioaugmentation as a solution to increase methane production from an ammonia-rich substrate. Environ. Sci. Technol. 2014, 48, 7669–7676. [Google Scholar] [CrossRef]
- Niu, Q.; Qiao, W.; Qiang, H.; Hojo, T.; Li, Y.-Y. Mesophilic methane fermentation of chicken manure at a wide range of ammonia concentration: Stability, inhibition and recovery. Bioresour. Technol. 2013, 137, 358–367. [Google Scholar] [CrossRef] [PubMed]
- van Velsen, A. Adaptation of methanogenic sludge to high ammonia-nitrogen concentrations. Water Res. 1979, 13, 995–999. [Google Scholar] [CrossRef]
- Sun, C.; Cao, W.; Banks, C.J.; Heaven, S.; Liu, R. Biogas production from undiluted chicken manure and maize silage: A study of ammonia inhibition in high solids anaerobic digestion. Bioresour. Technol. 2016, 218, 1215–1223. [Google Scholar] [CrossRef] [Green Version]
- Yao, Y.; Yu, L.; Ghogare, R.; Dunsmoor, A.; Davaritouchaee, M.; Chen, S. Simultaneous ammonia stripping and anaerobic digestion for efficient thermophilic conversion of dairy manure at high solids concentration. Energy 2017, 141, 179–188. [Google Scholar] [CrossRef]
- Nielsen, H.B.; Angelidaki, I. Strategies for optimizing recovery of the biogas process following ammonia inhibition. Bioresour. Technol. 2008, 99, 7995–8001. [Google Scholar] [CrossRef] [PubMed]
- Haag, N.L.; Nägele, H.-J.; Reiss, K.; Biertümpfel, A.; Oechsner, H. Methane formation potential of cup plant (Silphium perfoliatum). Biomass Bioenergy 2015, 75, 126–133. [Google Scholar] [CrossRef]
- VDI-Society Energy and Environment. VDI 4630 Fermentation of Organic Materials. Characterization of the Substrate, Sampling, Collection of Material Data, Fermentation Tests; VDI: Düsseldorf, Germany, 2006. [Google Scholar]
- Helffrich, D.; Oechsner, H. The Hohenheim Biogas Yield Test. Landtechnik 2003, 58, 148–149. [Google Scholar]
- Mittweg, G.; Oechsner, H.; Hahn, V.; Lemmer, A.; Reinhardt-Hanisch, A. Repeatability of a laboratory batch method to determine the specific biogas and methane yields. Eng. Life Sci. 2012, 12, 270–278. [Google Scholar] [CrossRef]
- Vintiloiu, A.; Lemmer, A.; Oechsner, H.; Jungbluth, T. Mineral substances and macronutrients in the anaerobic conversion of biomass: An impact evaluation. Eng. Life Sci. 2012, 12, 287–294. [Google Scholar] [CrossRef]
- Voelklein, M.A.; O’ Shea, R.; Jacob, A.; Murphy, J.D. Role of trace elements in single and two-stage digestion of food waste at high organic loading rates. Energy 2017, 121, 185–192. [Google Scholar] [CrossRef]
- Wintsche, B.; Glaser, K.; Sträuber, H.; Centler, F.; Liebetrau, J.; Harms, H.; Kleinsteuber, S. Trace Elements Induce Predominance among Methanogenic Activity in Anaerobic Digestion. Front. Microbiol. 2016, 7, 2034. [Google Scholar] [CrossRef] [PubMed]
- Choong, Y.Y.; Norli, I.; Abdullah, A.Z.; Yhaya, M.F. Impacts of trace element supplementation on the performance of anaerobic digestion process: A critical review. Bioresour. Technol. 2016, 209, 369–379. [Google Scholar] [CrossRef] [PubMed]
- American Public Health Association; American Water Works Association; Water Environment Federation. Standard Methods for the Examination of Water and Wastewater, 20th ed.; American Public Health Association: Washington, DC, USA, 1998. [Google Scholar]
- DIN EN 13650. Soil Improvers and Growing Media—Extraction of Aqua Regia Soluble Elements, German version EN 136502001; British Standards Institution: London, UK, 2001. [Google Scholar]
- DIN EN ISO 17294-2. Water Quality—Application of Inductively Coupled Plasma Mass Spectrometry. Determination of Selected Elements Including Uranium Isotopes, German version EN ISO 172942016; International Organization for Standardization: Geneva, Switzerland, 2017. [Google Scholar]
- DIN EN ISO 11885. Water Quality—Determination of Selected Elements by Inductively Coupled Plasma Optical Emission Spectrometry, German version EN ISO 118852009; International Organization for Standardization: Geneva, Switzerland, 2017. [Google Scholar]
- Piepho, H.P.; Buchse, A.; Richter, C. A Mixed Modelling Approach for Randomized Experiments with Repeated Measures. J. Agron. Crop. Sci. 2004, 190, 230–247. [Google Scholar] [CrossRef]
- Tian, H.; Fotidis, I.A.; Kissas, K.; Angelidaki, I. Effect of different ammonia sources on aceticlastic and hydrogenotrophic methanogens. Bioresour. Technol. 2018, 250, 390–397. [Google Scholar] [CrossRef] [Green Version]
- Dai, X.; Yan, H.; Li, N.; He, J.; Ding, Y.; Dai, L.; Dong, B. Metabolic adaptation of microbial communities to ammonium stress in a high solid anaerobic digester with dewatered sludge. Sci. Rep. 2016, 6, 28193. [Google Scholar] [CrossRef] [Green Version]
- Fotidis, I.A.; Treu, L.; Angelidaki, I. Enriched ammonia-tolerant methanogenic cultures as bioaugmentation inocula in continuous biomethanation processes. J. Clean. Prod. 2017, 166, 1305–1313. [Google Scholar] [CrossRef] [Green Version]




| Mesophilic conditions | |||||||||||
| TAN | Treated Substrate | Operating Temperature | Inhibition in CH4 Production | Reference | FAN | Treated Substrate | Operating Temperature | Inhibition in CH4 Production | Reference | ||
| ≤ | 1.00 | Mashed biowaste, residual food waste | Not indicated | Not indicated | [8] | ≤ | 0.03 | Mashed biowaste, residual food waste, steers manure | Not indicated, 35 °C | Not indicated | [8,21] |
| ≤ | 2.00 | Food waste | 37 °C | Not indicated | [17] | ≤ | 0.49 (b) | Animal manure, food waste | 37 ± 1 °C | Not indicated | [22] |
| ≤ | 2.40 | Chicken manure, spent poppy straw | 36 ± 1 °C | Not indicated | [27] | ≤ | 1.10 | Thin stillage | 38 °C | Not indicated | [28] |
| ≤ | 3.00 | Municipal wastewater biosolids | 36 ± 1 °C | Not indicated | [29] | ≤ | 1.20 | Pig slurry, maize silage, other | 38.0 ± 0.5 °C | Not indicated | [30] |
| ≤ | 3.20 (b) | Municipal wastewater | ~22 °C | Not indicated | [23] | agricultural wastes | |||||
| ≤ | 3.50 | Municipal wastewater biosolids | 37 ± 1 °C | Not indicated | [1] | ||||||
| ≤ | 4.56 (b) | Jatropha press cake | 37 °C | Not indicated | [31] | ||||||
| ≤ | 5.00 | Animal/poultry manure, organic waste, municipal wastewater | 30–38 °C | 50% inhibition at TAN of 3.0 g·L−1 | [11,32,33,34] | ||||||
| ≤ | 6.00 | Pig slurry, maize silage, other agricultural wastes | 38.0 ± 0.5 °C | Not indicated | [30] | ||||||
| ≤ | 7.00 | Chicken manure, maize silage | 37–41 °C | 10–20% at TAN ≥ 7.0 g·L−1, FAN ~ 600 mg·L−1; 50% at TAN ≥ 8.8 g·L−1 | [35] | ||||||
| ≤ | 10.00 (b) | Animal waste, food waste | 37 ± 1 °C | Not indicated | [4] | ||||||
| ≤ | 11.80 (b) | Beet-sugar factory wastewater | 30 ± 1 °C | Not indicated | [24] | ||||||
| Thermophilic Conditions | |||||||||||
| TAN | Treated Substrate | Operating Temperature | Inhibition in CH4 Production | Reference | FAN | Treated Substrate | Operating Temperature | Inhibition in CH4 Production | Reference | ||
| ≤ | 1.80–2.40 | Dairy manure | 55 °C | Not indicated | [36] | ≤ | 0.20 (a) | Steer manure | 55 °C | Not indicated | [21] |
| ≤ | 4.32 (b) | Animal manure, food industrial organic waste | 53 ± 1 °C | Not indicated | [22] | ≤ | 0.39 (b) | Steer manure | 55 °C | Not indicated | [21] |
| ≤ | 0.85 | Municipal wastewater biosolids | 55 °C | Not indicated | [1] | ||||||
| ≤ | 1.20 | Cattle manure | 53–55 °C | Not indicated | [37] | ||||||
| ≤ | 1.43 (b) | Animal manure, food industrial organic waste | 53 ± 1 °C | Not indicated | [22] | ||||||
| Parameter | Inoculum | Maize Silage | Soybean Meal | |
|---|---|---|---|---|
| #1 | #2 | |||
| DMFM (a) [g∙kg−1] | 59.80 (2.99) | 103.75 (5.18) | 377.61 (18.88) | 887.99 (18.46) |
| VSDM (b) [g∙kg−1] | 738.74 (36.93) | 789.74 (39.48) | 907.38 (45.37) | 927.19 (4.06) |
| TKNFM (c) [g∙kg−1] | 3.34 (0.70) | 7.14 (0.36) | 4.00 (0.20) | 67.83 (3.39) |
| NH4+FM (d) [g∙kg−1] | 1.35 (0.07) | 5.00 (0.25) | 0.60 (0.03) | 9.50 (0.48) |
| pH | 7.44 (0.37) | 8.42 (0.42) | NA | NA |
| SMYVSe) [L∙kg−1] | 25.78 (1.30) | 88.43 (4.42) | 330.66 (15.08) | 423.16 (21.11) |
| Inoculum | Feeding | Week | ||||
|---|---|---|---|---|---|---|
| Regime | 4 | 5 | 6 | 17 | 24 | |
| Fe | ||||||
| 1 | 0-increase | 1827.94 (91.40) | 1625.38 (81.27) | 1463.40 (73.17) | 936.00 (77.78) | 2105 (106.07) |
| 1 | 0.25-increase | 1515.01 (75.75) | 1512.09 (75.60) | 1449.05 (72.45) | 1120.00 (367.70) | 1810.00 (84.85) |
| 1 | 0.5-increase | 1625.33 (81.27) | 1368.86 (68.44) | 1402.16 (70.11) | 837.50 (74.25) | 1835.00 (134.35) |
| 2 | 0-increase | 3055.97 (102.47) | 2986.89 (246.08) | 2779.68 (138.98) | 2835.00 (473.76) | 3795.00 (700.04) |
| 2 | 0.25-increase | NA | NA | NA | 2840.00 (339.41) | 3540.00 (650.54) |
| 2 | 0.5-increase | 3116.76 (155.84) | 2846.76 (142.34) | 2705.63 (135.28) | 2910.00 (14.14) | 3380.00 (183.85) |
| Ni | ||||||
| 1 | 0-increase | 12.06 (0.60) | 11.70 (0.59) | 10.99 (0.55) | 5.33 (2.26) | 21.25 (0.21) |
| 1 | 0.25-increase | 6.81 (0.34) | 6.92 (0.35) | 6.57 (0.33) | 15.79 (14.02) | 21.15 (8.41) |
| 1 | 0.5-increase | 7.72 (0.39) | 6.29 (0.31) | 6.76 (0.34) | 8.17 (3.16) | 21.20 (1.27) |
| 2 | 0-increase | 8.14 (0.29) | 8.62 (0.06) | 8.45 (0.42) | 9.22 (1.05) | 21.10 (0.28) |
| 2 | 0.25-increase | NA | NA | NA | 9.97 (0.18) | 20.80 (0.85) |
| 2 | 0.5-increase | 13.82 (0.69) | 13.81 (0.69) | 13.1 (0.66) | 12.85 (0.49) | 20.45 (3.18) |
| Mo | ||||||
| 1 | 0-increase | 4.91 (0.25) | 4.83 (0.24) | 4.83 (0.24) | 2.85 (0.08) | 5.02 (0.15) |
| 1 | 0.25-increase | 4.32 (0.22) | 4.66 (0.23) | 4.62 (0.23) | 4.39 (0.92) | 6.49 (0.59) |
| 1 | 0.5-increase | 4.93 (0.25) | 4.39 (0.22) | 4.84 (0.24) | 4.06 (0.33) | 7.145 (0.49) |
| 2 | 0-increase | 5.18 (0.39) | 5.57 (0.34) | 5.59 (0.28) | 5.22 (0.66) | 7.32 (1.01) |
| 2 | 0.25-increase | NA | NA | NA | 5.29 (0.25) | 7.61 (0.95) |
| 2 | 0.5-increase | 5.70 (0.28) | 5.77 (0.29) | 5.78 (0.29) | 5.67 (0.40) | 7.56 (0.35) |
| Co | ||||||
| 1 | 0-increase | 1.14 (0.06) | 1.17 (0.06) | 1.12 (0.06) | 0.51 (0.02) | 1.90 (0.08) |
| 1 | 0.25-increase | 0.98 (0.05) | 1.07 (0.05) | 1.03 (0.05) | 0.76 (0.31) | 1.71 (0.15) |
| 1 | 0.5-increase | 1.05 (0.05) | 0.95 (0.05) | 1.04 (0.05) | 0.65 (0.09) | 1.83 (0.15) |
| 2 | 0-increase | 1.23 (0.10) | 1.28 (0.01) | 1.25 (0.06) | 0.91 (0.11) | 2.11 (0.25) |
| 2 | 0.25-increase | NA | NA | NA | 0.94 (0.02) | 1.90 (0.28) |
| 2 | 0.5-increase | 1.36 (0.07) | 1.38 (0.07) | 1.35 (0.07) | 0.99 (0.01) | 1.79 (0.06) |
| Se | ||||||
| 1 | 0-increase | 0.51 (0.03) | 0.49 (0.02) | 0.73 (0.04) | 0.34 (0.04) | 1.60 (0.14) |
| 1 | 0.25-increase | 0.62 (0.03) | 0.42 (0.02) | 0.64 (0.03) | 0.41 (0.05) | 1.85 (0.07) |
| 1 | 0.5-increase | 0.61 (0.03) | 0.50 (0.03) | 0.68 (0.03) | 0.40 (0.03) | 2.00 (0.28) |
| 2 | 0-increase | 1.19 (0.18) | 1.22 (0.07) | 1.12 (0.06) | 0.85 (0.13) | 2.20 (0.42) |
| 2 | 0.25-increase | NA | NA | NA | 0.85 (0.05) | 2.10 (0.28) |
| 2 | 0.5-increase | 1.25 (0.06) | 1.12 (0.06) | 1.18 (0.06) | 0.88 (0.02) | 1.90 (0.00) |
| Inoculum | Feeding Regime | DF | SMYt (L∙kgVS−1) | SMYm (L∙kgVS−1) | t-Value | p-Value |
|---|---|---|---|---|---|---|
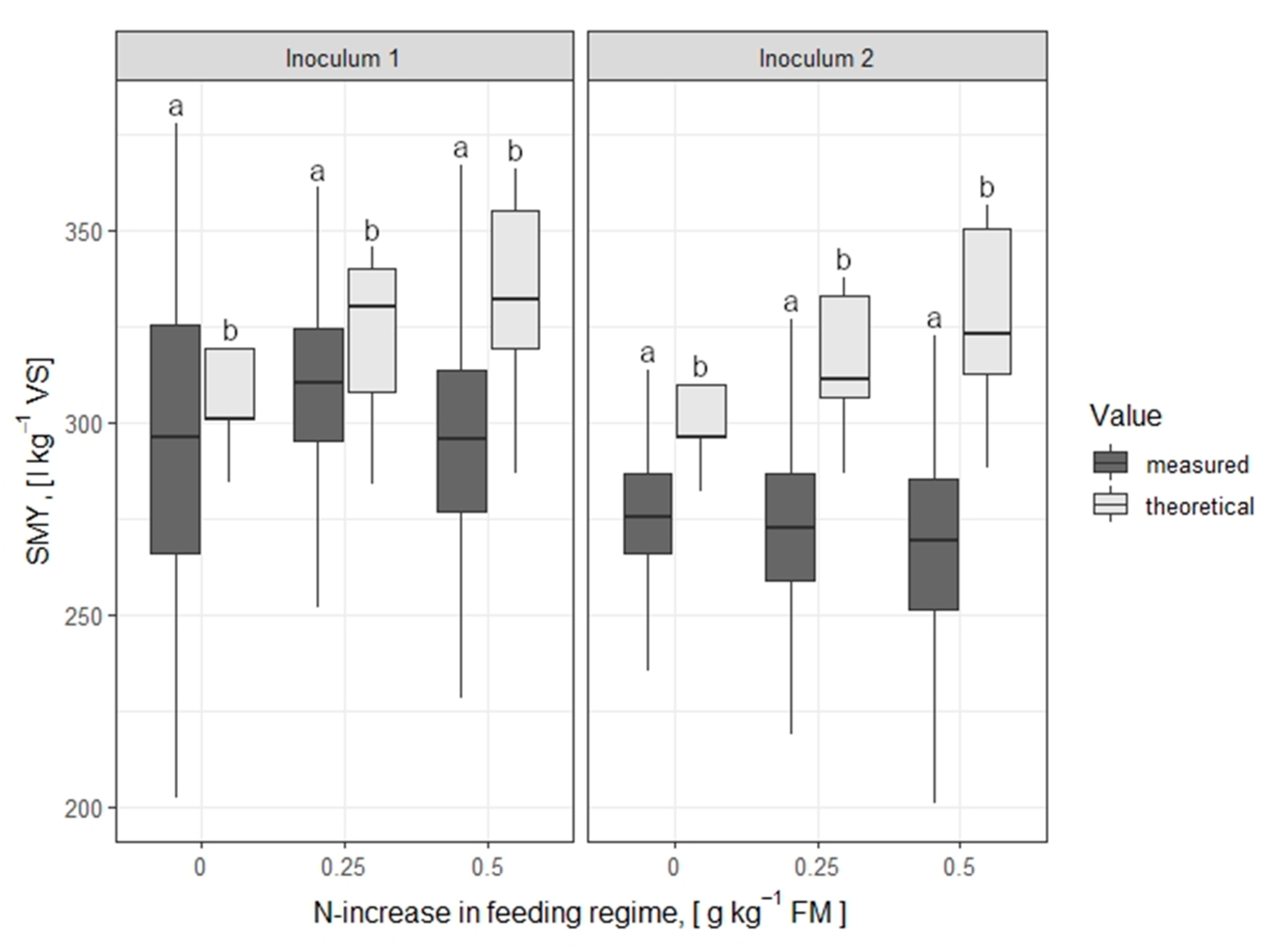
| 1 | 0-increase | 148 | 304.65 (11.80) | 298.68 (4.44) | 1.70 | 0.05 |
| 1 | 0.25-increase | 182 | 323.71 (18.17) | 302.41 (51.70) | 5.42 | 0.00 * |
| 1 | 0.5-increase | 182 | 333.36 (21.78) | 289.81 (55.09) | 9.53 | 0.00 * |
| 2 | 0-increase | 141 | 296.65 (10.57) | 264.50 (55.87) | 6.55 | 0.00 * |
| 2 | 0.25-increase | 141 | 313.72 (16.48) | 269.99 (46.99) | 10.29 | 0.00 * |
| 2 | 0.5-increase | 141 | 325.72 (22.40) | 257.87 (60.36) | 11.62 | 0.00 * |
| Dependent Variable | Effect | Numerator DF | Denominator DF | F-Value | R2 | p-Value |
|---|---|---|---|---|---|---|
| sqrt_Inhibition | TAN | 1 | 30.7 | 19.08 | 0.20 | 0.0001 |
| sqrt_Inhibition | FAN | 1 | 16.5 | 15.11 | 0.15 | 0.0012 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morozova, I.; Nikulina, N.; Oechsner, H.; Krümpel, J.; Lemmer, A. Effects of Increasing Nitrogen Content on Process Stability and Reactor Performance in Anaerobic Digestion. Energies 2020, 13, 1139. https://doi.org/10.3390/en13051139
Morozova I, Nikulina N, Oechsner H, Krümpel J, Lemmer A. Effects of Increasing Nitrogen Content on Process Stability and Reactor Performance in Anaerobic Digestion. Energies. 2020; 13(5):1139. https://doi.org/10.3390/en13051139
Chicago/Turabian StyleMorozova, Ievgeniia, Nadiia Nikulina, Hans Oechsner, Johannes Krümpel, and Andreas Lemmer. 2020. "Effects of Increasing Nitrogen Content on Process Stability and Reactor Performance in Anaerobic Digestion" Energies 13, no. 5: 1139. https://doi.org/10.3390/en13051139
APA StyleMorozova, I., Nikulina, N., Oechsner, H., Krümpel, J., & Lemmer, A. (2020). Effects of Increasing Nitrogen Content on Process Stability and Reactor Performance in Anaerobic Digestion. Energies, 13(5), 1139. https://doi.org/10.3390/en13051139