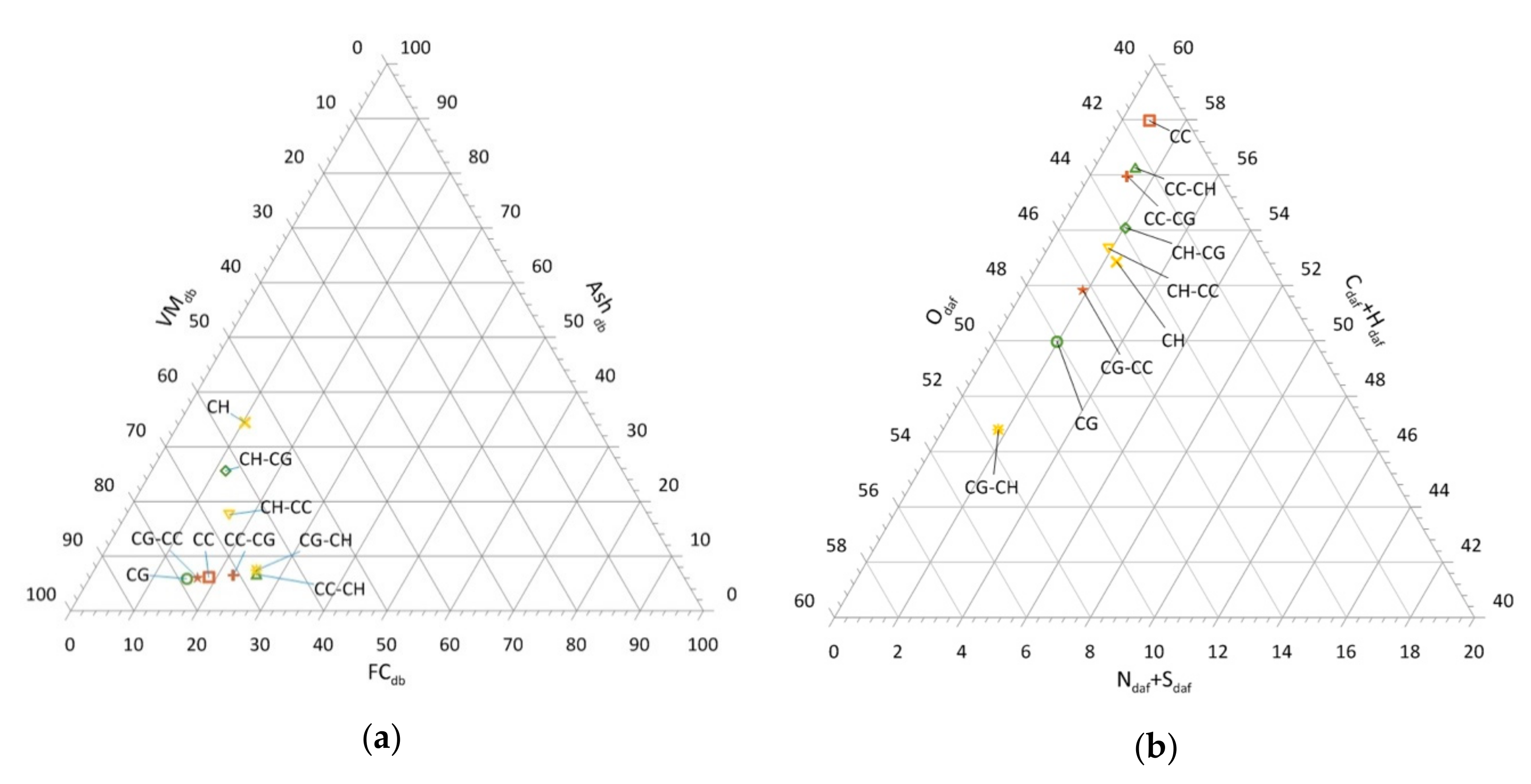
3.2. Results of Ashes’ Chemical Composition Examination
The assessment of the chemical composition of ash from biofuels (biomass) demonstrates the degree of impact of solid residues from the combustion process on the environment. Moreover, the assessment of ash in terms of macro- and microelements content makes it possible to demonstrate its degree of usefulness (e.g., for fertilizer (agricultural) purposes) [
28,
29]. On the basis of the analysis of the chemical composition of ash,
Table 1 presents the results of macroelements content in ashes from the analyzed biomass.
The analysis of macroelements content in the group of basic materials CC, CG, CH indicates their great diversity. The lowest content of particular elements was found in CG corn grain ashes. The content of phosphorus in the ashes of the biomass studied was in the range 21,452–47,778 ppm. The highest phosphorus content was recorded in the ash of CH and it was twice as high as in the ash of CG. Potassium content in ashes of basic materials was in a wide range of 25,970–258,259 ppm. It was found that CC ash was characterized by almost ten times higher potassium content than the lowest content obtained in CG ash. Calcium content ranged between 74,201–101,954 ppm and was the highest in CH ash, which was one and a half times higher than the lowest value obtained in CG ash. In addition, sulfur content almost twice as high was found in CH ash, compared to the lowest value (CG), where the results obtained were in the range 3438–6425 ppm.
The analysis of the macroelements content in the ashes of the created mixtures showed the dependence of their content on the content in ashes from the starting materials.
Figure 2 shows changes in the content of macroelements in all investigated ashes.
Phosphorus content in ash mixtures ranged from 22,688 to 41,719 ppm. For ash of CC-CH, CG-CH, and CG-CC mixtures, an increase in phosphorus content in relation to starting materials was recorded. In other cases, a decrease in the content of this element was shown. The content in the range 30,913–235,629 ppm was shown for potassium. An increase in potassium content in relation to ash from the starting materials was found in the ashes of CG-CC, CG-CH, and CH-CC mixtures. Calcium in mixtures from residues from corn grain drying was at a high level (i.e., in the range of 73,280–91,647 ppm). In this case, an increase in the ash content of this element can be observed for CC-CH, CG-CC, and CG-CH mixtures. The study carried out on the sulfur content in ash of the mixtures showed a large variation in the content of this element (i.e., 3726–6085 ppm). Only for the ashes of CG-CC and CG-CH mixtures, was an increase in the sulfur content in relation to the ashes from the starting materials recorded.
Analyzing the results presented in
Figure 2 for materials based mainly on CC, an increase in P, Ca, and S content for CC-CH mixture was observed compared to CC. In the case of potassium, a decrease in its concentration was noted compared to the initial material for both mixtures created. Comparing the obtained test results for mixtures based on CG, an increase of P, Ca and S content in relation to the CG starting material was noted. On the other hand, an almost three-fold increase in potassium concentration was noted in the ash of CG-CC mixture in relation to the ash from CG. For materials based on CH, a decrease in the concentration of P, Ca, and S was observed for all the mixtures in relation to CH. However, when potassium content was considered, it almost doubled in the mixture formed from CH-CC.
Table 2 shows the material series in descending order of the ash content of the individual macroelements.
As the results of the phosphorus content indicate, the lowest content was obtained in the ash of CG, while the highest concentration was in the ash of CH. The highest concentration of potassium among the materials studied was also found in the ash of CG and the highest in the ash of CC. In the case of calcium, the lowest content was recorded in the ash of CC-CG mixture, while the highest concentration was recorded in the ash of CH. The lowest sulfur content was found in the ash of CG and the highest in the ash of CH.
In relation to the mean potassium content in plant ashes (the world average for all parts of all plants), which is 387,755 ppm [
27], the examined ashes were characterized by a moderate and high content of this element. Vassilev et al. [
30] considered the aspects of chemical composition of different types of biomass constituting a potential source of energy (beech wood chips, corn cobs, marine macroalgae, plum pits, rice husks, switchgrass, sunflower shells, and walnut shells), found a similar K content in corn cobs ash, which was 277,900 ppm. High K content in corn cobs ash caused the material, together with the ash from marine macroalgae and sunflower shells, to be classified as one of the most problematic biomass resources from the technological and environmental point of view (biomass with similar chemical properties of ash, low acid–“K type”), for which the average K content was 211,600 ppm. However, the average content of this element in all ashes studied by these authors was 151,100 ppm.
Phosphorus was the second dominant element in the examined ashes. The content of this element in corn cobs ash, presented in the literature [
30], was 14,880 ppm, with an average content of 13,183 ppm in ash from this type of biomass and an average content of 10,439 ppm in ash from different types of biomass. The literature data [
27] show that the mean K content in plant ashes is much higher and amounts to 40,816 ppm.
Calcium content in corn cobs ash given in another study [
30] was lower than that found in this study and amounted to 10,700 ppm, while the mean Ca content in ash from this type of biomass and the mean Ca content in ash from various types of biomass were similar and amounted to 76,200 ppm and 110,800 ppm, respectively. The mean Ca content in plant ashes was 204,082 ppm [
27].
Vassilev et al. [
30] found a similar sulfur content in corn cobs ash, which amounted to 9700 ppm. The S content in ashes from this type of biomass was much higher and amounted to 47,200 ppm, while the average content in ashes from various types of biomass was also higher at 21,700 ppm. The average S content in plant ashes was also higher and amounted to 61,224 ppm [
27].
Table 3 presents the results of the study on the content of microelements in the ashes of the analyzed biomass.
In the case of microelements, also the lowest content was recorded in CG ash. The highest copper content was characteristic for ash obtained from CC and it was one and a half times higher than that obtained in CG ash. Ash with the highest iron content was recorded for CH with a value twice as high as the lowest one. CC ash was the richest in manganese, with the content two times higher in relation to CG. On the other hand, the highest zinc content was also found in CC ash with a value five times higher than the lowest in the group of starting raw materials.
The analysis of the content of microelements in the ashes of the created mixtures also showed the relationship of their content on the concentration in the ashes from the starting materials.
Figure 3 shows the changes of microelements content for all analyzed materials.
Copper content of the ash mixtures ranged from 145 to 195 ppm. An increase in copper content in relation to the ashes of the starting materials was recorded for the mixtures of CC-CH, CG-CH, and CH-CC. In the other cases, a decrease in the content of this element was shown. For iron, the content in the range 7859–13,014 ppm was shown. An increase in iron content in relation to the starting materials was shown in the ash of CC-CH, CG-CC, and CG-CH mixtures. Manganese in the ash of mixtures from the residues of drying corn grain was in the range 483–827 ppm. In this case, an increase in the content of this element in ash can be observed in the formation of CG-CC and CG-CH mixtures. The study carried out on the content of zinc in the ashes of mixtures showed large differences in the concentration of this element in ash (i.e., 187–699 ppm). For the ashes from CG-CC, CG-CH, and CH-CC mixtures, an increase in zinc content was recorded in relation to the ashes of the starting materials.
Analyzing the results of the study presented in
Figure 3 showing the content of microelements in ashes in materials based mainly on CC, a decrease in the content of Cu, Mn, and Zn in the ashes of both created mixtures (i.e., CC-CG and CC-CH in relation to CC), while an increase in the concentration of only Fe in the ash of CC-CG mixture can be observed. Comparing the results obtained for the ashes of CG-based mixtures, an increase in Cu, Mn, Zn, and Fe content in the ashes of both CG-CC and CG-CH mixtures was observed in relation to the ashes of the CG starting material. For ashes of CH-based materials, a decrease in Cu and Fe concentrations was noted for ashes of both formed mixtures, CH-CC and CH-CG; Mn and Zn for CH-CG ash as compared to CH ash. However, a higher content of Mn, Zn, and Cu in relation to CH ash was noted for ash of CH-CC mixture.
The analysis of the results allowed the ranking of mixtures in descending order depending on the concentration of particular microelements in ashes (
Table 4). For all analyzed microelements, the lowest concentration was found in CG ash. For copper content, the highest concentration was recorded in CC ash and for iron in CH ash. The highest content of manganese and zinc among the materials studied was found in the ash of CC.
Similar content of iron in CC ashes to that noted in the study can be found in the literature, and was 7400 ppm [
30]; the average content of this element in ashes from this type of biomass was 6100 ppm, and the average content in ashes from various types of biomass was 4000 ppm. On the other hand, the mean Fe content in plant ashes was lower and amounted to 3061 ppm [
27].
Manganese content in CC ash was slightly higher than that reported in the literature [
30], which was 404 ppm, but at the same time a similar content of this element was found in CG ash. The mean Mn content in ash from this type of biomass was lower and amounted to 312 ppm, while the mean content in ash from different types of biomass was 1862 ppm. The mean Mn content of plant ashes reported in the literature was much higher and amounted to 4082 ppm. [
27].
The content of copper in CC ash given in another study [
30] was similar to the results obtained in this study and amounted to 139 ppm; this element was found at a similar level of 169 ppm in ashes from biomass of this type. A similar average Cu content was also given for ashes from various types of biomass (232 ppm) and for plant ashes (204 ppm) [
27].
Zinc content found in the examined ashes did not differ from that given in the literature. Thus, the Zn content in corn cobs was slightly higher and amounted to 1,232 ppm, but the average content of this element in ashes from this type of biomass was much lower and amounted to 541 ppm. The mean Zn content in various types of biomass was found at 366 ppm [
30]. In turn, mean Zn content in plant ashes was 1020 ppm [
27].
Table 5 shows the results of the concentration of toxic elements in ashes of the biomass tested.
CH ash was the richest in nickel, which was one and a half times higher than the lowest obtained in CC ash. The lowest chromium content was recorded in CC ash, while the highest (one and a half times higher) was in CH ash. The ash of CC was also characterized by the lowest content of lead, while the highest content was found in CH ash. The lowest chromium content was found in the ash of CG, while the highest concentration was in the ash of CC (twice as high). In addition, the lowest arsenic content was found in the ash of CC and the highest concentration was in CH ash.
Figure 4 shows the distribution of toxic elements for all analyzed materials.
Analysis of the content of toxic elements in the ashes of the created mixtures again showed the relationship of their content to the content in ashes from the starting materials. Nickel content in the mixtures ranged from 322 to 482 ppm. An increase in nickel content in relation to the starting materials was recorded for the ash of CC-CH, CG-CH, and CG-CH mixtures. In other cases, a decrease in the content of this element was shown. For chromium, content in the range 208–283 ppm was shown. An increase in nickel content in relation to the starting materials was shown for CC-CH, CC-CG, and CG-CH. Lead in ashes of mixtures from residues of corn grain drying was in the range 27.49–38.97 ppm. In this case, an increase in this element in ash can be observed when composing CC-CH, CC-CG, and CG-CH mixtures. The study carried out on the arsenic content in ashes of the mixtures showed a variation in the concentration of this element (i.e., 2.09–4.22 ppm). For ash of CC-CH, CC-CG, CG-CH, and CG-CC mixtures, an increase in arsenic content in relation to ashes of the starting materials was noted.
Analyzing the results of the study presented in
Figure 4 showing the concentration of toxic elements in ashes of materials based mainly on CC, an increase in the content of all toxic elements for both created mixtures (i.e., CC-CG and CC-CH), compared to CC ash can be observed. Comparing the results obtained for the ashes of CG-based mixtures, an increase in the content of Ni, Pb, Cr, and As in the ash of CG-CH mixture was noted in relation to the ash of the CG starting material. On the other hand, a decrease in the concentration of Ni, Pb, Cr, and As in relation to the ash of CG was shown for the CG-CC mixture. For materials based on CH, only an increase in As concentration in the ash of CH-CG mixture in relation to CH ash and a decrease in the content of other toxic elements in ash of both created mixtures can be observed.
Table 6 shows the series of materials in decreasing order of individual toxic elements content in the ashes. The material with the highest concentration of toxic elements is CH ash. The lowest concentration of nickel, chromium, and arsenic was found in the ash of CC, whereas in the case of lead in the ash of CC-CG mixture.
The content of nickel in CC ash found in the study was similar to that reported in the literature, which was 286 ppm, while the other examined ashes were characterized by a higher content of this element. The mean Zn content in ashes from this type of biomass was found at the level of 301 ppm and the content in ashes from various types of biomass was 151 ppm [
30]. The mean Ni content in plant ashes was much lower and amounted to 31 ppm [
27].
Chromium content found in the examined ashes was significantly lower than that reported in the literature [
30]. Thus, the content of Cr in the ash from CC was 750 ppm, the average content of this element in ashes from this type of biomass was 733 ppm, and the average content in ashes from various types of biomass was 384 ppm. In turn, the mean Cr content in plant ashes was much lower and amounted to 31 ppm [
27].
Vassilev et al. [
30] found lead content in CC ashes at a much higher level of 68.3 ppm. In general, the content of this element in ash from biomass was observed within wide limits. The average content of this element in ashes from biomass of this type was 24.2 ppm, while the average content in ashes from various types of biomass was 0.44 ppm. The average content of Pb in plant ashes was 20 ppm [
27].
The arsenic content in CC ash found in the study was similar to that reported in the literature for the same biomass, which was 6.4 ppm. The average content of this element in ash from this type of biomass was 5.7 ppm and 6.3 ppm in ashes from different types of biomass [
30]. At the same time, the average As content in plant ashes was found at the level of 2 ppm [
27].